Preterm Birth and Small-for-Gestational Age Neonates among Prepregnancy Underweight Women: A Case-Controlled Study
Abstract
:1. Introduction
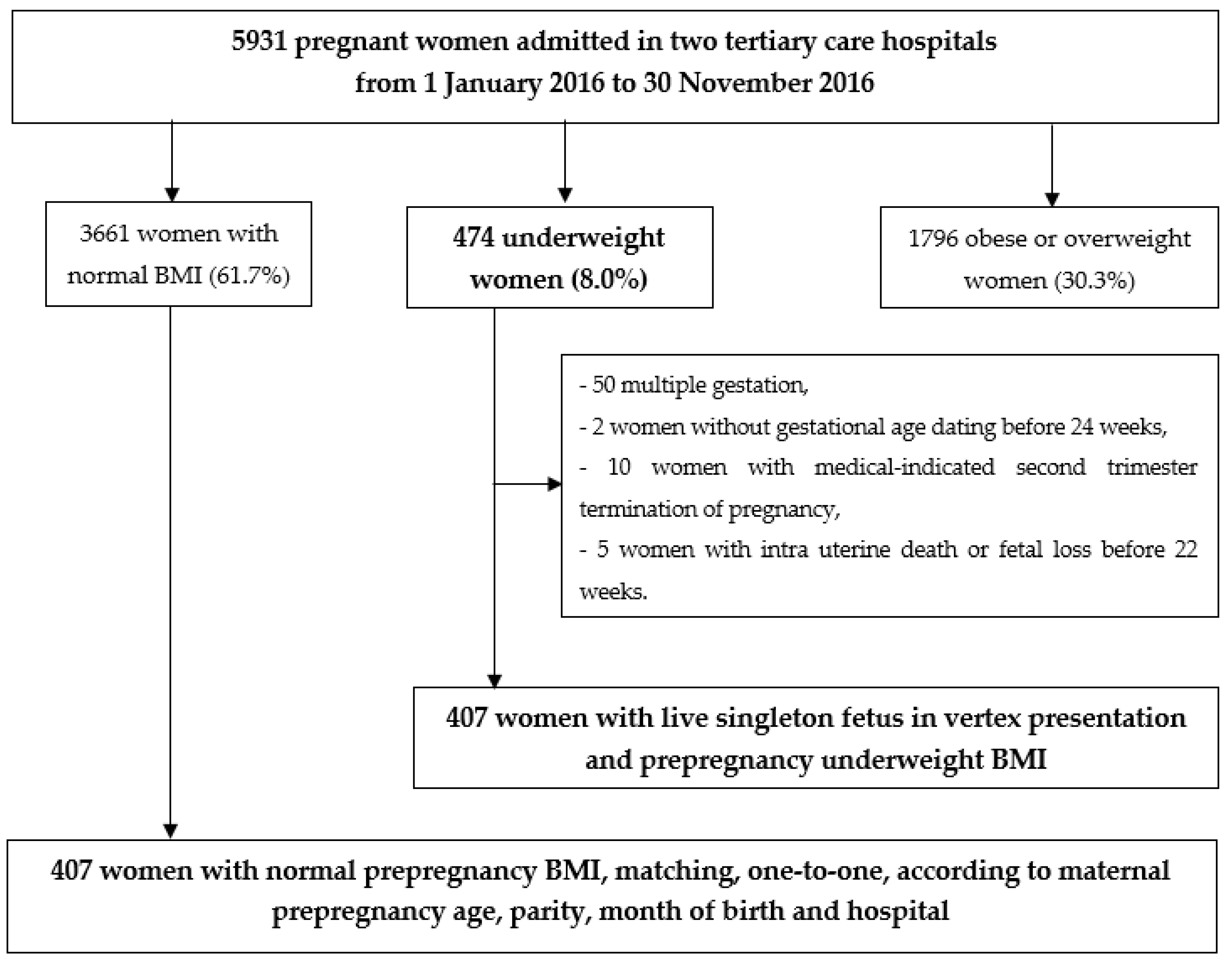
2. Materials and Methods
2.1. Patient Selection
2.2. Data Collection
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. International Classification of Adult Underweight, Overweight and Obesity According to BMI. Available online: http://www.assesmentpsychology.com/icbmi.htm (accessed on 8 November 2019).
