Metabolic Syndrome Severity Score, Comparable to Serum Creatinine, Could Predict the Occurrence of End-Stage Kidney Disease in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis
Abstract
:1. Introduction
2. Materials and Methods
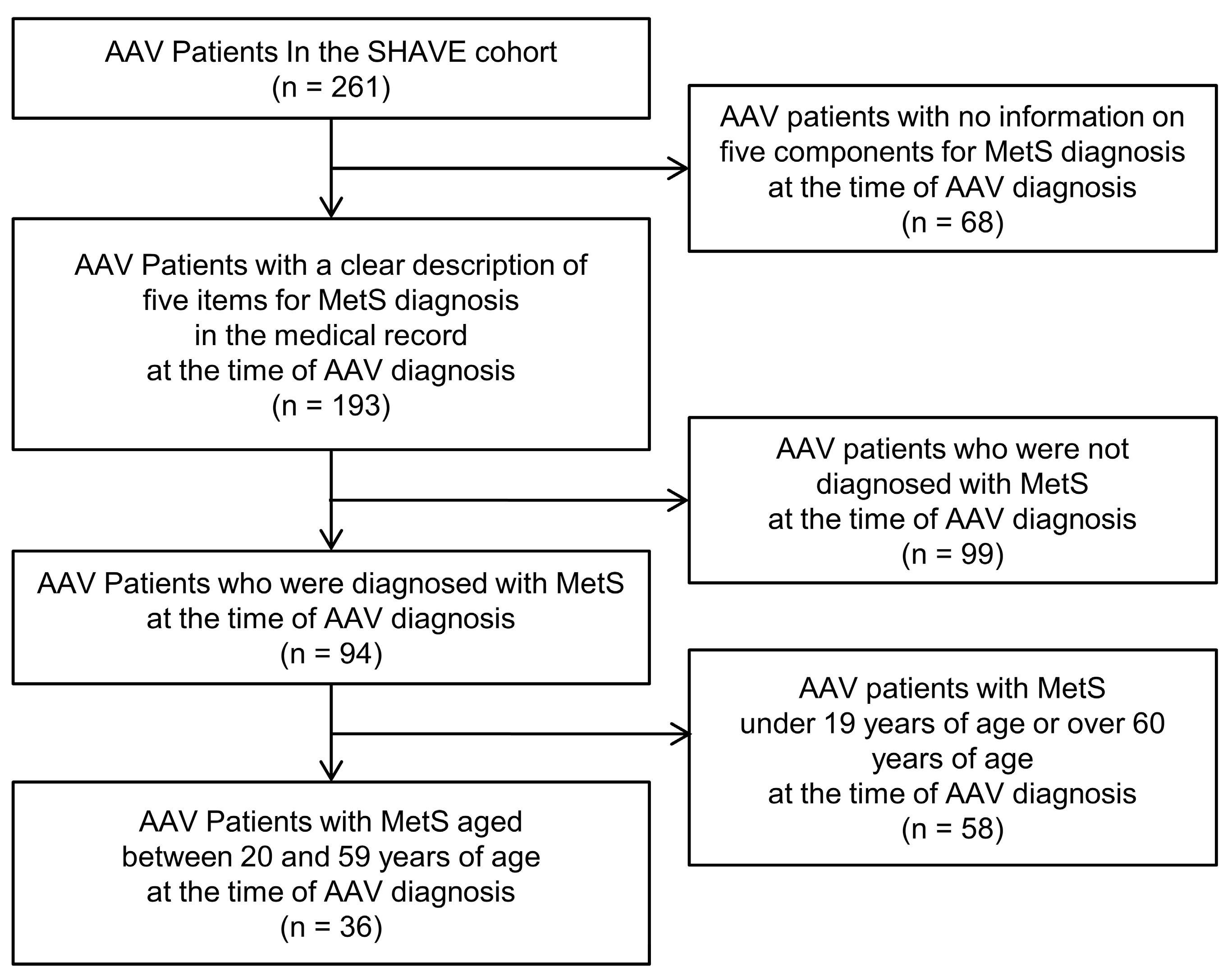
2.1. Patients
2.2. Definition of MetS
2.3. MSSS Equation
2.4. Variables
2.5. Statistical Analyses
3. Results
3.1. Characteristics of AAV Patients with MetS
3.2. Correlation Analysis
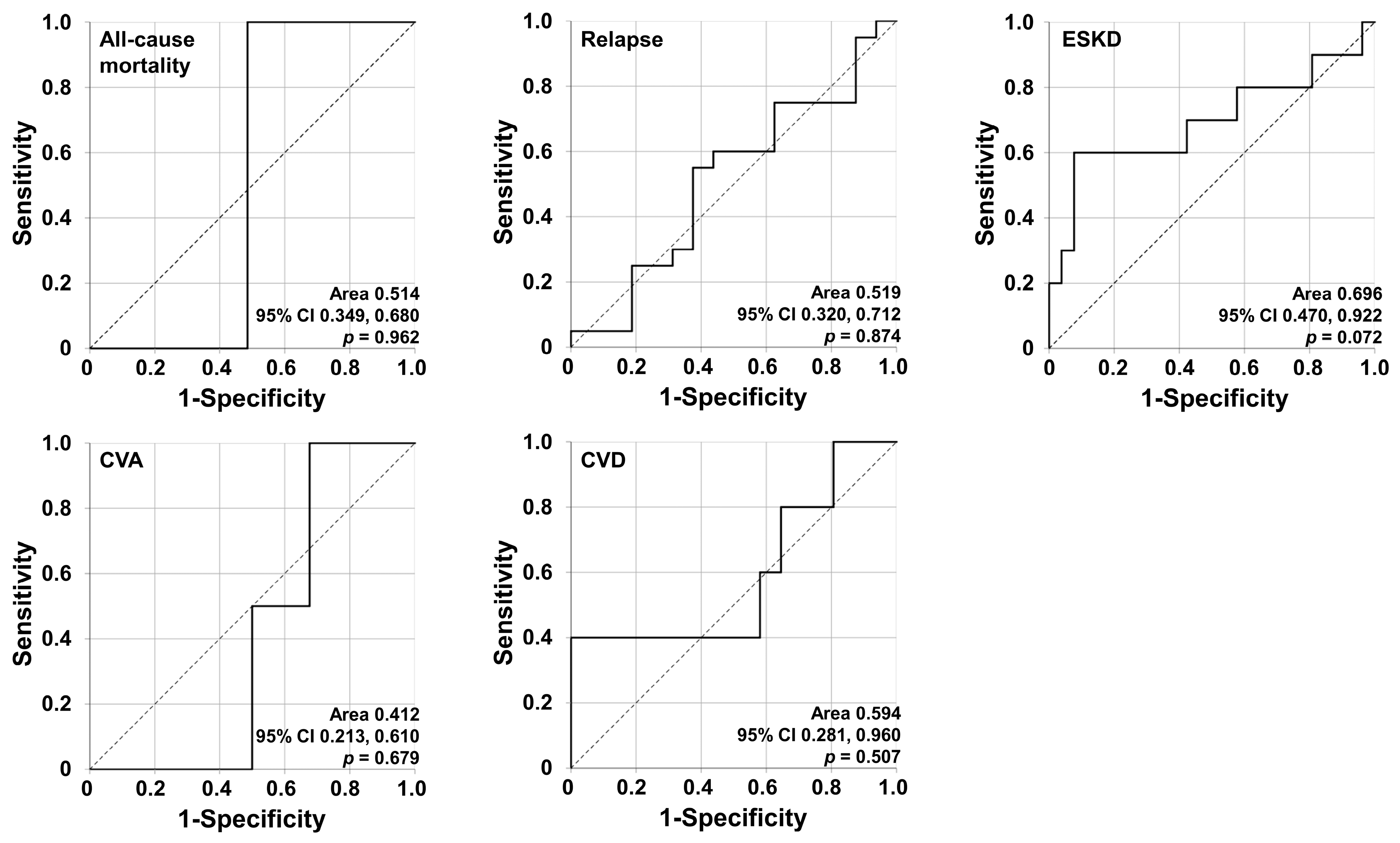
3.3. Determination of the Target Poor Outcome
3.4. Cox Hazards Model Analyses for the Occurrence of ESKD
3.5. Optimal MSSS Cut-Off and RR for ESKD
3.6. Comparisons between the Two Groups According to MSSS ≥ 1.72
3.7. Comparisons of Cumulative ESKD-Free Survival Rates between the Two Groups According to MSSS ≥ 1.72
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013, 65, 1–11. [Google Scholar] [CrossRef]
- Watts, R.; Lane, S.; Hanslik, T.; Hauser, T.; Hellmich, B.; Koldingsnes, W.; Mahr, A.; Segelmark, M.; Cohen-Tervaert, J.W.; Scott, D. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann. Rheum Dis. 2007, 66, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Cornec, D.; Cornec-Le Gall, E.; Fervenza, F.C.; Specks, U. ANCA-associated vasculitis—Clinical utility of using ANCA specificity to classify patients. Nat. Rev. Rheumatol. 2016, 12, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.A.; Dehghan, N.; Chen, W.; Xie, H.; Esdaile, J.M.; Avina-Zubieta, J.A. Mortality in ANCA-associated vasculitis: Ameta-analysis of observational studies. Ann. Rheum Dis 2017, 76, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Mukhtyar, C.; Flossmann, O.; Hellmich, B.; Bacon, P.; Cid, M.; Cohen-Tervaert, J.W.; Gross, W.L.; Guillevin, L.; Jayne, D.; Mahr, A.; et al. Outcomes from studies of antineutrophil cytoplasm antibody associated vasculitis: A systematic review by the European League Against Rheumatism systemic vasculitis task force. Ann. Rheum Dis. 2008, 67, 1004–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Monteiro, R.; Azevedo, I. Chronic inflammation in obesity and the metabolic syndrome. Mediat. Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Petermann Smits, D.R.; Wilde, B.; Kianersi Adegani, M.; de Jongh, H.; van Paassen, P.; Cohen Tervaert, J.W. Metabolic syndrome in ANCA-associated vasculitis. Rheumatology 2013, 52, 197–203. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.B.; Kwon, H.C.; Kang, M.I.; Park, Y.B.; Park, J.Y.; Lee, S.W. Increased prevalence rate of metabolic syndrome is an independent predictor of cardiovascular disease in patients with antineutrophil cytoplasmic antibody-associated vasculitis. Rheumatol. Int. 2021, 1–12. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Gurka, M.J.; Lilly, C.L.; Oliver, M.N.; DeBoer, M.D. An examination of sex and racial/ethnic differences in the metabolic syndrome among adults: A confirmatory factor analysis and a resulting continuous severity score. Metabolism 2014, 63, 218–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeBoer, M.D.; Gurka, M.J.; Woo, J.G.; Morrison, J.A. Severity of the metabolic syndrome as a predictor of type 2 diabetes between childhood and adulthood: The Princeton Lipid Research Cohort Study. Diabetologia 2015, 58, 2745–2752. [Google Scholar] [CrossRef] [Green Version]
- Huh, J.H.; Lee, J.H.; Moon, J.S.; Sung, K.C.; Kim, J.Y.; Kang, D.R. Metabolic Syndrome Severity Score in Korean Adults: Analysis of the 2010–2015 Korea National Health and Nutrition Examination Survey. J. Korean Med. Sci. 2019, 34, e48. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.N.; Lee, J.H.; Moon, J.S.; Kang, D.R.; Park, S.Y.; Cho, J.; Kim, J.Y.; Huh, J.H. Metabolic Syndrome Severity Score for Predicting Cardiovascular Events: A Nationwide Population-Based Study from Korea. Diabetes Metab. J. 2021, 45, 569–577. [Google Scholar] [CrossRef]
- Mukhtyar, C.; Lee, R.; Brown, D.; Carruthers, D.; Dasgupta, B.; Dubey, S.; Flossmann, O.; Hall, C.; Hollywood, J.; Jayne, D.; et al. Modification and validation of the Birmingham Vasculitis Activity Score (version 3). Ann. Rheum Dis. 2009, 68, 1827–1832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillevin, L.; Pagnoux, C.; Seror, R.; Mahr, A.; Mouthon, L.; Toumelin, P.L.; French Vasculitis Study Group (FVSG). The Five-Factor Score revisited: Assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine (Baltimore) 2011, 90, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Shin, H.; Song, J.H.; Kwak, S.H.; Kang, S.M.; Won Yoon, J.; Choi, S.H.; Cho, S.I.; Park, K.S.; Lee, H.K.; et al. Increasing prevalence of metabolic syndrome in Korea: The Korean National Health and Nutrition Examination Survey for 1998–2007. Diabetes