Association between Glucose-6-Phosphate Dehydrogenase Deficiency and Asthma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Eligibility Criteria
2.3. Exclusion Criteria
2.4. Diagnostic Criteria
2.5. Ethical Considerations
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Global Asthma Report. Available online: http://globalasthmareport.org/resources/Global_Asthma_Report_2018.pdf (accessed on 29 October 2021).
- 2D’Amato, G.; Holgate, S.T.; Pawankar, R.; Ledford, D.K.; Cecchi, L.; Al-Ahmad, M.; Al-Enezi, F.; Al-Muhsen, S.; Ansotegui, I.; Baena-Cagnani, C.E.; et al. Meteorological Conditions, Climate Change, New Emerging Factors, and Asthma and Related Allergic Disorders. A Statement of the World Allergy Organization. World Allergy Organ. J. 2015, 8, 25. [Google Scholar] [CrossRef] [Green Version]
- To, T.; Stanojevic, S.; Moores, G.; Gershon, A.S.; Bateman, E.D.; Cruz, A.A.; Boulet, L.P. Global Asthma Prevalence in Adults: Findings from the Cross-Sectional World Health Survey. BMC Public Health 2012, 12, 204. [Google Scholar] [CrossRef] [Green Version]
- Ridolo, E.; Incorvaia, C.; Martignago, I.; Caminati, M.; Canonica, G.W.; Senna, G. Sex in Respiratory and Skin Allergies. Clin. Rev. Allergy Immunol. 2019, 56, 322–332. [Google Scholar] [CrossRef]
- Holgate, S.T. Pathogenesis of Asthma. Clin. Exp. Allergy 2008, 38, 872–897. [Google Scholar] [CrossRef] [PubMed]
- Deschamps, K.; Cromlish, W.; Weicker, S.; Lamontagne, S.; Huszar, S.L.; Gauthier, J.Y.; Mudgett, J.S.; Guimond, A.; Romand, R.; Frossard, N.; et al. Genetic and Pharmacological Evaluation of Cathepsin s in a Mouse Model of Asthma. Am. J. Respir. Cell Mol. Biol. 2011, 45, 81–87. [Google Scholar] [CrossRef]
- Smit, J.J.; Lukacs, N.W. A Closer Look at Chemokines and Their Role in Asthmatic Responses. Eur. J. Pharmacol. 2006, 533, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Sun, Y.; Wu, Y. Lower Circulating Zinc and Selenium Levels Are Associated with an Increased Risk of Asthma: Evidence from a Meta-Analysis. Public Health Nutr. 2020, 23, 1555–1562. [Google Scholar] [CrossRef]
- Cook-Mills, J.; Gebretsadik, T.; Abdala-Valencia, H.; Green, J.; Larkin, E.K.; Dupont, W.D.; Shu, X.O.; Gross, M.; Bai, C.; Gao, Y.T.; et al. Interaction of Vitamin E Isoforms on Asthma and Allergic Airway Disease. Thorax 2016, 71, 954–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Li, J.; Liang, Y.; Han, W.; Tang, J.; Cheng, G.; Zheng, Y. Joint Effects of Carbon Black Exposure and Dietary Antioxidant Vitamin Intake on Small Airway Dysfunction. Front. Nutr. 2021, 8, 716398. [Google Scholar] [CrossRef] [PubMed]
- Carroll, K.N.; Gebretsadik, T.; Griffin, M.R.; Dupont, W.D.; Mitchel, E.F.; Wu, P.; Enriquez, R.; Hartert, T.V. Maternal Asthma and Maternal Smoking Are Associated with Increased Risk of Bronchiolitis during Infancy. Pediatrics 2007, 119, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Litonjua, A.A.; O’Connor, G.T.; Zeiger, R.S.; Bacharier, L.; Schatz, M.; Carey, V.J.; Weiss, S.T.; Mirzakhani, H. Effect of Early and Late Prenatal Vitamin D and Maternal Asthma Status on Offspring Asthma or Recurrent Wheeze. J. Allergy Clin. Immunol. 2021, 147, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Liew, Z.; Nohr, E.A.; Morgen, C.S.; Ernst, A.; Li, J.; Sorensen, T.I.A.; Olsen, J. Prenatal Exposure to Acetaminophen and Overweight in Childhood. Obesity 2019, 27, 1314–1322. [Google Scholar] [CrossRef]
