The Morphological Changes in Adjacent Segments Amongst Patients Receiving Anterior and Oblique Lumbar Interbody Fusion: A Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Operation Procedures
2.2.1. ALIF Procedure
2.2.2. OLIF Procedure
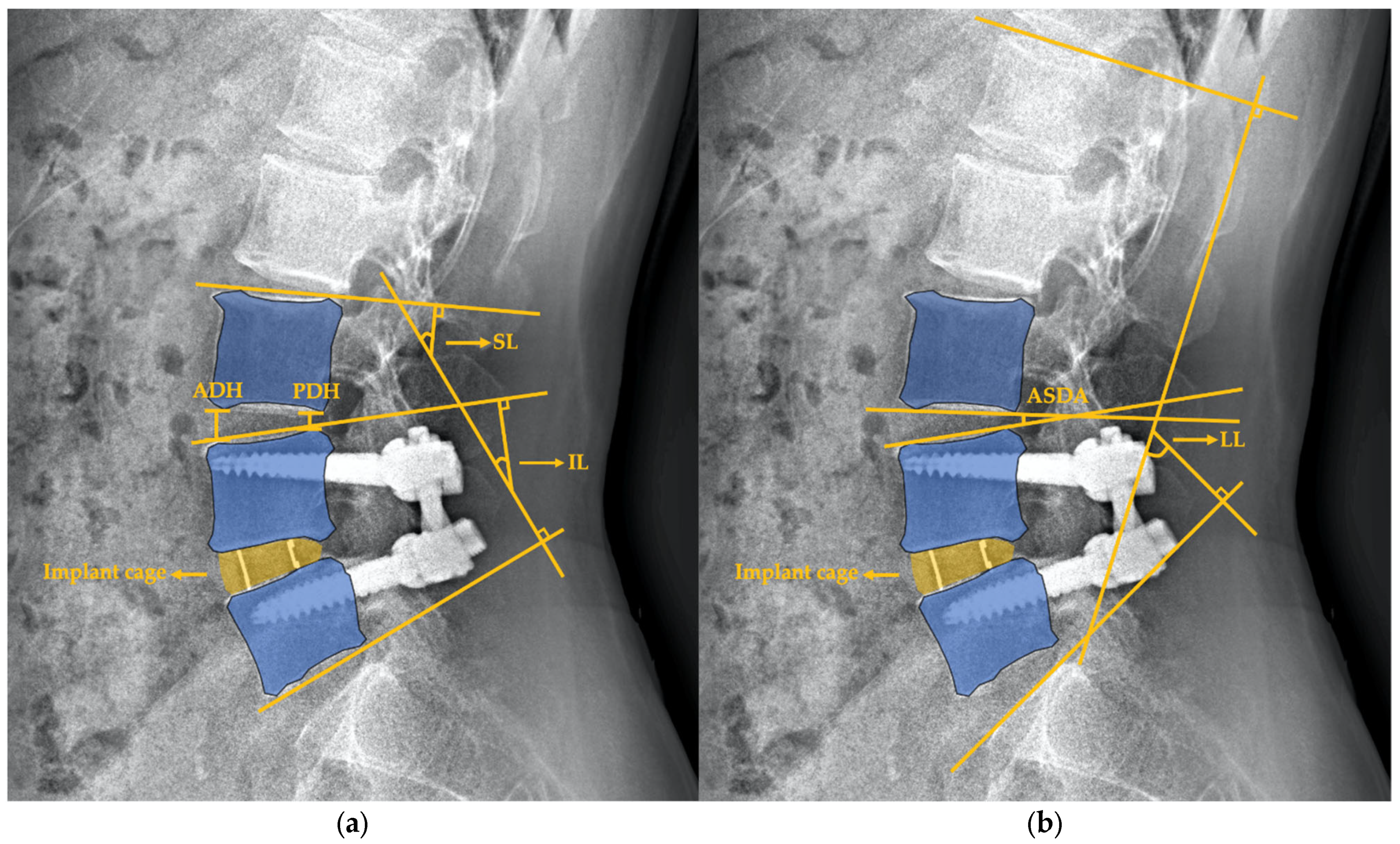
2.3. Radiographic Assessment
2.4. Outcome Measurements
2.5. Statistical Analyses
3. Results
3.1. Patient Population Demographics
3.2. Morphologic and Functional Outcomes of All Patients
3.3. Morphologic and Functional Outcomes in the ALIF and OLIF Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leone, A.; Guglielmi, G.; Cassar-Pullicino, V.N.; Bonomo, L. Lumbar intervertebral instability: A review. Radiology 2007, 245, 62–77. [Google Scholar] [CrossRef]
- He, B.; Yan, L.; Guo, H.; Liu, T.; Wang, X.; Hao, D. The difference in superior adjacent segment pathology after lumbar posterolateral fusion by using 2 different pedicle screw insertion techniques in 9-year minimum follow-up. Spine 2014, 39, 1093–1098. [Google Scholar] [CrossRef]
- Hilibrand, A.S.; Robbins, M. Adjacent segment degeneration and adjacent segment disease: The consequences of spinal fusion? Spine J. 2004, 4, 190s–194s. [Google Scholar] [CrossRef]
- Park, P.; Garton, H.J.; Gala, V.C.; Hoff, J.T.; McGillicuddy, J.E. Adjacent segment disease after lumbar or lumbosacral fusion: Review of the literature. Spine 2004, 29, 1938–1944. [Google Scholar] [CrossRef] [PubMed]
- Oda, I.; Cunningham, B.W.; Buckley, R.A.; Goebel, M.J.; Haggerty, C.J.; Orbegoso, C.M.; McAfee, P.C. Does spinal kyphotic deformity influence the biomechanical characteristics of the adjacent motion segments? An in vivo animal model. Spine 1999, 24, 2139–2146. [Google Scholar] [CrossRef] [Green Version]
- Schwab, F.J.; Blondel, B.; Bess, S.; Hostin, R.; Shaffrey, C.I.; Smith, J.S.; Boachie-Adjei, O.; Burton, D.C.; Akbarnia, B.A.; Mundis, G.M.; et al. Radiographical spinopelvic parameters and disability in the setting of adult spinal deformity: A prospective multicenter analysis. Spine 2013, 38, E803–E812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Huec, J.; Thompson, W.; Mohsinaly, Y.; Barrey, C.; Faundez, A. Sagittal balance of the spine. Eur. Spine J. 2019, 28, 1889–1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.B.; Jeon, T.S.; Heo, Y.M.; Lee, W.S.; Yi, J.W.; Kim, T.K.; Hwang, C.M. Radiographic