- Blondel, B.; Pierrat, V.; Foix-L’Hélias, L. Changes in conditions at birth in France from 1995 to 2016: Results of the National Perinatal Surveys. Arch. Pédiatr. 2018, 25, 245–246. [Google Scholar] [CrossRef] [PubMed]
- Girsen, A.I.; Mayo, J.A.; Carmichael, S.L.; Phibbs, C.S.; Shachar, B.Z.; Stevenson, D.K.; Lyell, D.J.; Shaw, G.M.; Gould, J.B. Women’s prepregnancy underweight as a risk factor for preterm birth: A retrospective study. BJOG Int. J. Obstet. Gynaecol. 2016, 123, 2001–2007. [Google Scholar] [CrossRef] [PubMed]
- Shaw, G.M.; Wise, P.H.; Mayo, J.; Carmichael, S.L.; Ley, C.; Lyell, D.J.; Shachar, B.Z.; Melsop, K.; Phibbs, C.S.; Stevenson, D.K.; et al. Maternal Prepregnancy Body Mass Index and Risk of Spontaneous Preterm Birth. Paediatr. Périnat. Epidemiol. 2014, 28, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Lynch, A.M.; Hart, J.E.; Agwu, O.C.; Fisher, B.M.; West, N.A.; Gibbs, R.S. Association of extremes of prepregnancy BMI with the clinical presentations of preterm birth. Am. J. Obstet. Gynecol. 2014, 210, 428.e1–428.e9. [Google Scholar] [CrossRef]
- Han, Z.; Mulla, S.; Beyene, J.; Liao, G.; McDonald, S.D. Maternal underweight and the risk of preterm birth and low birth weight: A systematic review and meta-analyses. Int. J. Epidemiol. 2010, 40, 65–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ESH/ESC. Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J. Hypertens. 2013, 31, 1925–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Institute of Medicine and National Research Council, Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines; Rasmussen, K.M., Yaktine, A.L., Eds.; National Academies Press: Washington, DC, USA, 2009.
- Goonewardene, M.; Shehata, M.; Hamad, A. Anaemia in pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2012, 26, 3–24. [Google Scholar] [CrossRef] [PubMed]
- International Association of Diabetes Pregnancy Study Groups. Recommendations on the diagnosis and classification of hyperglycaemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collège national des gynécologues et obstétriciens français; Société francophone du diabète. Gestational diabetes. J. Gynecol. Obstet. Biol. Reprod. 2010, 39, S338–S342. [Google Scholar]
- American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar]
- Ego, A. Definitions: Small for gestational age and intrauterine growth retardation. J. Gynecol. Obstet. Biol. Reprod. 2013, 42, 872–894. [Google Scholar] [CrossRef] [PubMed]
- Vayssière, C.; Sentilhes, L.; Ego, A.; Bernard, C.; Cambourieu, D.; Flamant, C.; Gascoin, G.; Gaudineau, A.; Grangé, G.; Houfflin-Debarge, V.; et al. Fetal growth restriction and intra-uterine growth restriction: Guidelines for clinical practice from the French College of Gynaecologists and Obstetricians. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 193, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Vayssiere, C.; Haumonte, J.B.; Chantry, A.; Coatleven, F.; Debord, M.P.; Gomez, C.; Le Ray, C.; Lopez, E.; Salomon, L.J.; Senat, M.V.; et al. Prolonged and post-term pregnancies: Guidelines for clinical practice from the French College of Gynecologists and Obstetricians (CNGOF). Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 169, 10–16. [Google Scholar] [CrossRef]