- Lai, T.; Wu, M.; Liu, J.; Luo, M.; He, L.; Wang, X.; Wu, B.; Ying, S.; Chen, Z.; Li, W.; et al. Acid-Suppressive Drug Use during Pregnancy and the Risk of Childhood Asthma: A Meta-analysis. Pediatrics 2018, 141, e20170889. [Google Scholar] [CrossRef] [Green Version]
- Kankaanranta, H.; Kauppi, P.; Tuomisto, L.E.; Ilmarinen, P. Emerging Comorbidities in Adult Asthma: Risks, Clinical Associations, and Mechanisms. Mediat. Inflamm. 2016, 2016, 3690628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thacher, J.D.; Gehring, U.; Gruzieva, O.; Standl, M.; Pershagen, G.; Bauer, C.P.; Berdel, D.; Keller, T.; Koletzko, S.; Koppelman, G.H.; et al. Maternal Smoking during Pregnancy and Early Childhood and Development of Asthma and Rhinoconjunctivitis - a MeDALL Project. Environ. Health Perspect. 2018, 126, 047005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greene, L.S. Asthma and Oxidant Stress: Nutritional, Environmental, and Genetic Risk Factors. J. Am. Coll. Nutr. 1995, 14, 317–324. [Google Scholar] [CrossRef]
- Tyran, W.; Wrzyszcz, M. Activity of Glucose-6-Phosphate Dehydrogenase in Erythrocytes in Patients with Atopic Asthma and Allergic Rhinitis. Pneumonol. Alergol. Pol. 1992, 60, 26–31. [Google Scholar]
- Luzzatto, L. Genetic Heterogeneity and Pathophysiology of G6PD Deficiency. Br. J. Haematol. 1974, 28, 151–155. [Google Scholar] [CrossRef]
- Luzzatto, L.; Ally, M.; Notaro, R. Glucose-6-Phosphate Dehydrogenase Deficiency. Blood 2020, 136, 1225–1240. [Google Scholar] [CrossRef]
- Parsanathan, R.; Jain, S.K. G6PD Deficiency Shifts Polarization of Monocytes/Macrophages towards a Proinflammatory and Profibrotic Phenotype. Cell Mol. Immunol. 2021, 18, 770–772. [Google Scholar] [CrossRef]
- Fiorelli, G.; Meloni, T.; Palomba, V.; Manoussakis, C.; Villa, S.; Cappellini, M.D. Gene Frequency of Glucose-6-Phosphate Dehydrogenase (G6PD) Polymorphic Variants in Sardinia. Gene. Geogr. 1990, 4, 139–142. [Google Scholar] [PubMed]
- de Marco, R.; Cappa, V.; Accordini, S.; Rava, M.; Antonicelli, L.; Bortolami, O.; Braggion, M.; Bugiani, M.; Casali, L.; Cazzoletti, L.; et al. Trends in the Prevalence of Asthma and Allergic Rhinitis in Italy between 1991 and 2010. Eur. Respir. J. 2012, 39, 883–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrante, G.; Baldissera, S.; Campostrini, S. Epidemiology of Chronic Respiratory Diseases and Associated Factors in the Adult Italian Population. Eur. J. Public Health 2017, 27, 1110–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Global Initiative for Asthma. Asthma Management and Prevention for Adults and Children Older Than 5 Years. A Pocket Guide for Health Professionals. 2019. Available online: https://books.google.com/books?id=ALVLAQAAIAAJ&dq=Asthma+Management+and+Prevention+for+Adults+and+Children+Older+Than+5+Years&lr=&source=gbs_navlinks_s (accessed on 27 November 2021).