results of single level transforaminal lumbar interbody fusion in degenerative lumbar spine disease: Focusing on changes of segmental lordosis in fusion segment. Clin. Orthop. Surg. 2009, 1, 207–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, T.; An, H.S.; Haughton, V.M.; Nowicki, B.H. Lumbar foraminal stenosis: Critical heights of the intervertebral discs and foramina. A cryomicrotome study in cadavera. J. Bone Jt. Surg. Am. 1995, 77, 32–38. [Google Scholar] [CrossRef]
- Griffith, J.F.; Wang, Y.-X.J.; Antonio, G.E.; Choi, K.C.; Yu, A.; Ahuja, A.T.; Leung, P.C. Modified pfirrmann grading system for lumbar intervertebral disc degeneration. Spine 2007, 32, E708–E712. [Google Scholar] [CrossRef]
- Pfirrmann, C.W.; Metzdorf, A.; Zanetti, M.; Hodler, J.; Boos, N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 2001, 26, 1873–1878. [Google Scholar] [CrossRef]
- Rabin, R.; de Charro, F. EQ-5D: A measure of health status from the EuroQol Group. Ann. Med. 2001, 33, 337–343. [Google Scholar] [CrossRef]
- Fairbank, J.C.; Pynsent, P.B. The Oswestry disability index. Spine 2000, 25, 2940–2953. [Google Scholar] [CrossRef]
- Nakashima, H.; Kawakami, N.; Tsuji, T.; Ohara, T.; Suzuki, Y.; Saito, T.; Nohara, A.; Tauchi, R.; Ohta, K.; Hamajima, N.; et al. Adjacent segment disease after posterior lumbar interbody fusion: Based on cases with a minimum of 10 years of follow-up. Spine 2015, 40, E831–E841. [Google Scholar] [CrossRef]
- Hutter, C.G. Posterior intervertebral body fusion. A 25-year study. Clin. Orthop. Relat. Res. 1983, 179, 86–96. [Google Scholar] [CrossRef]
- Santos, E.R.; Goss, D.G.; Morcom, R.K.; Fraser, R.D. Radiologic assessment of interbody fusion using carbon fiber cages. Spine 2003, 28, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Chen, J.; Fan, J.; Li, Q.; Yin, G.; Yu, L. Effect of zoledronic acid on lumbar spinal fusion in osteoporotic patients. Eur. Spine J. 2017, 26, 2969–2977. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yoon, K.; Ha, S. Which approach is advantageous to preventing the development of asd? A comparative analysis of 3 different lumbar interbody fusion techniques (alif, llif, and plif) in l4-5 spondylolisthesis. World Neurosurg. 2017, 105, 612–622. [Google Scholar] [CrossRef]
