One Year Assessment of the Hearing Preservation Potential of the EVO Electrode Array
Abstract
1. Introduction
2. Materials and Methods
2.1. Participant Selection
2.2. Surgical Procedure
2.3. Outcomes
2.4. Statistical Analyses
3. Results
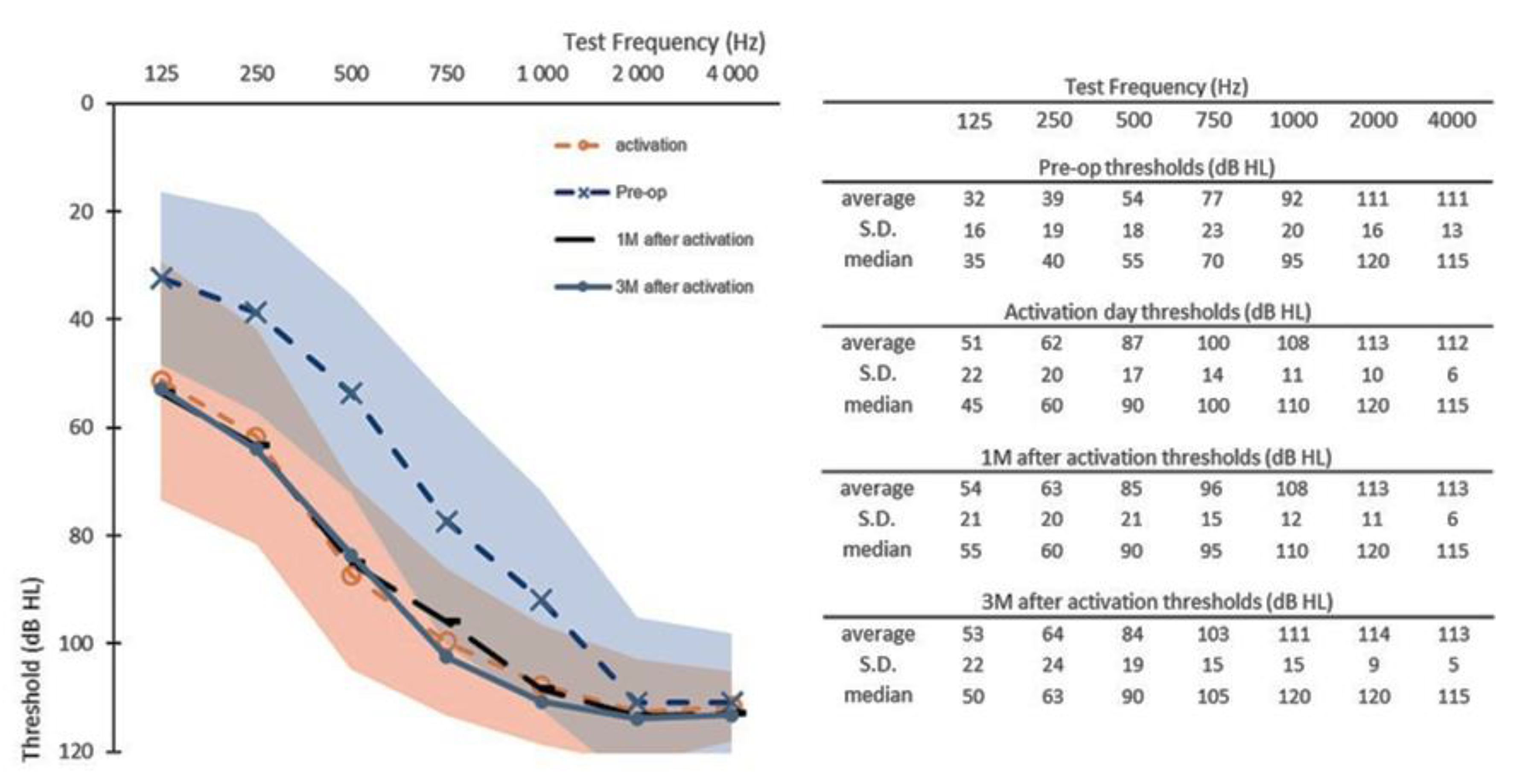
3.1. Early Follow-Up Data
3.2. Six-Month Follow-Up Data
3.3. Twelve-Month Follow-Up Data
3.4. Functional Hearing and Low-Frequency Hearing Preservation
4. Discussion
4.1. Hearing Preservation Stability over Follow-Up
4.2. Functional Hearing Preservation
4.3. Correlation with Age
4.4. EAS Adherence
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gantz, B.J.; Turner, C.W. Combining acoustic and electrical hearing. Laryngoscope 2010, 113, 1726–1730. [Google Scholar] [CrossRef] [PubMed]
- James, C.J.; Fraysse, B.; Deguine, O.; Lenarz, T.; Mawman, D.; Ramos, Á.; Ramsden, R.; Sterkers, O. Combined Electroacoustic Stimulation in Conventional Candidates for Cochlear Implantation. Audiol. Neurotol. 2006, 11, 57–62. [Google Scholar] [CrossRef]
- Mom, T.; Bachy, A.; Houette, A.; Pavier, Y.; Pastourel, R.; Gabrillargues, J.; Saroul, N.; Gilain, L.; Avan, P. Cochlear implantation through the round window with a straight slotted electrode array: Optimizing the surgical procedure. Eur. Arch. Oto-Rhino-Laryngol. 2015, 273, 853–858. [Google Scholar] [CrossRef]
- Lehnhardt, E. Intrakochleäre Plazierung der Cochlear-Implant-Elektroden in soft surgery technique [Intracochlear placement of cochlear implant electrodes in soft surgery technique]. HNO 1993, 41, 356–359. (In German) [Google Scholar] [PubMed]
- Sierra, C.; Calderón, M.; Bárcena, E.; Tisaire, A.; Raboso, E. Preservation of Residual Hearing After Cochlear Implant Surgery with Deep Insertion Electrode Arrays. Otol. Neurotol. 2019, 40, e373–e380. [Google Scholar] [CrossRef]
- Hodges, A.V.; Schloffman, J.; Balkany, T. Conservation of residual hearing with cochlear implantation. Am. J. Otol. 1997, 18, 179–183. [Google Scholar]
- Gantz, B.J.; Turner, C.; Gfeller, K.E.; Lowder, M.W. Preservation of Hearing in Cochlear Implant Surgery: Advantages of Combined Electrical and Acoustical Speech Processing. Laryngoscope 2005, 115, 796–802. [Google Scholar] [CrossRef]
- Mertens, G.; Punte, A.K.; Cochet, E.; De Bodt, M.; Van de Heyning, P. Long-term Follow-up of Hearing Preservation in Electric-Acoustic Stimulation Patients. Otol. Neurotol. 2014, 35, 1765–1772. [Google Scholar] [CrossRef]