- Dore, M.P.; Marras, G.; Rocchi, C.; Soro, S.; Pes, G.M. G6PD Deficiency Does Not Enhance Susceptibility for Acquiring Helicobacter pylori Infection in Sardinian Patients. PLoS ONE 2016, 11, e0160032. [Google Scholar] [CrossRef] [Green Version]
- Mosca, A.; Paderi, M.; Sanna, A.; Paleari, R.; Cao, A.; Galanello, R. Preliminary Experience with the Differential pH Technique for Glucose-6-Phosphate Dehydrogenase (G6PD) Measurement in Whole Blood: Application to an Area with High Prevalence of Thalassaemia and G6PD Deficiency. Haematologica 1990, 75, 397–399. [Google Scholar] [PubMed]
- Chung, K.F.; Wenzel, S.E.; Brozek, J.L.; Bush, A.; Castro, M.; Sterk, P.J.; Adcock, I.M.; Bateman, E.D.; Bel, E.H.; Bleecker, E.R.; et al. International ERS/ATS Guidelines on Definition, Evaluation and Treatment of Severe Asthma. Eur. Respir. J. 2014, 43, 343–373. [Google Scholar] [CrossRef] [Green Version]
- Quanjer, P.H.; Ruppel, G.L.; Langhammer, A.; Krishna, A.; Mertens, F.; Johannessen, A.; Menezes, A.M.B.; Wehrmeister, F.C.; Perez-Padilla, R.; Swanney, M.P.; et al. Bronchodilator Response in FVC Is Larger and More Relevant Than in FEV1 in Severe Airflow Obstruction. Chest 2017, 151, 1088–1098. [Google Scholar] [CrossRef]
- Pes, G.M.; Ganau, A.; Tognotti, E.; Errigo, A.; Rocchi, C.; Dore, M.P. The Association of Adult Height with the Risk of Cardiovascular Disease and Cancer in the Population of Sardinia. PLoS ONE 2018, 13, e0190888. [Google Scholar] [CrossRef]
- Ozmen, I. Evaluation of Effect of Some Corticosteroids on Glucose-6-Phosphate Dehydrogenase and Comparative Study of Antioxidant enzyme Activities. J. Enzyme Inhib. Med. Chem. 2005, 20, 19–24. [Google Scholar] [CrossRef]
- de Marco, R.; Poli, A.; Ferrari, M.; Accordini, S.; Giammanco, G.; Bugiani, M.; Villani, S.; Ponzio, M.; Bono, R.; Carrozzi, L.; et al. The Impact of Climate and Traffic-Related NO2 on the Prevalence of Asthma and Allergic Rhinitis in Italy. Clin. Exp. Allergy 2002, 32, 1405–1412. [Google Scholar] [CrossRef]
- Mehta, A.; Mason, P.J.; Vulliamy, T.J. Glucose-6-Phosphate Dehydrogenase Deficiency. Baillieres Best Pr. Res. Clin. Haematol. 2000, 13, 21–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battistuzzi, G.; D’Urso, M.; Toniolo, D.; Persico, G.M.; Luzzatto, L. Tissue-Specific Levels of Human Glucose-6-Phosphate Dehydrogenase Correlate with Methylation of Specific Sites at the 3′ End of the Gene. Proc. Natl. Acad. Sci. USA 1985, 82, 1465–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spolarics, Z.; Siddiqi, M.; Siegel, J.H.; Garcia, Z.C.; Stein, D.S.; Ong, H.; Livingston, D.H.; Denny, T.; Deitch, E.A. Increased Incidence of Sepsis and Altered Monocyte Functions in Severely Injured Type A- Glucose-6-Phosphate Dehydrogenase-Deficient African American Trauma Patients. Crit. Care Med. 2001, 29, 728–736. [Google Scholar] [CrossRef]