- Ducarme, G.; Pizzoferrato, A.; de Tayrac, R.; Schantz, C.; Thubert, T.; Le Ray, C.; Riethmuller, D.; Verspyck, E.; Gachon, B.; Pierre, F.; et al. Perineal prevention and protection in obstetrics: CNGOF clinical practice guidelines. J. Gynecol. Obstet. Hum. Reprod. 2019, 48, 455–460. [Google Scholar] [CrossRef]
- Sentilhes, L.; Vayssière, C.; Deneux-Tharaux, C.; Aya, A.G.; Bayoumeu, F.; Bonnet, M.P.; Djoudi, R.; Dolley, P.; Dreyfus, M.; Ducroux-Schouwey, C.; et al. Postpartum hemorrhage: Guidelines for clinical practice from the French Society of Anesthesiology and Intensive Care (SFAR). Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 198, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Salomon, L.J.; Bernard, J.P.; De Stavola, B.; Kenward, M.; Ville, Y. Birth weight and size: Charts and equations. J. Gynecol. Obstet. Biol. Reprod. 2007, 36, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Mamelle, N.; Munoz, F.; Grandjean, H. Fetal growth from the AUDIPOG study. I. Establishment of reference curves. J. Gynecol. Obstet. Biol. Reprod. 1996, 25, 61–70. [Google Scholar]
- Chabernaud, J.L. Neonatal resuscitation in delivery room: New advances. Arch. Pediatr. 2005, 12, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Adamkin, D.H. Nonatal hypoglycemia. Seminars Semin Fetal Neonatal Med. 2017, 22, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.J.; Liebling, R.E.; Verity, L.; Swingler, R.; Patel, R. Early maternal and neonatal morbidity associated with operative delivery in second stage of labour: A cohort study. Lancet 2001, 358, 1203–1207. [Google Scholar] [CrossRef]
- Sebire, N.J.; Jolly, M.; Harris, J.; Regan, L.; Robinson, S. Is maternal underweight really a risk factor for adverse pregnancy outcome? A population-based study in London. Br. J. Obstet. Gynaecol. 2001, 108, 61–66. [Google Scholar]
- Salihu, H.; Mbah, A.; Alio, A.; Clayton, H.; Lynch, O. Low pre-pregnancy body mass index and risk of medically indicated versus spontaneous preterm singleton birth. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 144, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Abe, S.K.; Kanda, M.; Narita, S.; Rahman, S.; Bilano, V.; Ota, E.; Gilmour, S.; Shibuya, K. Maternal body mass index and risk of birth and maternal health outcomes in low- and middle-income countries: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Ante, Z.; Luu, T.M.; Mph, J.H.; He, S.; Taddeo, D.; Lo, E.; Auger, N. Pregnancy outcomes in women with anorexia nervosa. Int. J. Eat. Disord. 2020, 53, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, U.; Papabathini, S.S.; Kawuki, J.; Obore, N.; Musa, T.H. Depression during pregnancy and the risk of low birth weight, preterm birth and intrauterine growth restriction- an updated meta-analysis. Early Hum. Dev. 2021, 152, 105243. [Google Scholar] [CrossRef]
