Clinical Spectrum of Schistosomiasis: An Update
Abstract
:1. Introduction
2. Cercarial Dermatitis or Swimmer’s Itch
3. Acute Schistosomiasis
4. Chronic Schistosomiasis
5. Intestinal Schistosomiasis
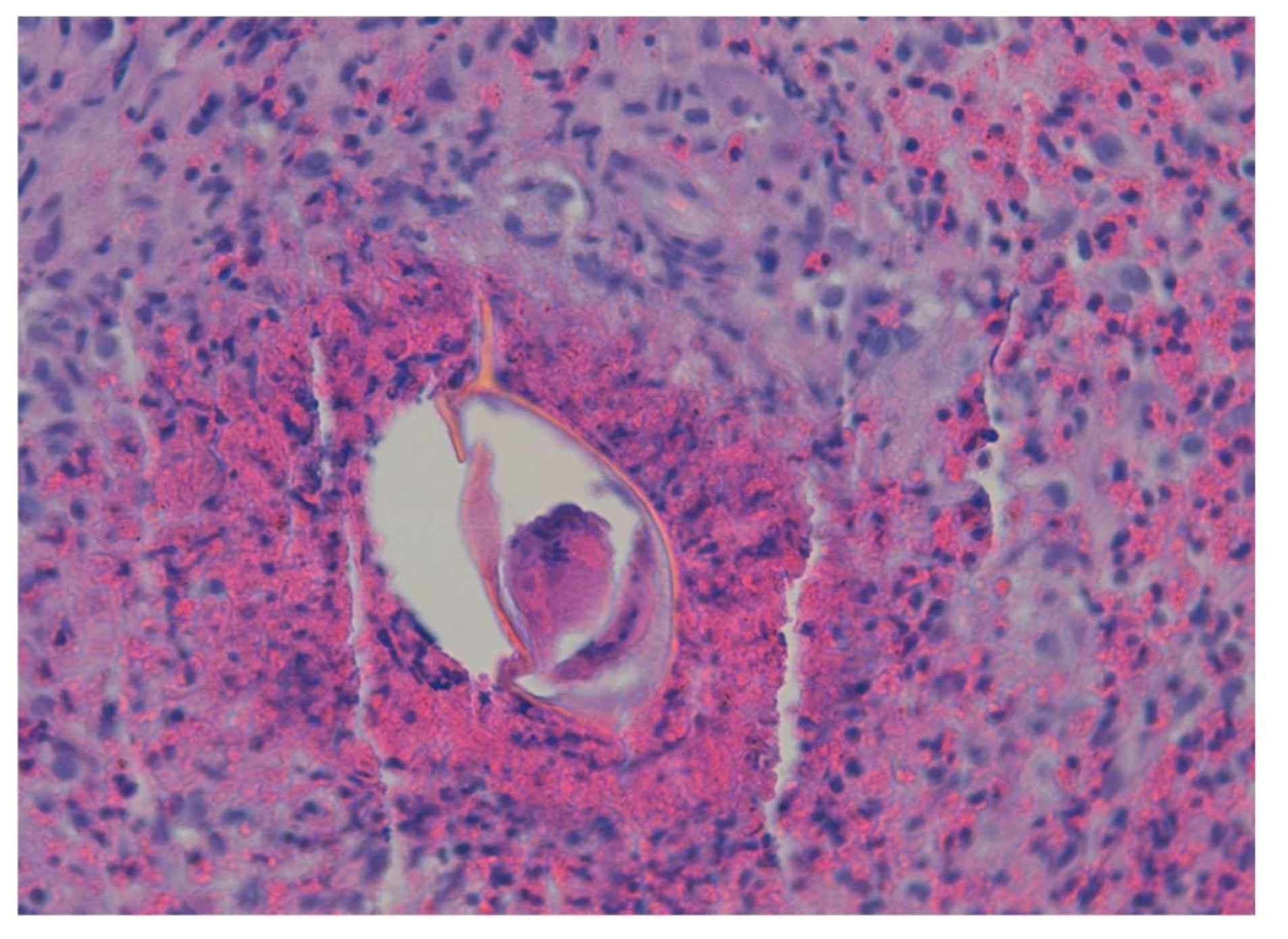
6. Hepatosplenic Schistosomiasis
7. Neuroschistosomiasis
8. Urogenital Schistosomiasis
8.1. Urinary Schistosomiasis
8.2. Genital Schistosomiasis
9. Pulmonary Schistosomiasis
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gautret, P.; Cramer, J.P.; Field, V.; Caumes, E.; Jensenius, M.; Gkrania-Klotsas, E.; de Vries, P.J.; Grobusch, M.P.; Lopez-Velez, R.; Castelli, F.; et al. Infectious diseases among travellers and migrants in Europe, EuroTravNet 2010. Eurosurveillance 2012, 17, 20205. [Google Scholar] [CrossRef] [PubMed]
- Tracz, E.; Al-Jubury, A.; Buchmann, K.; Bygum, A. Outbreak of Swimmer’s Itch in Denmark. Acta Derm-Venereol. 2019, 99, 1116–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leshem, E.; Maor, Y.; Meltzer, E.; Assous, M.; Schwartz, E. Acute Schistosomiasis Outbreak: Clinical Features and Economic Impact. Clin. Infect. Dis. 2008, 47, 1499–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colebunders, R.; Verstraeten, T.; Van Gompel, A.; Ende, J.V.D.; De Roo, A.; Polderman, A.; Visser, L. Acute Schistosomiasis in Travelers Returning from Mali. J. Travel Med. 1995, 2, 235–238. [Google Scholar] [CrossRef] [Green Version]
- Ross, A.G.; Vickers, D.; Olds, G.R.; Shah, S.M.; McManus, D.P. Katayama syndrome. Lancet Infect. Dis. 2007, 7, 218–224. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Wang, X.; Xu, X.; Pan, W. Evaluation of six novel antigens as potential biomarkers for the early immunodiagnosis of schistosomiasis. Parasite Vector 2015, 8, 447. [Google Scholar] [CrossRef] [Green Version]
- Meurs, L.; Brienen, E.; Mbow, M.; Ochola, E.A.; Mboup, S.; Karanja, D.M.S.; Secor, W.E.; Polman, K.; Van Lieshout, L. Is PCR the Next Reference Standard for the Diagnosis of Schistosoma in Stool? A Comparison with Microscopy in Senegal and Kenya. PLoS Neglect. Trop. D 2015, 9, e0003959. [Google Scholar] [CrossRef]
- Lingscheid, T.; Witzenrath, M.; Bouchaud, O.; Kern, P.; Da Cunha, J.S.; Beltrame, A.; Zammarchi, L.; Paul, M.; Kosina, P.; Marocco, S.; et al. in European Travelers and Migrants: Analysis of 14 Years TropNet Surveillance Data. Am. J. Trop. Med. Hyg. 2017, 97, 567–574. [Google Scholar] [CrossRef] [Green Version]
- Jauréguiberry, S.; Paris, L.; Caumes, E. Acute schistosomiasis, a diagnostic and therapeutic challenge. Clin. Microbiol. Infect. 2010, 16, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Marchese, V.; Beltrame, A.; Angheben, A.; Monteiro, G.B.; Giorli, G.; Perandin, F.; Buonfrate, D.; Bisoffi, Z. Schistosomiasis in immigrants, refugees and travellers in an Italian referral centre for tropical diseases. Infect. Dis. Poverty 2018, 7, 55. [Google Scholar] [CrossRef] [Green Version]