- Helbig, S.; Adel, Y.; Rader, T.; Stöver, T.; Baumann, U. Long-term Hearing Preservation Outcomes After Cochlear Implantation for Electric-Acoustic Stimulation. Otol. Neurotol. 2016, 37, e353–e359. [Google Scholar] [CrossRef] [PubMed]
- Bourn, S.; Goldstein, M.R.; Jacob, A. Hearing Preservation in Elderly Cochlear Implant Recipients. Otol. Neurotol. 2020, 41, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, M.B.; Sagi, E.; Jackson, M.; Shapiro, W.H.; Roland, J.J.T.; Waltzman, S.; Svirsky, M. Reimplantation of Hybrid Cochlear Implant Users with a Full-Length Electrode After Loss of Residual Hearing. Otol. Neurotol. 2008, 29, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Adunka, O.; Unkelbach, M.H.; Mack, M.G.; Radeloff, A.; Gstoettner, W. Predicting Basal Cochlear Length for Electric-Acoustic Stimulation. Arch. Otolaryngol. Head Neck Surg. 2005, 131, 488–492. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gstoettner, W.K.; Helbig, S.; Maier, N.; Kiefer, J.; Radeloff, A.; Adunka, O.F. Ipsilateral Electric Acoustic Stimulation of the Auditory System: Results of Long-Term Hearing Preservation. Audiol. Neurotol. 2006, 11, 49–56. [Google Scholar] [CrossRef]
- Gstoettner, W.; Helbig, S.; Settevendemie, C.; Baumann, U.; Wagenblast, J.; Arnoldner, C. A new electrode for residual hearing preservation in cochlear implantation: First clinical results. Acta Oto-Laryngol. 2009, 129, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Sipari, S.; Iso-Mustajärvi, M.; Matikka, H.; Tervaniemi, J.; Koistinen, A.; Aarnisalo, A.; Sinkkonen, S.T.; Löppönen, H.; Dietz, A. Cochlear Implantation with a Novel Long Straight Electrode: The Insertion Results Evaluated by Imaging and Histology in Human Temporal Bones. Otol. Neurotol. 2018, 39, e784–e793. [Google Scholar] [CrossRef]
- Nguyen, Y.; Miroir, M.; Kazmitcheff, G.; Sutter, J.; Bensidhoum, M.; Ferrary, E.; Sterkers, O.; Grayeli, A.B. Cochlear Implant Insertion Forces in Microdissected Human Cochlea to Evaluate a Prototype Array. Audiol. Neurotol. 2012, 17, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Bento, R.F.; Danieli, F.; Magalhães, A.T.D.M.; Gnansia, D.; Hoen, M. Residual Hearing Preservation with the Evo® Cochlear Implant Electrode Array: Preliminary Results. Int. Arch. Otorhinolaryngol. 2016, 20, 353–358. [Google Scholar] [CrossRef][Green Version]