- Atay, E.; Bozaykut, A.; Ipek, I.O. Glucose-6-Phosphate Dehydrogenase Deficiency in Neonatal Indirect Hyperbilirubinemia. J. Trop. Pediatr. 2006, 52, 56–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dore, M.P.; Parodi, G.; Portoghese, M.; Pes, G.M. The Controversial Role of Glucose-6-Phosphate Dehydrogenase Deficiency on Cardiovascular Disease: A Narrative Review. Oxid. Med. Cell Longev. 2021, 2021, 5529256. [Google Scholar] [CrossRef]
- Dore, M.P.; Portoghese, M.; Pes, G.M. The Elderly with Glucose-6-Phosphate Dehydrogenase Deficiency are More Susceptible to Cardiovascular Disease. J. Atheroscler. Thromb. 2021, 28, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, M.; Qin, Y.; Liu, H.; Tu, C.; Shen, B.; Xu, X.; Chen, H. Differentially Expressed Serum Proteins in Children with or without Asthma as Determined Using Isobaric Tags for Relative and Absolute Quantitation Proteomics. PeerJ 2020, 8, e9971. [Google Scholar] [CrossRef]
- Chen, Y.; Qiao, J. Protein-Protein Interaction Network Analysis and Identifying Regulation microRNAs in Asthmatic Children. Allergol. Immunopathol. 2015, 43, 584–592. [Google Scholar] [CrossRef]
- Beutler, E. G6PD Deficiency. Blood 1994, 84, 3613–3636. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.H.; Chiu, D.T.; Lin, H.R.; Tang, H.Y.; Cheng, M.L.; Ho, H.Y. Glucose-6-Phosphate Dehydrogenase Enhances Antiviral Response through Downregulation of NADPH Sensor HSCARG and Upregulation of NF-kappaB Signaling. Viruses 2015, 7, 6689–6706. [Google Scholar] [CrossRef] [Green Version]
- Fanucchi, M.V.; Plopper, C.G.; Evans, M.J.; Hyde, D.M.; Van Winkle, L.S.; Gershwin, L.J.; Schelegle, E.S. Cyclic Exposure to Ozone Alters Distal Airway Development in Infant Rhesus Monkeys. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 291, L644–L650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malig, B.J.; Pearson, D.L.; Chang, Y.B.; Broadwin, R.; Basu, R.; Green, R.S.; Ostro, B. A Time-Stratified Case-Crossover Study of Ambient Ozone Exposure and Emergency Department Visits for Specific Respiratory Diagnoses in California (2005–2008). Env. Health Perspect. 2016, 124, 745–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, F.; Menendez, S.; Wong, R. Decrease of Blood Cholesterol and Stimulation of Antioxidative Response in Cardiopathy Patients Treated with Endovenous Ozone Therapy. Free Radic. Biol. Med. 1995, 19, 115–119. [Google Scholar] [CrossRef]