- Salvana, E.M.T.; King, C.H. Schistosomiasis in travelers and immigrants. Curr. Infect. Dis. Rep. 2008, 10, 42–49. [Google Scholar] [CrossRef]
- Norman, F.F.; Monge-Maillo, B.; Martínez-Pérez, Á.; Perez-Molina, J.A.; López-Vélez, R. Parasitic infections in travelers and immigrants: Part II helminths and ectoparasites. Future Microbiol. 2015, 10, 87–99. [Google Scholar] [CrossRef]
- Agbessi, C.-A.; Bourvis, N.; Fromentin, M.; Jaspard, M.; Teboul, F.; Bougnoux, M.-E.; Hanslik, T. La bilharziose d’importation chez les voyageurs: Enquête en France métropolitaine. Rev. Méd. Interne 2006, 27, 595–599. [Google Scholar] [CrossRef]
- Nicolls, D.J.; Weld, L.H.; Schwartz, E.; Reed, C.; von Sonnenburg, F.; Freedman, D.O.; Kozarsky, P.E. Characteristics of schistosomiasis in travelers reported to the GeoSentinel Surveillance Network 1997–2008. Am. J. Trop. Med. Hyg. 2008, 79, 729–734. [Google Scholar] [CrossRef]
- Grobusch, M.P.; Mühlberger, N.; Jelinek, T.; Bisoffi, Z.; Corachán, M.; Harms, G.; Matteelli, A.; Fry, G.; Hatz, C.; Gjørup, I.; et al. Imported schistosomiasis in Europe: Sentinel surveillance data from TropNetEurop. J. Travel Med. 2003, 10, 164–169. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Menéndez, M.; Molina, J.A.P.; Serre, N.; Treviño, B.; Torrús, D.; Matarranz, M.; Martín, E.; Rojo-Marcos, G.; Aguilera, P.; Rico, A.; et al. Imported diseases by immigrants and travellers: Results from the Cooperative Network for the study of Imported Diseases by Immigrants and Travellers +Redivi. Enferm. Infecc. Microbiol. Clín. 2012, 30, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Ben-Chetrit, E.; Lachish, T.; Mørch, K.; Atias, D.; Maguire, C.; Schwartz, E. Schistosomiasis in Pregnant Travelers: A Case Series. J. Travel Med. 2015, 22, 94–98. [Google Scholar] [CrossRef] [Green Version]
- Beltrame, A.; Zammarchi, L.; Zuglian, G.; Gobbi, F.; Angheben, A.; Marchese, V.; Degani, M.; Mantella, A.; Bianchi, L.; Montagnani, C.; et al. Schistosomiasis Screening of Travelers from Italy with Possible Exposure in Corsica, France. Emerg. Infect. Dis. 2015, 21, 1887–1889. [Google Scholar] [CrossRef] [Green Version]
- Rochat, L.; Bizzini, A.; Senn, N.; Bochud, P.; Genton, B.; de Vallière, S. Acute Schistosomiasis: A Risk Underestimated by Travelers and a Diagnosis Frequently Missed by General Practitioners—A Cluster Analysis of 42 Travelers. J. Travel Med. 2015, 22, 168–173. [Google Scholar] [CrossRef] [Green Version]
- Pavlin, B.I.; Kozarsky, P.; Cetron, M.S. Acute pulmonary schistosomiasis in travelers: Case report and review of the literature. Travel Med. Infect. Dis. 2012, 10, 209–219. [Google Scholar] [CrossRef]
- Norman, F.; Chamorro, S.; Comeche, B.; Pérez-Molina, J.; López-Vélez, R. Update on the major imported helminth infections in travelers and migrants. Future Microbiol. 2020, 15, 437–444. [Google Scholar] [CrossRef]