- Skarzynski, H.; Van de Heyning, P.; Agrawal, S.; Arauz, S.L.; Atlas, M.; Baumgartner, W.; Caversaccio, M.; DE Bodt, M.; Gavilan, J.; Godey, B.; et al. Towards a consensus on a hearing preservation classification system. Acta Oto-Laryngol. 2013, 133, 3–13. [Google Scholar] [CrossRef]
- Iso-Mustajärvi, M.; Sipari, S.; Löppönen, H.; Dietz, A. Preservation of residual hearing after cochlear implant surgery with slim modiolar electrode. Eur. Arch. Oto-Rhino-Laryngol. 2019, 277, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Haber, K.; Neagu, A.; Konopka, W.; Amernik, K.; Gheorghe, D.C.; Drela, M.; Wrukowska-Niemczewska, I.; Mierzwiński, J. The influence of Slim Modiolar electrode on residual hearing in pediatric patients. Eur. Arch. Oto-Rhino-Laryngol. 2020, 278, 2723–2732. [Google Scholar] [CrossRef]
- Nguyen, Y.; Mosnier, I.; Borel, S.; Ambert-Dahan, E.; Bouccara, D.; Bozorg-Grayeli, A.; Ferrary, E.; Sterkers, O. Evolution of electrode array diameter for hearing preservation in cochlear implantation. Acta Oto-Laryngol. 2012, 133, 116–122. [Google Scholar] [CrossRef]
- James, C.; Albegger, K.; Battmer, R.; Burdo, S.; Deggouj, N.; Deguine, O.; Dillier, N.; Gersdorff, M.; Laszig, R.; Lenarz, T.; et al. Preservation of residual hearing with cochlear implantation: How and why. Acta Oto-Laryngol. 2005, 125, 481–491. [Google Scholar] [CrossRef]
- Nguyen, Y.; Bernardeschi, D.; Kazmitcheff, G.; Miroir, M.; Vauchel, T.; Ferrary, E.; Sterkers, O. Effect of Embedded Dexamethasone in Cochlear Implant Array on Insertion Forces in an Artificial Model of Scala Tympani. Otol. Neurotol. 2015, 36, 354–358. [Google Scholar] [CrossRef]
- Derinsu, U.; Serin, G.M.; Akdaş, F.; Batman, Ç. Cochlear Implantation: Is hearing preservation necessary in severe to profound hearing loss? J. Craniofacial Surg. 2011, 22, 520–522. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Lee, S.; Chung, J.-H.; Choi, J.W. Preimplant Hearing Threshold: An Important Predictor of Hearing Preservation in Cochlear Implantation with Lateral Wall Electrodes. Otol. Neurotol. 2020, 42, e145–e152. [Google Scholar] [CrossRef]
- Harrison, L.; Manjaly, J.G.; Ellis, W.; Lavy, J.A.; Shaida, A.; Khalil, S.S.; Saeed, S.R. Hearing Preservation Outcomes with Standard Length Electrodes in Adult Cochlear Implantation and the Uptake of Electroacoustic Stimulation. Otol. Neurotol. 2020, 41, 1060–1065. [Google Scholar] [CrossRef]