Care 2011, 34, 1323–1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerchman, F.; Tong, J.; Utzschneider, K.M.; Zraika, S.; Udayasankar, J.; McNeely, M.J.; Carr, D.B.; Leonetti, D.L.; Young, B.A.; de Boer, I.H.; et al. Body mass index is associated with increased creatinine clearance by a mechanism independent of body fat distribution. J. Clin. Endocrinol. Metab. 2009, 94, 3781–3788. [Google Scholar] [CrossRef] [Green Version]
- Koh, E.S.; Do Han, K.; Kim, M.K.; Kim, E.S.; Lee, M.K.; Nam, G.E.; Hong, O.K.; Kwon, H.S. Changes in metabolic syndrome status affect the incidence of end-stage renal disease in the general population: A nationwide cohort study. Sci. Rep. 2021, 11, 1957. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, S.D.; Schold, J.D.; Kirwan, J.P.; Arrigain, S.; Jolly, S.E.; Poggio, E.D.; Beddhu, S.; Nally, J.V., Jr. Metabolic syndrome, ESRD, and death in CKD. Clin. J. Am. Soc. Nephrol. 2013, 8, 945–952. [Google Scholar] [CrossRef] [Green Version]
- Burrows, N.R.; Hora, I.; Geiss, L.S.; Gregg, E.W.; Albright, A. Incidence of End-Stage Renal Disease Attributed to Diabetes Among Persons with Diagnosed Diabetes—United States and Puerto Rico, 2000–2014. MMWR Morb. Mortal. Wkly Rep. 2017, 66, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; McCulloch, C.E.; Darbinian, J.; Go, A.S.; Iribarren, C. Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Arch. Intern. Med. 2005, 165, 923–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huh, J.H.; Yadav, D.; Kim, J.S.; Son, J.W.; Choi, E.; Kim, S.H.; Shin, C.; Sung, K.C.; Kim, J.Y. An association of metabolic syndrome and chronic kidney disease from a 10-year prospective cohort study. Metabolism 2017, 67, 54–61. [Google Scholar] [CrossRef]
- Berden, A.E.; Ferrario, F.; Hagen, E.C.; Jayne, D.R.; Jennette, J.C.; Joh, K.; Neumann, I.; Noël, L.H.; Pusey, C.D.; Waldherr, R.; et al. Histopathologic classification of ANCA- associated glomerulonephritis. J. Am. Soc. Nephrol. 2010, 21, 1628–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berti, A.; Cornec-Le Gall, E.; Cornec, D.; Casal Moura, M.; Matteson, E.L.; Crowson, C.S.; Ravindran, A.; Sethi, S.; Fervenza, F.C.; Specks, U. Incidence, prevalence, mortality and chronic renal damage of anti-neutrophil cytoplasmic antibody-associated glomerulonephritis in a 20-year population-based cohort. Nephrol. Dial Transpl. 2019, 34, 1508–1517. [Google Scholar] [CrossRef]