- Bottieau, E.; Clerinx, J.; de Vega, M.R.; den Enden, E.V.; Colebunders, R.; Van Esbroeck, M.; Vervoort, T.; Van Gompel, A.; Ende, J.V.D. Imported Katayama fever: Clinical and biological features at presentation and during treatment. J. Infect. 2006, 52, 339–345. [Google Scholar] [CrossRef]
- Leshem, E.; Meltzer, E.; Marva, E.; Schwartz, E. Travel-related Schistosomiasis Acquired in Laos. Emerg. Infect. Dis. 2009, 15, 1823–1826. [Google Scholar] [CrossRef] [PubMed]
- Jauréguiberry, S.; Caumes, E. Neurological involvement during Katayama syndrome. Lancet Infect. Dis. 2008, 8, 9–10. [Google Scholar] [CrossRef]
- Granier, H.; Potard, M.; Diraison, P.; Nicolas, X.; Laborde, J.P.; Talarmin, F. Acute encephalitis concurrent with primary infection by Schistosoma mansoni. Med. Trop. Rev. Corps Sante Colon. 2003, 63, 60–63. [Google Scholar]
- Caumes, E.; Perez, L.; Bricaire, F.; Danis, M.; Jauréguiberry, S.; Ansart, S. Acute neuroschistosomiasis: Two cases associated with cerebral vasculitis. Am. J. Trop. Med. Hyg. 2007, 76, 964–966. [Google Scholar] [CrossRef]
- Camuset, G.; Wolff, V.; Marescaux, C.; Abou-Bacar, A.; Candolfi, E.; Lefebvre, N.; Christmann, D.; Hansmann, Y. Cerebral vasculitis associated with Schistosoma mansoniinfection. BMC Infect. Dis. 2012, 12, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jauréguiberry, S.; Epelboin, L.; Estève, J.-B.; Danis, M.; Komajda, M.; Caumes, E.; Bricaire, F. Myocarditis during Acute Schistosomiasis in Two Travelers. Am. J. Trop. Med. Hyg. 2010, 82, 365–367. [Google Scholar] [CrossRef]
- Most, H.; Kane, C.A.; Lavietes, P.H.; Schroeder, E.F.; Behm, A.; Blum, L.; Katzin, B.; Hayman, J.M., Jr. Schistosomiasis Japonica in American Military Personnel: Clinical Studies of 600 Cases during the First Year after Infection 1,2. Am. J. Trop. Med. Hyg. 1950, 30, 239–299. [Google Scholar] [CrossRef]
- De Jesus, A.R.; Silva, A.; Santana, L.B.; Magalhães, A.; de Jesus, A.A.; de Almeida, R.P.; Rego, M.A.V.; Burattini, M.N.; Pearce, E.J.; Carvalho, E.M. Clinical and Immunologic Evaluation of 31 Patients with Acute Schistosomiasis mansoni. J. Infect. Dis. 2002, 185, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Baird, T.; Cooper, C.L.; Wong, R.; Runnegar, N.; Keir, G. Pulmonary schistosomiasis mimicking IgG4-related lung disease. Respirol. Case Rep. 2018, 6, e00276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, E.; Rozenman, J.; Perelman, M. Pulmonary manifestations of early schistosome infection among nonimmune travelers. Am. J. Med. 2000, 109, 718–722. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2013, 380, 2197–2223. [Google Scholar] [CrossRef]
- King, C.H. Parasites and poverty: The case of schistosomiasis. Acta Trop. 2010, 113, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Zoni, A.C.; Catalá, L.; Ault, S.K. Schistosomiasis Prevalence and Intensity of Infection in Latin America and the Caribbean Countries, 1942-2014: A Systematic Review in the Context of a Regional Elimination Goal. PLoS Neglect. Trop. D 2016, 10, e0004493. [Google Scholar] [CrossRef] [Green Version]