- Ekman, P.; Möller, H.; Shalabi, A.; Yu, Y.X.; Hedlund, R. A prospective randomised study on the long-term effect of lumbar fusion on adjacent disc degeneration. Eur. Spine J. 2009, 18, 1175–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imagama, S.; Kawakami, N.; Matsubara, Y.; Kanemura, T.; Tsuji, T.; Ohara, T. Preventive effect of artificial ligamentous stabilization on the upper adjacent segment impairment following posterior lumbar interbody fusion. Spine 2009, 34, 2775–2781. [Google Scholar] [CrossRef]
- Huang, Y.P.; Du, C.F.; Cheng, C.K.; Zhong, Z.C.; Chen, X.W.; Wu, G.; Li, Z.C.; Ye, J.D.; Lin, J.H.; Wang, L.Z. Preserving posterior complex can prevent adjacent segment disease following posterior lumbar interbody fusion surgeries: A finite element analysis. PLoS ONE 2016, 11, e0166452. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ma, L.; Yang, D.; Wang, T.; Liu, S.; Yang, S.; Ding, W. Incidence and risk factors of adjacent segment disease following posterior decompression and instrumented fusion for degenerative lumbar disorders. Medicine 2017, 96, e6032. [Google Scholar] [CrossRef] [PubMed]
- Okuda, S.; Yamashita, T.; Matsumoto, T.; Nagamoto, Y.; Sugiura, T.; Takahashi, Y.; Maeno, T.; Iwasaki, M. Adjacent segment disease after posterior lumbar interbody fusion: A case series of 1000 patients. Glob. Spine J. 2018, 8, 722–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsuura, A.; Hukuda, S.; Saruhashi, Y.; Mori, K. Kyphotic malalignment after anterior cervical fusion is one of the factors promoting the degenerative process in adjacent intervertebral levels. Eur. Spine J. 2001, 10, 320–324. [Google Scholar] [CrossRef] [Green Version]
- Park, M.S.; Kelly, M.P.; Lee, D.H.; Min, W.K.; Rahman, R.K.; Riew, K.D. Sagittal alignment as a predictor of clinical adjacent segment pathology requiring surgery after anterior cervical arthrodesis. Spine J. 2014, 14, 1228–1234. [Google Scholar] [CrossRef] [Green Version]
- Scheer, J.K.; Tang, J.A.; Smith, J.S.; Acosta, F.L., Jr.; Protopsaltis, T.S.; Blondel, B.; Bess, S.; Shaffrey, C.I.; Deviren, V.; Lafage, V.; et al. Cervical spine alignment, sagittal deformity, and clinical implications: A review. J. Neurosurg. Spine 2013, 19, 141–159. [Google Scholar] [CrossRef] [Green Version]