- Margerison, C.E.; Luo, Z.; Li, Y. Economic conditions during pregnancy and preterm birth: A maternal fixed-effects analysis. Paediatr. Périnat. Epidemiol. 2019, 33, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.G.; Berger, H.; Park, A.L. Population-based study of serum ferritin in early pregnancy and adverse perinatal outcomes. Paediatr. Perinat. Epidemiol. 2020, 34, 706–712. [Google Scholar] [CrossRef]
- Salmon, C.; Thibon, P.; Prime, L.; Renouf, S.; Dreyfus, M.; Dolley, P. Impact of maternal underweight on obstetric and neonatal prognosis: A retrospective study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 260, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Ancel, P.-Y.; Saurel-Cubizolles, M.-J.; Di Renzo, G.C.; Papiernik, E.; Breart, G. A complete list of members of the Europop Group may be found on page 1169 Very and moderate preterm births: Are the risk factors different? BJOG Int. J. Obstet. Gynaecol. 1999, 106, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Doherty, D.; Magann, E.; Francis, J.; Morrison, J.; Newnham, J. Pre-pregnancy body mass index and pregnancy outcomes. Int. J. Gynecol. Obstet. 2006, 95, 242–247. [Google Scholar] [CrossRef] [PubMed]
| Underweight BMI (<18.5 kg/m2) n = 407 | Normal BMI (18.5–24.9 kg/m2) n = 407 | p-Value | |
|---|---|---|---|
| Maternal characteristics | |||
| Maternal age (years) | |||
| Less than 20 years | 18 (4.4) | 18 (4.4) | - |
| Between 20 and 39 years | 376 (92.4) | 376 (92.4) | - |
| Over 39 years | 13 (3.2) | 13 (3.2) | - |
| BMI before pregnancy (kg/m2) | 17.5 ± 0.9 | 21.5 ± 1.8 | <0.001 |
| Nulliparity | 165 (40.5) | 165 (40.5) | - |
| Geographic origin | |||
| Caucasian | 376 (92.4) | 366 (89.9) | 0.22 |
| North Africa | 11 (2.7) | 15 (3.7) | 0.43 |
| Sub-Saharan Africa | 11 (2.7) | 11 (2.7) | >0.99 |
| Asian | 6 (1.5) | 9 (2.2) | 0.45 |
| Overseas Departments | 3 (0.7) | 6 (1.5) | 0.34 |
| Hispanic | 0 | 0 | - |
| Smoking | 122 (30.0) | 81 (19.9) | 0.001 |
| Previous caesarean delivery | 44 (10.8) | 31 (7.6) | 0.12 |
| Previous hypertension | 3 (0.7) | 0 | 0.25 |
| Pregestational diabetes mellitus | 3 (0.7) | 5 (1.2) | 0.22 |
| History of depression | 28 (6.9) | 12 (3.0) | 0.01 |
| History of eating behaviour disorder | 25 (6.1) | 10 (2.5) | 0.01 |
| ART-conceived pregnancy | 26 (6.4) | 24 (5.9) | 0.77 |
| Gestational weight gain (kg) | 13.1 ± 4.2 | 12.3 ± 4.7 | 0.02 |
| Inadequate gestational weight gain | 179 (46.4) | 174 (44.7) | 0.65 |
| Gestational diabetes mellitus | 29 (7.1) | 25 (6.1) | 0.57 |
| Intrahepatic cholestasis of pregnancy | 2 (0.5) | 9 (2.2) | 0.03 |
| Anaemia | 198 (48.8) | 190 (47.2) | 0.64 |
| Threatened preterm labour | 35 (8.6) | 15 (3.7) | <0.01 |
| Pregnancy-associated hypertensive disorders | 4 (1.0) | 6 (1.5) | 0.52 |