- Asundi, A.; Beliavsky, A.; Liu, X.J.; Akaberi, A.; Schwarzer, G.; Bisoffi, Z.; Mendez, A.R.; Shrier, I.; Greenaway, C. Prevalence of strongyloidiasis and schistosomiasis among migrants: A systematic review and meta-analysis. Lancet Global Health 2019, 7, e236–e248. [Google Scholar] [CrossRef] [Green Version]
- McLellan, J.; Gill, M.J.; Vaughan, S.; Meatherall, B. Schistosoma and Strongyloides screening in migrants initiating HIV Care in Canada: A cross sectional study. BMC Infect. Dis. 2020, 20, 76. [Google Scholar] [CrossRef]
- Williams, B.; Boullier, M.; Cricks, Z.; Ward, A.; Naidoo, R.; Williams, A.; Robinson, K.; Eisen, S.; Cohen, J. Screening for infection in unaccompanied asylum-seeking children and young people. Arch. Dis. Child. 2020, 105, 530–532. [Google Scholar] [CrossRef]
- Buonfrate, D.; Tamarozzi, F.; Gobbi, F. Imported chronic schistosomiasis: Screening and management issues. J. Travel Med. 2020, 27, taaa005. [Google Scholar] [CrossRef]
- Salas-Coronas, J.; Vázquez-Villegas, J.; Lozano-Serrano, A.B.; Soriano-Pérez, M.J.; Cabeza-Barrera, I.; Cabezas-Fernández, M.T.; Villarejo-Ordóñez, A.; Sánchez-Sánchez, J.C.; Vivas-Pérez, J.I.A.; Vázquez-Blanc, S.; et al. Severe complications of imported schistosomiasis, Spain: A retrospective observational study. Travel Med. Infect. Dis. 2020, 35, 101508. [Google Scholar] [CrossRef]
- Potters, I.; Duffel, L.V.; Broeckx, G.; Bottieau, E. Intestinal schistosomiasis: A very long-lived tropical parasite. Clin. Microbiol. Infect. 2019, 25, 696–698. [Google Scholar] [CrossRef]
- Qin, X.; Liu, C.-Y.; Xiong, Y.-L.; Bai, T.; Zhang, L.; Hou, X.-H.; Song, J. The clinical features of chronic intestinal schistosomiasis-related intestinal lesions. BMC Gastroenterol. 2021, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- Combes, A.D.; Magdy, M.; Morris, D.L. Intestinal schistosomiasis mimicking caecal malignancy. ANZ J. Surg. 2020, 90, 2576–2577. [Google Scholar] [CrossRef]
- Diab, R.G.; Tolba, M.M.; Ghazala, R.A.; Abu-Sheasha, G.A.; Webster, B.L.; Mady, R.F. Intestinal schistosomiasis: Can a urine sample decide the infection? Parasitol. Int. 2021, 80, 102201. [Google Scholar] [CrossRef] [PubMed]
- Tamarozzi, F.; Fittipaldo, V.A.; Orth, H.M.; Richter, J.; Buonfrate, D.; Riccardi, N.; Gobbi, F.G. Diagnosis and clinical management of hepatosplenic schistosomiasis: A scoping review of the literature. PLoS Neglect. Trop. D 2021, 15, e0009191. [Google Scholar] [CrossRef] [PubMed]
- Abdela, S.G.; Hassen, N.G.; Hussien, F.M.; Yesuf, A.M.; van Griensven, J.; van Henten, S. Hepatosplenic schistosomiasis, the ignored morbidity: Experience from a referral hospital in Ethiopia. Trans. R. Soc. Trop. Med. Hyg. 2020, 115, 57–62. [Google Scholar] [CrossRef]
- Baekby, M.; Glerup, H.; Stribolt, K.; Tarp, B. Hepatosplenic schistosomiasis: Playing hide-and-seek with an elusive parasite. BMJ Case Rep. 2017, 2017, bcr-2017. [Google Scholar] [CrossRef]