- Yamasaki, K.; Hoshino, M.; Omori, K.; Igarashi, H.; Nemoto, Y.; Tsuruta, T.; Matsumoto, K.; Iriuchishima, T.; Ajiro, Y.; Matsuzaki, H. Risk factors of adjacent segment disease after transforaminal inter-body fusion for degenerative lumbar disease. Spine 2017, 42, E86–E92. [Google Scholar] [CrossRef]
- Umehara, S.; Zindrick, M.; Patwardhan, A.; Havey, R.; Vrbos, L.; Knight, G.; Miyano, S.; Kirincic, M.; Kaneda, K.; Lorenz, M. The biomechanical effect of postoperative hypolordosis in instrumented lumbar fusion on instrumented and adjacent spinal segments. Spine 2000, 25, 1617–1624. [Google Scholar] [CrossRef]
- Xu, D.S.; Walker, C.T.; Godzik, J.; Turner, J.D.; Smith, W.; Uribe, J.S. Minimally invasive anterior, lateral, and oblique lumbar interbody fusion: A literature review. Ann. Transl. Med. 2018, 6, 104. [Google Scholar] [CrossRef]
- Horsting, P.P.; Pavlov, P.W.; Jacobs, W.C.; Obradov-Rajic, M.; de Kleuver, M. Good functional outcome and adjacent segment disc quality 10 years after single-level anterior lumbar interbody fusion with posterior fixation. Glob. Spine J. 2012, 2, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Jin, C.; Xie, M.; He, L.; Xu, W.; Han, W.; Liang, W.; Qian, Y. Oblique lumbar interbody fusion for adjacent segment disease after posterior lumbar fusion: A case-controlled study. J. Orthop. Surg. Res. 2019, 14, 216. [Google Scholar] [CrossRef]
- Allain, J.; Dufour, T. Anterior lumbar fusion techniques: ALIF, OLIF, DLIF, LLIF, IXLIF. Orthop. Traumatol. Surg. Res. 2020, 106, S149–S157. [Google Scholar] [CrossRef]
- Xi, Z.; Chou, D.; Mummaneni, P.V.; Ruan, H.; Eichler, C.; Chang, C.-C.; Burch, S. Anterior lumbar compared to oblique lumbar interbody approaches for multilevel fusions to the sacrum in adults with spinal deformity and degeneration. J. Neurosurg. Spine SPI 2020, 33, 461–470. [Google Scholar] [CrossRef]
- Safaee, M.M.; Tenorio, A.; Osorio, J.A.; Choy, W.; Amara, D.; Lai, L.; Molinaro, A.M.; Zhang, Y.; Hu, S.S.; Tay, B. The impact of obesity on perioperative complications in patients undergoing anterior lumbar interbody fusion. J. Neurosurg. Spine 2020, 33, 332–341. [Google Scholar] [CrossRef]
- Miscusi, M.; Trungu, S.; Ricciardi, L.; Forcato, S.; Ramieri, A.; Raco, A. The anterior-to-psoas approach for interbody fusion at the L5–S1 segment: Clinical and radiological outcomes. Neurosurg. Focus 2020, 49, E14. [Google Scholar] [CrossRef] [PubMed]