| Antenatal suspicion of SGA * | 13 (3.2) | 5 (1.2) | 0.06 |
| Labour characteristics | |||
| Gestational age at delivery (w) | 39.2 ± 2.2 | 39.6 ± 1.7 | 0.001 |
| Preterm birth | 41 (10.1) | 23 (5.7) | 0.02 |
| Less than 28 weeks | 2 (0.5) | 1 (0.3) | |
| 28 to less than 32 | 5 (1.2) | 2 (0.5) | |
| 32 to less than 36 | 34 (8.4) | 20 (4.9) | |
| Iatrogenic preterm birth | 17 (4.2) | 6 (1.5) | 0.02 |
| Planned caesarean delivery | 22 (5.4) | 19 (4.7) | 0.64 |
| Induced labour | 65 (16.0) | 78 (19.2) | 0.23 |
| Spontaneous vaginal delivery | 316 (77.6) | 309 (75.9) | 0.56 |
| Operative vaginal delivery | 47 (11.6) | 51 (12.5) | 0.67 |
| Caesarean section during labour | 44 (10.8) | 47 (11.6) | 0.74 |
| Maternal outcome | |||
| 3rd or 4th-degree perineal lacerations | 4 (1.1) | 3 (0.8) | 0.53 |
| Postpartum haemorrhage (PPH) | 30 (7.6) | 33 (8.2) | 0.50 |
| Severe PPH (blood loss > 1000 mL) | 12 (3.1) | 16 (4.0) | 0.47 |
| Neonatal outcome | |||
| Birth weight (g) | 3055 ± 580 | 3281 ± 511 | <0.001 |
| Birth weight (centile) | 35 ± 27 | 46 ± 27 | <0.001 |
| Birth weight Z-score | −0.2 ± 1.0 | 0.14 ± 1.0 | <0.001 |
| SGA † | 93 (22.9) | 45 (11.1) | <0.001 |
| Birth weight 2500 g or less | 58 (14.3) | 24 (5.9) | 0.001 |
| LGA ‡ | 9 (2.2) | 26 (6.4) | <0.01 |
| Birth weight 4000 g or more | 13 (3.2) | 27 (6.6) | 0.02 |
| 5-min Apgar score less than 7 | 5 (1.2) | 3 (0.7) | 0.73 |
| pH < 7.10 | 8 (2.0) | 20 (5.3) | 0.02 |
| Transfer to NICU | 53 (13.0) | 44 (10.8) | 0.33 |
| NICU hospitalization longer than 24 h | 26 (6.4) | 21 (5.2) | 0.45 |
| Respiratory distress syndrome | 29 (7.1) | 33 (8.1) | 0.60 |
| Neonatal hyperbilirubinemia | 17 (4.2) | 6 (1.5) | 0.02 |
| Neonatal hypoglycaemia | 7 (1.7) | 3 (0.7) | 0.20 |
| Intraventricular haemorrhage greater than grade 2 | 0 | 3 (0.7) | 0.25 |
| Need for resuscitation or intubation | 3 (0.7) | 2 (0.5) | 0.98 |
| Sepsis | 4 (1.0) | 5 (1.2) | 0.96 |
| Seizures | 0 | 1 (0.3) | 0.87 |
| Neonatal death | 1 (0.3) | 3 (0.7) | 0.62 |
| Preterm Birth * | |||
|---|---|---|---|
| No (n = 750) | Yes (n = 64) | p | |
| Underweight women (BMI, <18.5 kg/m2) | 366 (48.8) | 41 (64.1) | 0.02 |
| Categories of underweight women | 0.02 | ||
| Severe thinness (BMI, <16 kg/m2) | 277 (36.9) | 27 (42.2) | |
| Moderate thinness (BMI, 16–16.99 kg/m2) | 69 (9.2) | 9 (14.1) | |
| Mild thinness (BMI, 17–18.49 kg/m2) | 20 (2.7) | 5 (7.8) | |
| Normal prepregnancy BMI women (BMI, >18 kg/m2) | 384 (51.2) | 23 (36.0) | 0.02 |
| Maternal age 40 years or greater | 25 (3.3) | 1 (1.6) | 0.45 |
| Nulliparity | 302 (40.3) | 28 (43.8) | 0.59 |
| Smoking | 173 (23.1) | 30 (46.9) | <0.001 |
| ART-conceived pregnancy | 22 (2.9) | 2 (3.1) | 0.93 |
| Inadequate GWG | 322 (44.2) | 31 (67.4) | <0.01 |
| Pregnancy-associated hypertensive disorders | 13 (1.8) | 3 (4.7) | 0.15 |
| Threatened preterm labour required hospitalization | 25 (3.3) | 25 (39.1) | <0.001 |
| GDM | 45 (6.0) | 9 (14.1) | 0.02 |