- Brix, S.R.; Noriega, M.; Tennstedt, P.; Vettorazzi, E.; Busch, M.; Nitschke, M.; Jabs, W.J.; Özcan, F.; Wendt, R.; Hausberg, M.; et al. Development and validation of a renal risk score in ANCA-associated glomerulonephritis. Kidney Int. 2018, 94, 1177–1188. [Google Scholar] [CrossRef] [PubMed]


| Variables | Values |
|---|---|
| At the time of diagnosis | |
| Demographic data | |
| Age (years) | 51.2 (11.2) |
| Sex (n, (%)) | |
| Male | 13 (36.1) |
| Female | 23 (63.9) |
| Body mass index (kg/m2) | 23.1 (2.9) |
| AAV Subtypes (n, (%)) | |
| MPA | 18 (50.0) |
| GPA | 8 (22.2) |
| EGPA | 10 (27.8) |
| ANCA type (n, (%)) | |
| MPO-ANCA (or P-ANCA) positive | 25 (69.4) |
| PR3-ANCA (or C-ANCA) positive | 5 (13.9) |
| Both ANCA positive | 0 (0) |
| ANCA positive | 30 (83.3) |
| AAV-specific indices | |
| BVAS | 12.5 (13.0) |
| FFS | 1.0 (2.0) |
| Laboratory results | |
| White blood cell count (/mm3) | 1820.0 (6170.0) |
| Haemoglobin (g/dL) | 11.1 (3.5) |
| Platelet count (×1000/mm3) | 320.0 (113.0) |
| Blood urea nitrogen (mg/dL) | 21.7 (32.6) |
| Serum creatinine (mg/dL) | 1.1 (2.8) |
| eGFR | 60.8 (76.1) |
| Serum albumin (g/dL) | 3.6 (0.8) |
| Acute-phase reactants | |
| ESR (mm/hr) | 62.0 (51.0) |
| CRP (mg/L) | 8.5 (82.4) |
| MSSS variables | |
| Waist circumference (cm) | 84.3 (9.1) |
| Systolic blood pressure (mmHg) | 130.0 (10.0) |
| Triglyceride (mg/dL) | 146.0 (81.0) |
| HDL-cholesterol (mg/dL) | 43.0 (17.0) |
| Fasting plasma glucose (mg/dL) | 100.5 (38.5) |
| MSSS | 1.1 (1.0) |
| During follow-up | |
| Poor outcomes and follow-up duration | |
| All-cause mortality (n, (%)) | 1 (2.8) |
| Follow-up duration based on all-cause mortality (months) | 61.0 (100.8) |
| Relapse (n, (%)) | 20 (55.6) |
| Follow-up duration based on relapse (months) | 15.9 (49.1) |
| ESKD (n, (%)) | 10 (27.8) |
| Follow-up duration based on ESKD (months) | 20.4 (107.6) |
| CVA (n, (%)) | 2 (5.6) |
| Follow-up duration based on CVA (months) | 58.2 (101.3) |
| CVD (n, (%)) | 5 (13.9) |
| Follow-up duration based on CVD (months) | 55.5 (95.8) |
| Medications administered during follow-up (n, (%)) | |
| Glucocorticoid | 36 (100) |
| Cyclophosphamide | 20 (55.6) |
| Rituximab | 8 (22.2) |
| Mycophenolate mofetil | 7 (19.4) |
| Azathioprine | 17 (47.2) |
| Tacrolimus | 1 (2.8) |