- Turel, M.K.; Kerolus, M.G.; David, B.T.; Fessler, R.G. Minimally invasive options for surgical management of adjacent segment disease of the lumbar spine. Neurol. India 2018, 66, 755. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Hwang, C.J.; Lee, S.W.; Ahn, Y.J.; Kim, Y.T.; Lee, D.H.; Lee, M.Y. Risk factors for adjacent segment disease after lumbar fusion. Eur. Spine J. 2009, 18, 1637–1643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mobbs, R.J.; Phan, K.; Malham, G.; Seex, K.; Rao, P.J. Lumbar interbody fusion: Techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J. Spine Surg. 2015, 1, 2–18. [Google Scholar]
- Park, S.W.; Ko, M.J.; Kim, Y.B.; Le Huec, J.C. Correction of marked sagittal deformity with circumferential minimally invasive surgery using oblique lateral interbody fusion in adult spinal deformity. J. Orthop. Surg. Res. 2020, 15, 13. [Google Scholar] [CrossRef]
- Damasceno, L.H.F.; Catarin, S.R.G.; Campos, A.D.; Defino, H.L.A. Lumbar lordosis: A study of angle values and of vertebral bodies and intervertebral discs role. Acta Ortop. Bras. 2006, 14, 193–198. [Google Scholar] [CrossRef]




| KERRYPNX | ALIF | OLIF | Overall | p-Value |
|---|---|---|---|---|
| Patient number | 25 | 25 | 50 | |
| Total correction levels | 30 | 27 | 57 | 0.85 |
| Age (years) | 57.2 ± 3.7 | 58.1 ± 4.2 | 56.6 ± 5.0 | 0.63 |
| BMI (Kg/m2) | 24.9 ± 2.9 | 25.9 ± 3.0 | 25.1 ± 3.8 | 0.82 |
| Hospital stays (days) | 7.0 ± 1.5 | 7.0 ± 2.5 | 7.0 ± 2.0 | 0.90 |
| Gender | 1 | |||
| Male | 10 (40%) | 10 (40%) | 20 (40%) | |
| Female | 15 (60%) | 15 (60%) | 30 (60%) | |
| Pre-OP diagnosis | 0.87 | |||
| Spondylolisthesis | 18 (72%) | 20 (80%) | 38 (76%) | |
| Spondylosis | 7 (28%) | 5 (20%) | 12 (24%) | |
| Fusion levels | 0.88 | |||
| 1 | 20 (80%) | 21 (84%) | 41 (82%) | |
| 2 | 5 (20%) | 3 (12%) | 8 (16%) | |
| 3 | 0 (0%) | 1 (4%) | 1 (2%) | |
| Index fusion level | 0.09 | |||
| L2/L3 | 0 (0%) | 1 (3.7%) | 1 (1.8%) | |
| L3/L4 | 4 (13.3%) | 11 (40.7%) | 15 (26.3%) | |
| L4/L5 | 9 (30%) | 15 (55.6%) | 24 (42.1%) | |
| L5/S1 | 17 (56.7%) | 0 (0%) | 17 (29.8%) | |
| Fusion status | 0.72 | |||
| Grade I | 0 (0%) | 0 (0%) | 0 (0%) | |
| Grade II | 4 (13%) | 5 (19%) | 9 (16%) | |
| Grade III | 26 (87%) | 22 (71%) | 48 (84%) | |
| Pre-OP adjacent disc degeneration status | ||||
| Grade 1 | 1 (4%) | 0 (0%) | 1 (2%) | 0.68 |
| Grade 2 | 2 (8%) | 3 (12%) | 5 (10%) | |