| Antenatal suspicion of SGA | 36 (4.8) | 14 (21.9) | <0.001 |
| Anaemia | 360 (48.3) | 28 (44.4) | 0.56 |
| ICP | 5 (0.7) | 6 (9.4) | <0.001 |
| Preterm Birth (n = 64) | ||
|---|---|---|
| Variable * | Adjusted OR (95% CI) * | p-Value |
| Underweight women (BMI less than 18.5 kg/m2) | 2.32 (1.12–4.81) | 0.02 |
| Maternal age 40 years or greater | 0.40 (0.04–3.48) | 0.06 |
| Nulliparity | 1.78 (0.87–3.61) | 0.11 |
| Smoking | 3.01 (1.45–6.25) | 0.01 |
| Inadequate GWG | 4.48 (2.04–9.82) | 0.001 |
| Pregnancy-associated hypertensive disorders | 7.26 (0.71–74.42) | 0.09 |
| GDM | 2.00 (0.72–5.60) | 0.19 |
| Antenatal suspicion of SGA | 36.44 (8.95–148.30) | <0.001 |
| ICP | 28.46 (5.91–137.13) | <0.001 |
| SGA Neonates * | |||
|---|---|---|---|
| No (n = 676) | Yes (n = 138) | p | |
| Underweight women (BMI, <18.5 kg/m2) | 314 (46.5) | 93 (67.4) | <0.001 |
| Categories of underweight women | <0.0001 | ||
| Severe thinness (BMI, <16 kg/m2) | 16 (2.4) | 9 (6.5) | |
| Moderate thinness (BMI, 16–16.99 kg/m2) | 56 (8.3) | 22 (15.9) | |
| Mild thinness (BMI, 17–18.49 kg/m2) | 242 (35.8) | 62 (44.9) | |
| Normal prepregnancy BMI women (BMI, >18 kg/m2) | 362 (53.5) | 45 (32.6) | <0.001 |
| Maternal age 40 years or greater | 19 (2.8) | 7 (5.1) | 0.17 |
| Nulliparity | 266 (39.4) | 64 (46.4) | 0.13 |
| Smoking | 145 (21.5) | 58 (42.0) | <0.001 |
| History of eating behavior disorders | 24 (3.6) | 11 (8.0) | 0.02 |
| History of depression | 25 (3.7) | 15 (10.9) | <0.001 |
| ART-conceived pregnancy | 41 (6.1) | 9 (6.5) | 0.84 |
| Inadequate GWG | 274 (42.7) | 79 (59.4) | <0.001 |
| Pregnancy-associated hypertensive disorders | 7 (1.0) | 3 (2.2) | 0.28 |
| GDM | 48 (7.1) | 6 (4.4) | 0.24 |
| Anaemia | 331 (49.3) | 57 (41.6) | 0.10 |
| ICP | 11 (1.6) | 0 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lefizelier, E.; Misbert, E.; Brooks, M.; Le Thuaut, A.; Winer, N.; Ducarme, G. Preterm Birth and Small-for-Gestational Age Neonates among Prepregnancy Underweight Women: A Case-Controlled Study. J. Clin. Med. 2021, 10, 5733. https://doi.org/10.3390/jcm10245733
Lefizelier E, Misbert E, Brooks M, Le Thuaut A, Winer N, Ducarme G. Preterm Birth and Small-for-Gestational Age Neonates among Prepregnancy Underweight Women: A Case-Controlled Study. Journal of Clinical Medicine. 2021; 10(24):5733. https://doi.org/10.3390/jcm10245733
Chicago/Turabian StyleLefizelier, Emelyne, Emilie Misbert, Marion Brooks, Aurélie Le Thuaut, Norbert Winer, and Guillaume Ducarme. 2021. "Preterm Birth and Small-for-Gestational Age Neonates among Prepregnancy Underweight Women: A Case-Controlled Study" Journal of Clinical Medicine 10, no. 24: 5733. https://doi.org/10.3390/jcm10245733
APA StyleLefizelier, E., Misbert, E., Brooks, M., Le Thuaut, A., Winer, N., & Ducarme, G. (2021). Preterm Birth and Small-for-Gestational Age Neonates among Prepregnancy Underweight Women: A Case-Controlled Study. Journal of Clinical Medicine, 10(24), 5733. https://doi.org/10.3390/jcm10245733