| Methotrexate | 2 (5.6) |
| Variables | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Age | 1.019 | 0.944, 1.099 | 0.635 | |||
| Male sex | 0.932 | 0.293, 3.638 | 0.920 | |||
| Body mass index | 0.779 | 0.624, 0.974 | 0.028 | |||
| MPA | 2.266 | 0.585, 8.771 | 0.236 | |||
| GPA | 2.090 | 0.533, 8192 | 0.290 | |||
| EGPA | 0.027 | 0.000, 7.064 | 0.203 | |||
| MPO-ANCA (or P-ANCA) positivity | 4.810 | 0.607, 38.140 | 0.137 | |||
| PR3-ANCA (or C-ANCA) positivity | 0.675 | 0.086, 5.334 | 0.710 | |||
| BVAS | 1.114 | 1.017, 1.221 | 0.020 | 1.178 | 0.945, 1.469 | 0.144 |
| FFS | 2.726 | 1.309, 5.677 | 0.007 | 1.030 | 0.258, 4.115 | 0.966 |
| White blood cell count | 1.000 | 1.000, 1.000 | 0.980 | |||
| Haemoglobin | 0.665 | 0.471, 0.940 | 0.021 | 1.186 | 0.782, 1.800 | 0.422 |
| Platelet count | 0.988 | 0.992, 1.004 | 0.998 | |||
| Blood urea nitrogen | 1.023 | 1.010, 1.035 | <0.001 | 0.976 | 0.941, 1.012 | 0.191 |
| Serum creatinine | 2.508 | 1.604, 3.919 | <0.001 | 3.713 | 1.560, 8.838 | 0.003 |
| Serum albumin | 0.555 | 0.198, 1555 | 0.263 | |||
| ESR | 1.019 | 0998, 1.040 | 0.074 | 1.015 | 0.979, 1.054 | 0.416 |
| CRP | 1.006 | 0.997, 1.015 | 0.209 | |||
| MSSS | 1.399 | 0.975, 2.007 | 0.068 | 1.971 | 1.071, 3.630 | 0.029 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, P.G.; Pyo, J.Y.; Ahn, S.S.; Song, J.J.; Park, Y.-B.; Huh, J.H.; Lee, S.-W. Metabolic Syndrome Severity Score, Comparable to Serum Creatinine, Could Predict the Occurrence of End-Stage Kidney Disease in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. J. Clin. Med. 2021, 10, 5744. https://doi.org/10.3390/jcm10245744
Park PG, Pyo JY, Ahn SS, Song JJ, Park Y-B, Huh JH, Lee S-W. Metabolic Syndrome Severity Score, Comparable to Serum Creatinine, Could Predict the Occurrence of End-Stage Kidney Disease in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Journal of Clinical Medicine. 2021; 10(24):5744. https://doi.org/10.3390/jcm10245744
Chicago/Turabian StylePark, Pil Gyu, Jung Yoon Pyo, Sung Soo Ahn, Jason Jungsik Song, Yong-Beom Park, Ji Hye Huh, and Sang-Won Lee. 2021. "Metabolic Syndrome Severity Score, Comparable to Serum Creatinine, Could Predict the Occurrence of End-Stage Kidney Disease in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis" Journal of Clinical Medicine 10, no. 24: 5744. https://doi.org/10.3390/jcm10245744