| Grade 3 | 7 (28%) | 7 (28%) | 14 (28%) | |
| Grade 4 | 12 (48%) | 10 (40%) | 22 (44%) | |
| Grade 5 | 3 (12%) | 5 (20%) | 8 (16%) | |
| ALIF | OLIF | Overall | p-Value | |
|---|---|---|---|---|
| PT (°) | ||||
| Pre-OP | 13.9 ± 12.1 | 16.1 ± 11.3 | 15.1 ± 11.6 | 0.69 |
| Post-OP | 18.1 ± 9.6 | 17.9 ± 6.7 | 18 ± 8.1 | 0.98 |
| 2Y | 16.4 ± 8.7 | 16.9 ± 6.4 | 16.7 ± 7.5 | 0.91 |
| PI (°) | ||||
| Pre-OP | 55.2 ± 15.3 | 50.6 ± 10.5 | 52.8 ± 13 | 0.31 |
| Post-OP | 55 ± 15.6 | 52.5 ± 9.6 | 53.7 ± 12.7 | 0.99 |
| 2Y | 54.9 ± 10.7 | 49.1 ± 14.3 | 51.8 ± 13 | 0.13 |
| LL (°) | ||||
| Pre-OP | 50.6 ± 17.6 | 43.5 ± 15.8 | 46.8 ± 16.9 | 0.31 |
| Post-OP | 44.7 ± 19.2 | 42.7 ± 14.1 | 43.6 ± 16.5 | 0.84 |
| 2Y | 50.2 ± 14.4 | 44.2 ± 12.9 | 47 ± 13.8 | 0.16 |
| PI-LL (°) | ||||
| Pre-OP | 14.6 ± 12.8 | 10.9 ± 14.8 | 12.6 ± 14.1 | 0.11 |
| Post-OP | 10.3 ± 12 | 9.8 ± 12 | 10 ± 11.9 | 0.78 |
| 2Y | 4.6 ± 12.8 | 7.6 ± 7.5 | 6.2 ± 10.3 | 0.26 |
| SS (°) | ||||
| Pre-OP | 41.3 ± 11.7 | 36.3 ± 11.1 | 38.7 ± 11.6 | 0.3 |
| Post-OP | 36.9 ± 12.6 | 34.6 ± 7.9 | 35.7 ± 10.3 | 0.67 |
| 2Y | 38.5 ± 8.9 | 33.8 ± 11.8 | 36 ± 10.7 | 0.07 |
| TK (°) | ||||
| Pre-OP | 17.6 ± 14.1 | 28.7 ± 14.4 | 22.6 ± 15 | 0.16 |
| Post-OP | 17.6 ± 13.9 | 22.4 ± 9 | 20.7 ± 11 | 0.45 |
| 2Y | 23 ± 10.3 | 21.6 ± 11.5 | 22.2 ± 10.9 | 1 |
| SVA (mm) | ||||
| Pre-OP | 52.8 ± 37.9 | 81.9 ± 34.7 | 65.1 ± 38.6 | 0.12 |
| Post-OP | 40 ± 33.5 | 37.2 ± 26.2 | 38.2 ± 28.3 | 0.84 |
| 2Y | 39.6 ± 31.6 | 47.3 ± 25.8 | 44 ± 28.3 | 0.37 |
| IL (°) | ||||
| Pre-OP | 25.1 ± 11.2 | 21.1 ± 10.2 | 23.1 ± 10.6 | 0.09 |
| Post-OP | 31.9 ± 8.9 | 28.0 ± 15.1 | 30.0 ± 12.2 | 0.03 * |
| 2Y | 32.6 ± 10.4 | 28.4 ± 12.2 | 30.5 ± 11 | <0.01 ** |
| SL (°) | ||||
| Pre-OP | 29.7 ± 13.7 | 21.1 ± 10.2 | 25.1 ± 12.6 | 0.08 |
| Post-OP | 31.9 ± 9.2 | 22.8 ± 10.5 | 27 ± 10.8 | 0.01 * |
| 2Y | 32.7 ± 10.1 | 22.6 ± 11 | 27.3 ± 11.6 | <0.01 ** |
| ADH (mm) | ||||
| Pre-OP | 12.8 ± 3.1 | 10.6 ± 3.1 | 11.6 ± 3.3 | 0.07 |
| Post-OP | 11.7 ± 2.7 | 10.7 ± 2.4 | 11.2 ± 2.6 | 0.29 |
| 2Y | 12 ± 2.7 | 9.5 ± 2.1 | 10.7 ± 2.7 | <0.01 ** |
| PDH (mm) | ||||
| Pre-OP | 6.6 ± 1.2 | 5.8 ± 2.1 | 6.2 ± 1.8 | 0.05 |
| Post-OP | 6.2 ± 1.3 | 4.9 ± 1.1 | 5.5 ± 1.4 | <0.01 ** |
| 2Y | 6.1 ± 1.3 | 5 ± 1.4 | 5.5 ± 1.5 | <0.01 ** |
| ASDA (°) | ||||
| Pre-OP | 10 ± 3.3 | 8.5 ± 5.8 | 9.2 ± 4.8 | 0.05 |