- Linn, W.S.; Buckley, R.D.; Spier, C.E.; Blessey, R.L.; Jones, M.P.; Fischer, D.A.; Hackney, J.D. Health Effects of Ozone Exposure in Asthmatics. Am. Rev. Respir. Dis. 1978, 117, 835–843. [Google Scholar] [CrossRef]
- Mochitate, K.; Miura, T. Metabolic Enhancement and Increase of Alveolar Macrophages Induced by Ozone. Environ. Res. 1989, 49, 79–92. [Google Scholar] [CrossRef]
- Varghese, M.V.; James, J.; Rafikova, O.; Rafikov, R. Glucose-6-Phosphate Dehydrogenase Deficiency Contributes to Metabolic Abnormality and Pulmonary Hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2021, 320, L508–L521. [Google Scholar] [CrossRef]
- Kurata, M.; Suzuki, M. Glutathione Regeneration in Calcium-Loaded Erythrocytes: A Possible Relationship among Calcium Accumulation, ATP Decrement and Oxidative Damage. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1994, 109, 305–312. [Google Scholar] [CrossRef]
- Wang, Q.; Li, A.; Zheng, Y.; Zhang, S.; Wang, P. Glutathione ethyl Ester Supplementation Prevents Airway Hyper-Responsiveness in Mice. Ann. Transl. Med. 2020, 8, 1519. [Google Scholar] [CrossRef]
- Ahmad, A.; Shameem, M.; Husain, Q. Relation of Oxidant-Antioxidant Imbalance with Disease Progression in Patients with Asthma. Ann. Thorac. Med. 2012, 7, 226–232. [Google Scholar] [CrossRef]
- Parsanathan, R.; Jain, S.K. Glucose-6-Phosphate Dehydrogenase Deficiency Increases Cell Adhesion Molecules and Activates Human Monocyte-Endothelial Cell Adhesion: Protective Role of l-Cysteine. Arch. Biochem. Biophys. 2019, 663, 11–21. [Google Scholar] [CrossRef]
- Prado, C.M.; Martins, M.A.; Tiberio, I.F. Nitric Oxide in Asthma Physiopathology. ISRN Allergy 2011, 2011, 832560. [Google Scholar] [CrossRef] [Green Version]
- Pourbagher-Shahri, A.M.; Farkhondeh, T.; Talebi, M.; Kopustinskiene, D.M.; Samarghandian, S.; Bernatoniene, J. An Overview of NO Signaling Pathways in Aging. Molecules 2021, 26, 4533. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.C.; Cheng, M.L.; Hua, Y.S.; Wu, Y.H.; Lin, H.R.; Liu, H.Y.; Ho, H.Y.; Chiu, D.T. Glucose 6-Phosphate Dehydrogenase Knockdown Enhances IL-8 Expression in HepG2 Cells via Oxidative Stress and NF-kappaB Signaling Pathway. J. Inflamm. 2015, 12, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, S.; Kato, M.; Koike, T.; Kama, Y.; Suzuki, K.; Enseki, M.; Tabata, H.; Hirai, K.; Yamada, Y.; Mochizuki, H. Differences in Virus Detection and Cytokine Profiles between First Wheeze and Childhood Asthma. Tokai J. Exp. Clin. Med. 2020, 45, 10–17. [Google Scholar] [PubMed]