- Lambertucci, J.R. Revisiting the concept of hepatosplenic schistosomiasis and its challenges using traditional and new tools. Rev. Soc. Bras. Med. Trop. 2014, 47, 130–136. [Google Scholar] [CrossRef] [Green Version]
- Berkowitz, A.L.; Raibagkar, P.; Pritt, B.S.; Mateen, F.J. Neurologic manifestations of the neglected tropical diseases. J. Neurol. Sci. 2015, 349, 20–32. [Google Scholar] [CrossRef]
- Ferrari, T.C.A.; Moreira, P.R.R. Neuroschistosomiasis: Clinical symptoms and pathogenesis. Lancet Neurol. 2011, 10, 853–864. [Google Scholar] [CrossRef]
- Alves, W. The distribution of Schistosoma eggs in human tissues. Bull. World Health Organ. 1958, 18, 1092–1097. [Google Scholar] [PubMed]
- Ross, A.G.; McManus, D.P.; Farrar, J.; Hunstman, R.J.; Gray, D.J.; Li, Y.-S. Neuroschistosomiasis. J. Neurol. 2012, 259, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Koibuchi, T.; Kumagai, T.; Maeda, T.; Osada, Y.; Ohta, N.; Koga, M.; Nakamura, H.; Miura, T.; Iwamoto, A.; et al. Cerebral Schistosomiasis Due to Schistosoma haematobium Confirmed by PCR Analysis of Brain Specimen. J. Clin. Microbiol. 2011, 49, 3703–3706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barsoum, R.S. Urinary Schistosomiasis: Review. J. Adv. Res. 2013, 4, 453–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salas-Coronas, J.; Vázquez-Villegas, J.; Soriano-Pérez, M.J.; Cabezas-Fernández, M.T. Esquistosomiasis urinaria. Med. Clín. 2014, 142, 281. [Google Scholar] [CrossRef]
- Hotez, P.J.; Engels, D.; Gyapong, M.; Ducker, C.; Malecela, M.N. Female Genital Schistosomiasis. N. Engl. J. Med. 2019, 381, 2493–2495. [Google Scholar] [CrossRef]
- Sturt, A.; Webb, E.; Francis, S.; Hayes, R.; Bustinduy, A. Beyond the barrier: Female Genital Schistosomiasis as a potential risk factor for HIV-1 acquisition. Acta Trop. 2020, 209, 105524. [Google Scholar] [CrossRef]
- Kjetland, E.F.; Leutscher, P.D.C.; Ndhlovu, P.D. A review of female genital schistosomiasis. Trends Parasitol. 2012, 28, 58–65. [Google Scholar] [CrossRef]
- Sturt, A.S.; Webb, E.L.; Phiri, C.R.; Mweene, T.; Chola, N.; van Dam, G.J.; Corstjens, P.L.A.M.; Wessels, E.; Stothard, J.R.; Hayes, R.; et al. Genital self-sampling compared with cervicovaginal lavage for the diagnosis of female genital schistosomiasis in Zambian women: The BILHIV study. PLoS Neglect. Trop. D 2020, 14, e0008337. [Google Scholar] [CrossRef]
- Fernandes, C.J.C.D.S.; Jardim, C.V.P.; Hovnanian, A.; Hoette, S.; Morinaga, L.K.; Souza, R. Schistosomiasis and pulmonary hypertension. Expert Rev. Respir. Med. 2014, 5, 675–681. [Google Scholar] [CrossRef]
- Sarwat, A.K.; Din, M.A.T.E.; Bassiouni, M.; Ashmawi, S.S. Schistosomiasis of the lung. J. Egypt. Soc. Parasitol. 1986, 16, 359–366. [Google Scholar] [PubMed]
- Andrade, Z.A.; Andrade, S.G. Pathogenesis of Schistosomal Pulmonary Arteritis. Am. J. Trop. Med. Hyg. 1970, 19, 305–310. [Google Scholar] [CrossRef] [PubMed]