- Schart-Morén, N.; Erixon, E.; Li, H.; Rask-Andersen, H. Cochlear implantation and residual hearing preservation long-term follow-up of the first consecutively operated patients using the round window approach in Uppsala, Sweden. Cochlea- Implant. Int. 2020, 21, 246–259. [Google Scholar] [CrossRef]
- Mamelle, E.; Granger, B.; Sterkers, O.; Lahlou, G.; Ferrary, E.; Nguyen, Y.; Mosnier, I. Long-term residual hearing in cochlear implanted adult patients who were candidates for electro-acoustic stimulation. Eur. Arch. Oto-Rhino-Laryngol. 2019, 277, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Helbig, S.; Baumann, U.; Hey, C.; Helbig, M. Hearing Preservation After Complete Cochlear Coverage in Cochlear Implantation with the Free-Fitting FLEXSOFT Electrode Carrier. Otol. Neurotol. 2011, 32, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Lenarz, T.; Buechner, A.; Lesinski-Schiedat, A.; Timm, M.; Salcher, R. Hearing Preservation with a New Atraumatic Lateral Wall Electrode. Otol. Neurotol. 2020, 41, e993–e1003. [Google Scholar] [CrossRef] [PubMed]
- Moran, M.; Dowell, R.C.; Iseli, C.; Briggs, R.J.S. Hearing Preservation Outcomes for 139 Cochlear Implant Recipients Using a Thin Straight Electrode Array. Otol. Neurotol. 2017, 38, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Jr, J.T.R.; Gantz, B.J.; Waltzman, S.B.; Parkinson, A.J. Long-term outcomes of cochlear implantation in patients with high-frequency hearing loss. Laryngoscope 2017, 128, 1939–1945. [Google Scholar] [CrossRef]
- Lenarz, T.; James, C.; Cuda, D.; O’Connor, A.F.; Frachet, B.; Frijns, J.H.; Klenzner, T.; Laszig, R.; Manrique, M.; Marx, M.; et al. European multi-centre study of the Nucleus Hybrid L24 cochlear implant. Int. J. Audiol. 2013, 52, 838–848. [Google Scholar] [CrossRef]
- Friedmann, D.; Peng, R.; Fang, Y.; McMenomey, S.O.; Roland, J.J.T.; Waltzman, S.B. Effects of loss of residual hearing on speech performance with the CI422 and the Hybrid-L electrode. Cochlea- Implant. Int. 2015, 16, 277–284. [Google Scholar] [CrossRef]
- Zanetti, D.; Nassif, N.; DE Zinis, L.O.R. Fattori influenzanti la conservazione dei residui uditivi negli impianti cocleari. Acta Otorhinolaryngol. Ital. 2015, 35, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Causon, A.; Verschuur, C.; Newman, T.A. A Retrospective Analysis of the Contribution of Reported Factors in Cochlear Implantation on Hearing Preservation Outcomes. Otol. Neurotol. 2015, 36, 1137–1145. [Google Scholar] [CrossRef]