- Sanna, F.; Bonatesta, R.R.; Frongia, B.; Uda, S.; Banni, S.; Melis, M.P.; Collu, M.; Madeddu, C.; Serpe, R.; Puddu, S.; et al. Production of Inflammatory Molecules in Peripheral Blood Mononuclear Cells from Severely Glucose-6-Phosphate Dehydrogenase-Deficient Subjects. J. Vasc. Res. 2007, 44, 253–263. [Google Scholar] [CrossRef]
- Lynch, K.R.; O’Neill, G.P.; Liu, Q.; Im, D.S.; Sawyer, N.; Metters, K.M.; Coulombe, N.; Abramovitz, M.; Figueroa, D.J.; Zeng, Z.; et al. Characterization of the Human Cysteinyl Leukotriene CysLT1 Receptor. Nature 1999, 399, 789–793. [Google Scholar] [CrossRef]
- Duroudier, N.P.; Strachan, D.P.; Blakey, J.D.; Hall, I.P. Association of the Cysteinyl Leukotriene Receptor 1 Gene with Atopy in the British 1958 Birth Cohort. J. Allergy Clin. Immunol. 2009, 124, 566–572. [Google Scholar] [CrossRef] [PubMed]
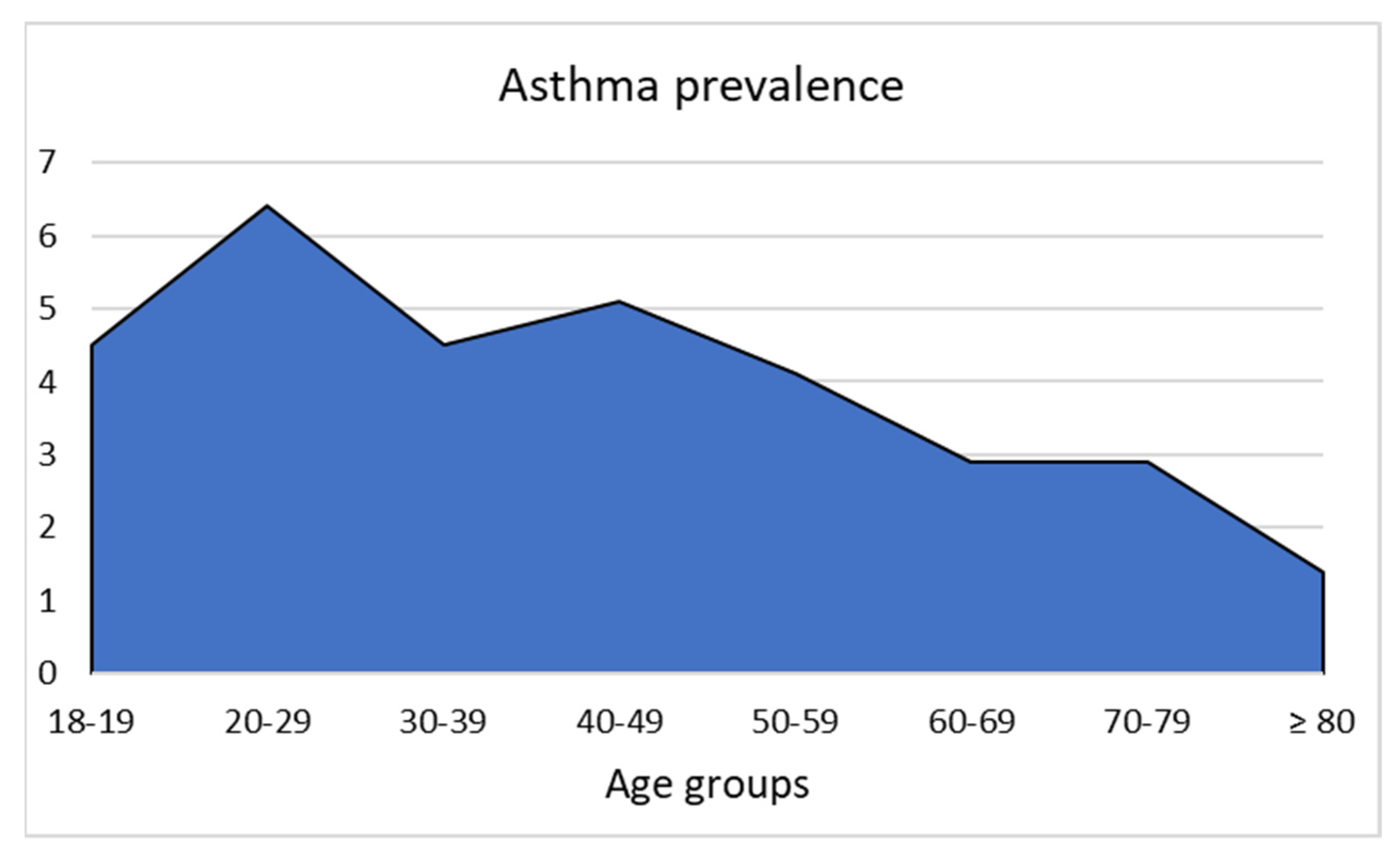


| Covariates | Asthma (Cases) | No Asthma (Controls) | p-Value |
|---|---|---|---|
| Age, n (%) | |||
| <60 | 322 (4.8) | 6352 (95.2) | |
| ≥60 | 133 (2.6) | 5022 (97.4) | <0.0001 |
| Sex, n (%) | |||
| Female | 315 (4.3) | 7008 (95.7) | |
| Male | 140 (3.1) | 4366 (96.9) | 0.001 |
| SES 1, n (%) | |||
| Low | 230 (3.3) | 6807 (96.7) | |
| High | 225 (4.7) | 4567 (95.3) | <0.0001 |
| BMI 2, kg/m2 | |||
| <25 | 211 (3.6) | 5652 (96.4) | |
| ≥25 | 244 (4.1) | 5722 (95.9) | 0.166 |
| Smoke | |||
| No | 316 (3.5) | 8602 (96.5) | |
| Yes | 139 (4.8) | 2772 (95.2) | 0.003 |
| G6PD 3 status | |||
| Normal | 378 (3.6) | 10,132 (96.4) | |
| Deficiency | 77 (5.8) | 1242 (94.2) | <0.0001 |
| Cases (Asthma) | Controls (No Asthma) | OR § (95% CI †) | |||
|---|---|---|---|---|---|
| G6PD # Normal | G6PD Deficient | G6PD Normal | G6PD Deficient | ||
| Sex, n (%) | |||||
| Female | 253 | 62 (19.6) | 6097 | 911 (12.9) | 1.64 (1.23–2.18) ** |
| Male | 124 | 16 (11.4) | 4035 | 331 (7.6) | 1.57 (0.92–2.68) |
| Age, n (%) | |||||
| <60 | 271 | 51 (15.8) | 5629 | 723 (11.4) | 1.47 (1.08–2.00) * |
| ≥60 | 106 | 27 (20.3) | 4503 | 519 (10.3) | 2.21 (1.43–3.40) ** |
| Total patients | 377 | 78 (17.1) | 10,132 | 1242 (10.9) | 1.69 (1.31–2.17) ** |
| Covariates | Unadjusted OR ‡s and 95% CI | Adjusted ORs and 95% CI |
|---|---|---|
| G6PD # status | ||
| Normal | Ref | Ref |