| Types of Presentations | Clinical Manifestations |
|---|---|
| Swimmer’s itch | Local inflammation of the cercariae entry zone, most frequently caused by non-human pathogenic species that cannot migrate |
| Cercarial dermatitis | Maculopapular skin rash. It develops in previously sensitized people when they are reinfected by non-human pathogenic species |
| Katayama syndrome | Delayed systemic hypersensitivity reaction (3 and 8 weeks after exposure) It affects more than 50% of infected people. Fever, arthralgia, and cutaneous vasculitis and eosinophilia are the most common clinical manifestations. Spontaneous resolution after 2 to 10 weeks A minority develop persistent disease (weight loss, dyspnoea and diarrhoea, abdominal pain, hepatosplenomegaly) |
| Pulmonary form | Pulmonary symptoms resulting from the systemic immunoallergic reaction of acute schistosomiasis in non-immune patients. It presents as dyspnoea, bronchospasm, productive cough, haemoptysis, and/or chest pain, which may appear in isolation or within the clinical picture of Katayama fever |
| Non-Endemic Area Resident | Endemic Area Resident | |
|---|---|---|
| Most common form of disease | Acute schistosomiasis | Chronic infections |
| Age group | Adult | Children–adolescents–young adult |
| Most common clinical manifestations | Skin lesions (pruritus, skin eruption), fever, cough, abdominal pain, and diarrhoea | Anaemia, haematuria, abdominal pain, hepatomegaly |
| Diagnostic clues | History of exposure to fresh water in an area of endemicity | Abdominal pain, haematuria or/and genito-urinary symptoms More frequent ova identification and increased IgE |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbonell, C.; Rodríguez-Alonso, B.; López-Bernús, A.; Almeida, H.; Galindo-Pérez, I.; Velasco-Tirado, V.; Marcos, M.; Pardo-Lledías, J.; Belhassen-García, M. Clinical Spectrum of Schistosomiasis: An Update. J. Clin. Med. 2021, 10, 5521. https://doi.org/10.3390/jcm10235521
Carbonell C, Rodríguez-Alonso B, López-Bernús A, Almeida H, Galindo-Pérez I, Velasco-Tirado V, Marcos M, Pardo-Lledías J, Belhassen-García M. Clinical Spectrum of Schistosomiasis: An Update. Journal of Clinical Medicine. 2021; 10(23):5521. https://doi.org/10.3390/jcm10235521
Chicago/Turabian StyleCarbonell, Cristina, Beatriz Rodríguez-Alonso, Amparo López-Bernús, Hugo Almeida, Inmaculada Galindo-Pérez, Virginia Velasco-Tirado, Miguel Marcos, Javier Pardo-Lledías, and Moncef Belhassen-García. 2021. "Clinical Spectrum of Schistosomiasis: An Update" Journal of Clinical Medicine 10, no. 23: 5521. https://doi.org/10.3390/jcm10235521
APA StyleCarbonell, C., Rodríguez-Alonso, B., López-Bernús, A., Almeida, H., Galindo-Pérez, I., Velasco-Tirado, V., Marcos, M., Pardo-Lledías, J., & Belhassen-García, M. (2021). Clinical Spectrum of Schistosomiasis: An Update. Journal of Clinical Medicine, 10(23), 5521. https://doi.org/10.3390/jcm10235521