- Wanna, G.B.; O’Connell, B.P.; Francis, D.O.; Gifford, R.; Hunter, J.; Holder, J.; Bennett, M.; Rivas, A.; Labadie, R.F.; Haynes, D.S. Predictive factors for short- and long-term hearing preservation in cochlear implantation with conventional-length electrodes. Laryngoscope 2017, 128, 482–489. [Google Scholar] [CrossRef]
- Spitzer, E.R.; Waltzman, S.; Landsberger, D.M.; Friedmann, D. Acceptance and Benefits of Electro-Acoustic Stimulation for Conventional-Length Electrode Arrays. Audiol. Neurotol. 2020, 26, 17–26. [Google Scholar] [CrossRef] [PubMed]



| ID. | Age | Gender | Age at Deafness Onset (Years) | Etiology | HL Evolution Severe/Profound | ||
|---|---|---|---|---|---|---|---|
| R | L | R | L | ||||
| 1 | 76 | M | 66 | 1 | Presbyacousis | progressive | progressive |
| 2 | 55 | F | 37 | 37 | Genetic | sudden | sudden |
| 3 | 53 | M | 1 | 1 | Unknown | progressive | progressive |
| 4 | 78 | F | 61 | 66 | Unknown | n.a | n.a |
| 5 | 39 | F | 4 | 4 | Meningitis | progressive | progressive |
| 6 | 69 | F | 42 | 42 | Unknown | progressive | progressive |
| 7 | 34 | M | 5 | 5 | Unknown | sudden | progressive |
| 8 | 71 | F | 30 | 1 | Unknown | progressive | sudden |
| 9 | 86 | F | 47 | 47 | Unknown | sudden | sudden |
| 10 | 36 | F | n.a | n.a | n.a | n.a | n.a |
| 11 | 61 | M | 34 | 34 | Unknown | progressive | progressive |
| 12 | 46 | F | 1 | 1 | Ototoxic | progressive | progressive |
| 13 | 57 | M | 45 | 45 | Genetic | sudden | sudden |
| 14 | 81 | M | 69 | 69 | Unknown | progressive | progressive |
| 15 | 72 | F | 53 | 53 | Unknown | progressive | progressive |
| ID | CI Ear | Low-Frequency PTA [125–500 Hz] | Full Range PTA (dB HL) [125–4000 Hz] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-op | Act | 1M | 3M | 6M | 12M | Pre-op | Act | 1M | 3M | 6M | 12M | ||
| 1 | right | 56,7 | 66.7 | 68.3 | 70.0 | 85.0 | x | 77.7 | 83 | 83.4 | 88.6 | 90.5 | x |
| 2 | left | 46.7 | 45.0 | 65.0 | 53.3 | 73.3 | 85.0 | 78.2 | 79.1 | 85 | 88 | 93.4 | 96.6 |
| 3 | left | 43.3 | 76.7 | 78.3 | 63.3 | 60.0 | x | 81.1 | 90.9 | 93.6 | 88.4 | 85.9 | x |
| 4 | left | 48.3 | 80.0 | 61.7 | 70.0 | 63.3 | 63.3 | 79.1 | 93 | 88.9 | 84.5 | 86.8 | 85.9 |
| 5 | left | 60.0 | 58.3 | 48.3 | 43.3 | 46.7 | 51.7 | 89.8 | 89.2 | 84.8 | 85.2 | 86.1 | 87.5 |
| 6 | left | 15.0 | 66.7 | 75.0 | 80.0 | 73.3 | x | 63 | 80.2 | 81.1 | 93.9 | 83.2 | x |
| 7 | right | 30.0 | 38.3 | 48.3 | 46.7 | 36.7 | 40.0 | 81.6 | 83.9 | 84.8 | 84.3 | 82 | 82.5 |