| Deficiency | 1.69 (1.31–2.17) ** | 1.63 (1.27–2.10) ** |
| Age, yrs | ||
| <60 | Ref | Ref |
| ≥60 | 0.52 (0.42–0.64) ** | 0.49 (0.39–0.61) ** |
| Sex | ||
| Male | Ref | Ref |
| Female | 1.40 (1.14–1.72) ** | 1.66 (1.34–2.06) ** |
| SES § | ||
| Low | Ref. | Ref. |
| High | 1.46 (1.21–1.76) ** | 1.40 (1.16–1.70) ** |
| BMI, kg/m2 | ||
| <30 | Ref. | Ref. |
| ≥30 | 1.14 (0.94–1.38) | 1.56 (1.27–1.92) ** |
| Smoking | ||
| No | Ref. | Ref. |
| Yes | 1.36 (1.11–1.67) ** | 1.44 (1.17–1.77) ** |
| Severity of Asthma According to GINA # Guidelines | Drugs Used to Treat Asthma | G6PD-Normal No. (%) | G6PD-Deficient No. (%) |
|---|---|---|---|
| Intermittent asthma | Low dose ICS §-formoterol as needed, with rapid onset LABA ¶ as needed, or low-dose ICS whenever SABA $ used | 206 (85.5) | 35 (14.5) |
| Mild persistent asthma | Daily low dose ICS with SABA or low dose ICS-formoterol as needed, or low-dose ICS plus SABA $ concomitantly as needed, or LTRA‡ daily and SABA $ as needed | 74 (84.1) | 14 (15.9) |
| Moderate persistent asthma | Low dose ICS-LABA as maintenance and reliever therapy, or low-dose ICS plus LTRA daily, +/− SABA as needed | 84 (76.4) | 26 (23.6) ** |
| Severe persistent asthma | Medium or high-dose ICS-LABA daily and SABA as needed, or high dose of ICS plus tiotropium, or LTRA +/− short course of oral glucocorticoids +/− add-on therapy (e.g., tiotropium, zileuton, anti-IgE, anti-IL-5, anti-IL-5R, anti-IL-4R), or oral glucocorticoids, or addiction of biologics | 14 (87.5) | 2 (12.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fois, A.; Dore, M.P.; Manca, A.; Scano, V.; Pirina, P.; Pes, G.M. Association between Glucose-6-Phosphate Dehydrogenase Deficiency and Asthma. J. Clin. Med. 2021, 10, 5639. https://doi.org/10.3390/jcm10235639
Fois A, Dore MP, Manca A, Scano V, Pirina P, Pes GM. Association between Glucose-6-Phosphate Dehydrogenase Deficiency and Asthma. Journal of Clinical Medicine. 2021; 10(23):5639. https://doi.org/10.3390/jcm10235639
Chicago/Turabian StyleFois, Alessandro, Maria Pina Dore, Andrea Manca, Valentina Scano, Pietro Pirina, and Giovanni Mario Pes. 2021. "Association between Glucose-6-Phosphate Dehydrogenase Deficiency and Asthma" Journal of Clinical Medicine 10, no. 23: 5639. https://doi.org/10.3390/jcm10235639
APA StyleFois, A., Dore, M. P., Manca, A., Scano, V., Pirina, P., & Pes, G. M. (2021). Association between Glucose-6-Phosphate Dehydrogenase Deficiency and Asthma. Journal of Clinical Medicine, 10(23), 5639. https://doi.org/10.3390/jcm10235639