| Post-OP | 9.3 ± 3.7 | 8.8 ± 3.6 | 9 ± 3.6 | 0.52 |
| 2Y | 10.1 ± 3.4 | 9.4 ± 6.2 | 9.7 ± 5.1 | 0.12 |
| ALIF | OLIF | Overall | p-Value | |
|---|---|---|---|---|
| EQ-5D | ||||
| Pre-OP | 11.3 ± 1.6 | 11.6 ± 0.7 | 11.5 ± 1.2 | 0.49 |
| Post-OP | 6.6 ± 2.2 | 7.3 ± 1.9 | 7 ± 2.1 | 0.25 |
| 2Y | 7.5 ± 2.1 | 7.1 ± 2.8 | 7.4 ± 2.4 | 0.37 |
| ODI | ||||
| Pre-OP | 49.3 ± 11.9 | 49.4 ± 11.5 | 49.4 ± 11.5 | 0.98 |
| Post-OP | 20.2 ± 16.8 | 27.4 ± 15.4 | 23.7 ± 16.3 | 0.15 |
| 2Y | 27.6 ± 17.4 | 29.1 ± 18.7 | 28.2 ± 17.9 | 0.69 |
| VASP-Total | ||||
| Pre-OP | 8.2 ± 1.5 | 10.3 ± 11.8 | 9.4 ± 8.7 | 0.43 |
| Post-OP | 2.3 ± 2.2 | 4.1 ± 6.3 | 3.2 ± 4.8 | 0.22 |
| 2Y | 3.3 ± 2.1 | 2.8 ± 1.6 | 3.1 ± 1.9 | 0.25 |
| VASP-Back | ||||
| Pre-OP | 6 ± 3.2 | 7.3 ± 2.4 | 6.72 ± 2.8 | 0.1 |
| Post-OP | 1.6 ± 1.7 | 2.4 ± 1.9 | 2 ± 1.8 | 0.15 |
| 2Y | 2.3 ± 2.05 | 2.2 ± 2.13 | 2.3 ± 2.07 | 0.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tung, K.-K.; Hsu, F.-W.; Ou, H.-C.; Chen, K.-H.; Pan, C.-C.; Lu, W.-X.; Chin, N.-C.; Shih, C.-M.; Wu, Y.-C.; Lee, C.-H. The Morphological Changes in Adjacent Segments Amongst Patients Receiving Anterior and Oblique Lumbar Interbody Fusion: A Retrospective Study. J. Clin. Med. 2021, 10, 5533. https://doi.org/10.3390/jcm10235533
Tung K-K, Hsu F-W, Ou H-C, Chen K-H, Pan C-C, Lu W-X, Chin N-C, Shih C-M, Wu Y-C, Lee C-H. The Morphological Changes in Adjacent Segments Amongst Patients Receiving Anterior and Oblique Lumbar Interbody Fusion: A Retrospective Study. Journal of Clinical Medicine. 2021; 10(23):5533. https://doi.org/10.3390/jcm10235533
Chicago/Turabian StyleTung, Kuan-Kai, Fang-Wei Hsu, Hsien-Che Ou, Kun-Hui Chen, Chien-Chou Pan, Wen-Xian Lu, Ning-Chien Chin, Cheng-Min Shih, Yun-Che Wu, and Cheng-Hung Lee. 2021. "The Morphological Changes in Adjacent Segments Amongst Patients Receiving Anterior and Oblique Lumbar Interbody Fusion: A Retrospective Study" Journal of Clinical Medicine 10, no. 23: 5533. https://doi.org/10.3390/jcm10235533
APA StyleTung, K.-K., Hsu, F.-W., Ou, H.-C., Chen, K.-H., Pan, C.-C., Lu, W.-X., Chin, N.-C., Shih, C.-M., Wu, Y.-C., & Lee, C.-H. (2021). The Morphological Changes in Adjacent Segments Amongst Patients Receiving Anterior and Oblique Lumbar Interbody Fusion: A Retrospective Study. Journal of Clinical Medicine, 10(23), 5533. https://doi.org/10.3390/jcm10235533