| 8 | right | 63.3 | 85.0 | 85.0 | 88.3 | x | x | 60.2 | 83.6 | 84.5 | 85.7 | x | x |
| 9 | left | 55.0 | 86.7 | 83.3 | x | x | x | 88.4 | 97 | 96.1 | x | x | x |
| 10 | left | 31.7 | 61.7 | 70.0 | 68.3 | 55.0 | 51.7 | 74.5 | 89.8 | 92 | 91.6 | 88.4 | 84.1 |
| 11 | right | 21.7 | 38.3 | 23.3 | 28.3 | 25.0 | x | 65.9 | 71.4 | 68.6 | 66.8 | 66.6 | x |
| 12 | right | 20.0 | 53.3 | 56.7 | 55.0 | 65.0 | 56.7 | 71.6 | 83.9 | 83 | 86.6 | 86.8 | 88.9 |
| 13 | left | 41.7 | 96.7 | 95.0 | 101.7 | 101.7 | x | 75.2 | 99.8 | 99.3 | 101.1 | 100.7 | x |
| 14 | left | 60.0 | 83.3 | 88.3 | 91.7 | 91.7 | x | 69.5 | 88 | 90.5 | 91.6 | 97.5 | x |
| 15 | left | 30.0 | 65.0 | 63.3 | 75.0 | 68.3 | x | 70.7 | 88.2 | 88.4 | 93.9 | 92 | x |
| Time Point: | Activation | 1M | 3M | 6M |
|---|---|---|---|---|
| Full range Hearing loss (25–4000 Hz) (dB) | ||||
| average | 11 | 0.2 | 1.6 | −0.4 |
| S.D. | 7.8 | 2.9 | 4.7 | 4.2 |
| Low-Frequency Hearing loss (125–500 Hz) (dB) | ||||
| average | 25 | 0.9 | 0.4 | −0.1 |
| S.D. | 18.1 | 10.6 | 7.7 | 9.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guevara, N.; Parietti-Winkler, C.; Godey, B.; Franco-Vidal, V.; Gnansia, D.; Ardoint, M.; Hoen, M.; Karoui, C.; Truy, E.; Vincent, C.; et al. One Year Assessment of the Hearing Preservation Potential of the EVO Electrode Array. J. Clin. Med. 2021, 10, 5604. https://doi.org/10.3390/jcm10235604
Guevara N, Parietti-Winkler C, Godey B, Franco-Vidal V, Gnansia D, Ardoint M, Hoen M, Karoui C, Truy E, Vincent C, et al. One Year Assessment of the Hearing Preservation Potential of the EVO Electrode Array. Journal of Clinical Medicine. 2021; 10(23):5604. https://doi.org/10.3390/jcm10235604
Chicago/Turabian StyleGuevara, Nicolas, Cécile Parietti-Winkler, Benoit Godey, Valerie Franco-Vidal, Dan Gnansia, Marine Ardoint, Michel Hoen, Chadlia Karoui, Eric Truy, Christophe Vincent, and et al. 2021. "One Year Assessment of the Hearing Preservation Potential of the EVO Electrode Array" Journal of Clinical Medicine 10, no. 23: 5604. https://doi.org/10.3390/jcm10235604
APA StyleGuevara, N., Parietti-Winkler, C., Godey, B., Franco-Vidal, V., Gnansia, D., Ardoint, M., Hoen, M., Karoui, C., Truy, E., Vincent, C., Mosnier, I., & Nguyen, Y. (2021). One Year Assessment of the Hearing Preservation Potential of the EVO Electrode Array. Journal of Clinical Medicine, 10(23), 5604. https://doi.org/10.3390/jcm10235604







