Percutaneous Image-Guided Ablation of Lung Tumors
Abstract
:1. Introduction
2. Methods
3. Indications
3.1. Non-Small Cell Lung Cancer
| Modality | 5-Year Survival Stage I NSCLC | Local Recurrence | Sample Age (Mean, Median, Range) |
|---|---|---|---|
| Lobar resection [14] | 60–80% | 3–9% | NA |
| Sub-lobar resection [15,16] | 60–74% | 17% | 67.3, 68, 20–101 [15] 76.9, NA, 72–85 [16] |
| SBRT [17,18,19] | 42–55% | 14% | 74.2, NA, NA [18] |
| External beam radiation [20] | 10–27% | 50–55% | NA, 70, 34–90 |
| Radiofrequency ablation [21,22] | 27–56% | 22% | 68.5, NA, 17–94 [21] 70, NA, 48–84 [22] |
| Cryoablation [23] | 68% | 36% (locoregional) | 74.8, NA, 49–85 |
3.2. Metastases to the Lungs
3.3. Palliative
3.4. Pleural Lesions
4. Techniques
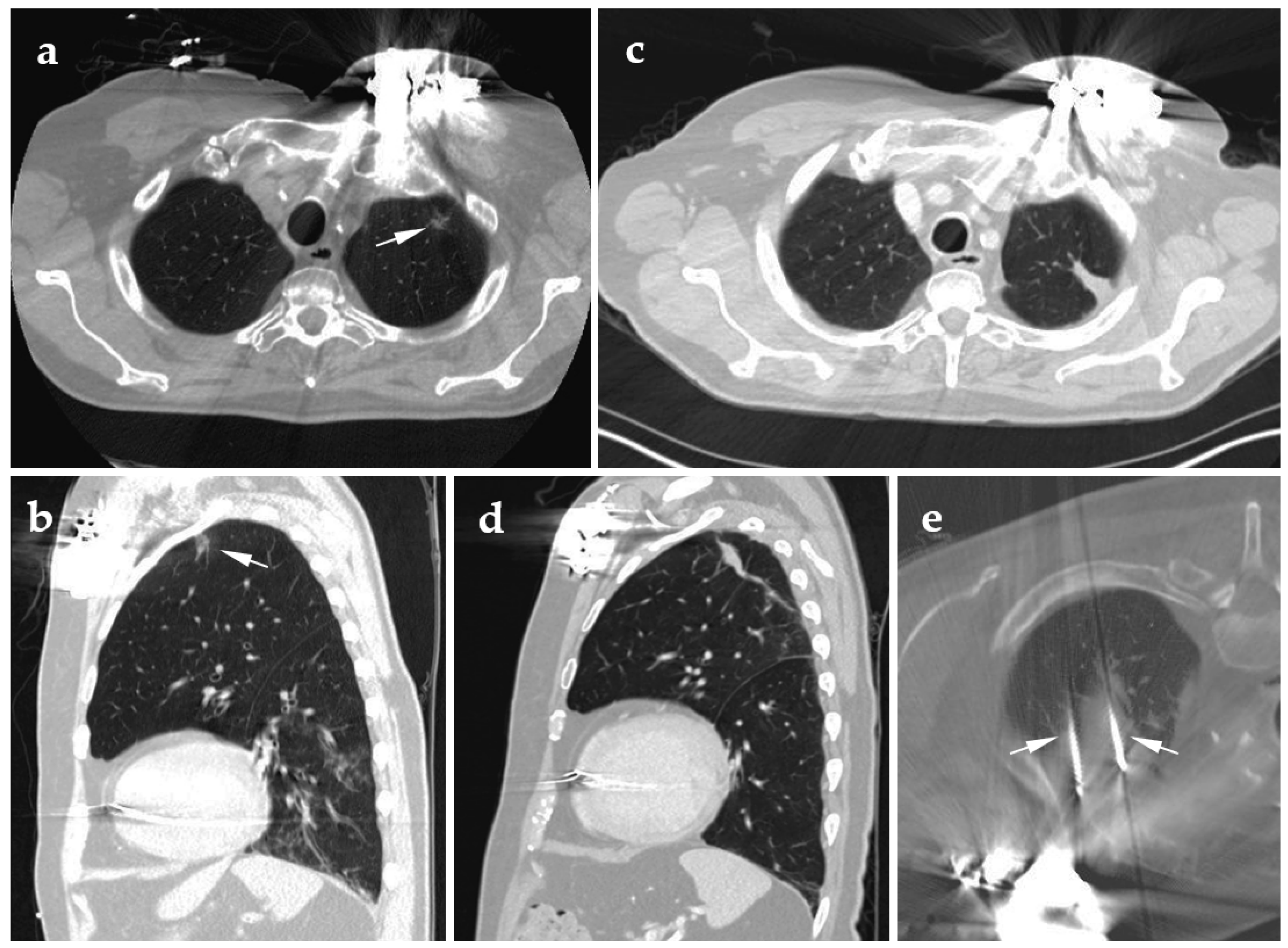
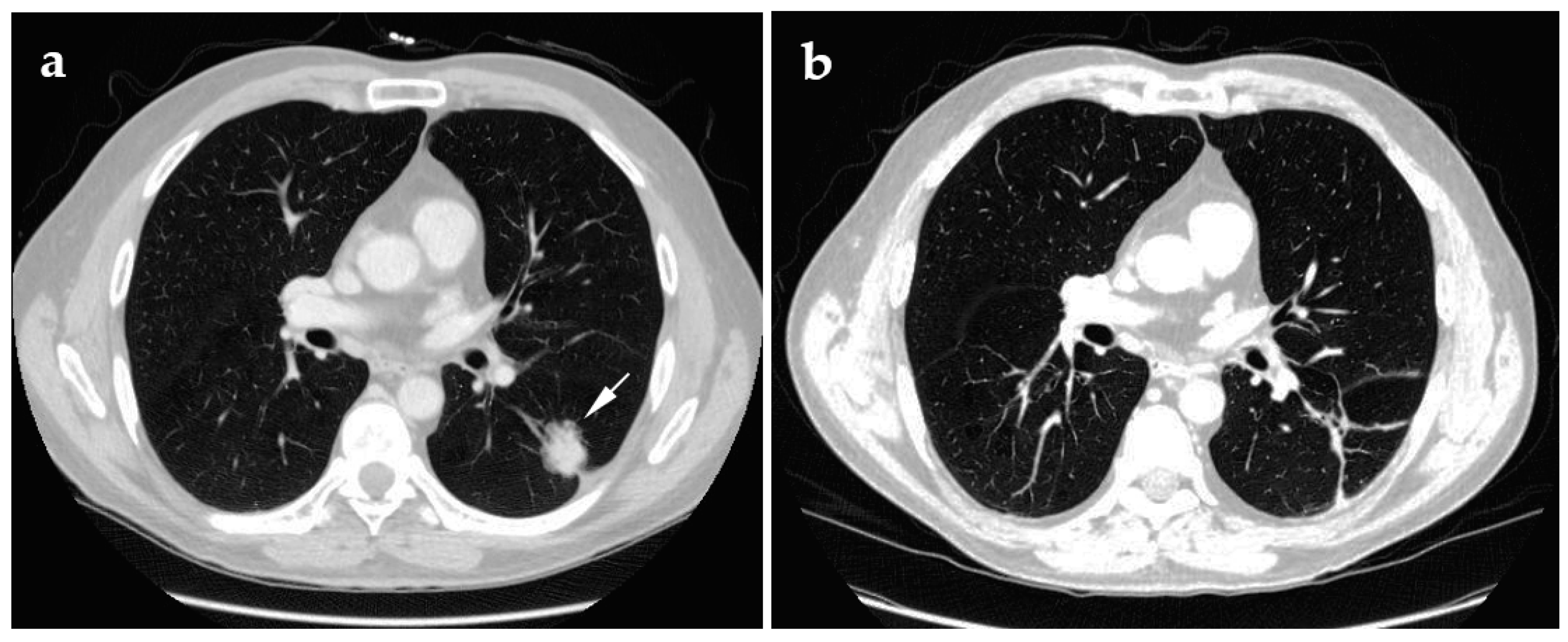
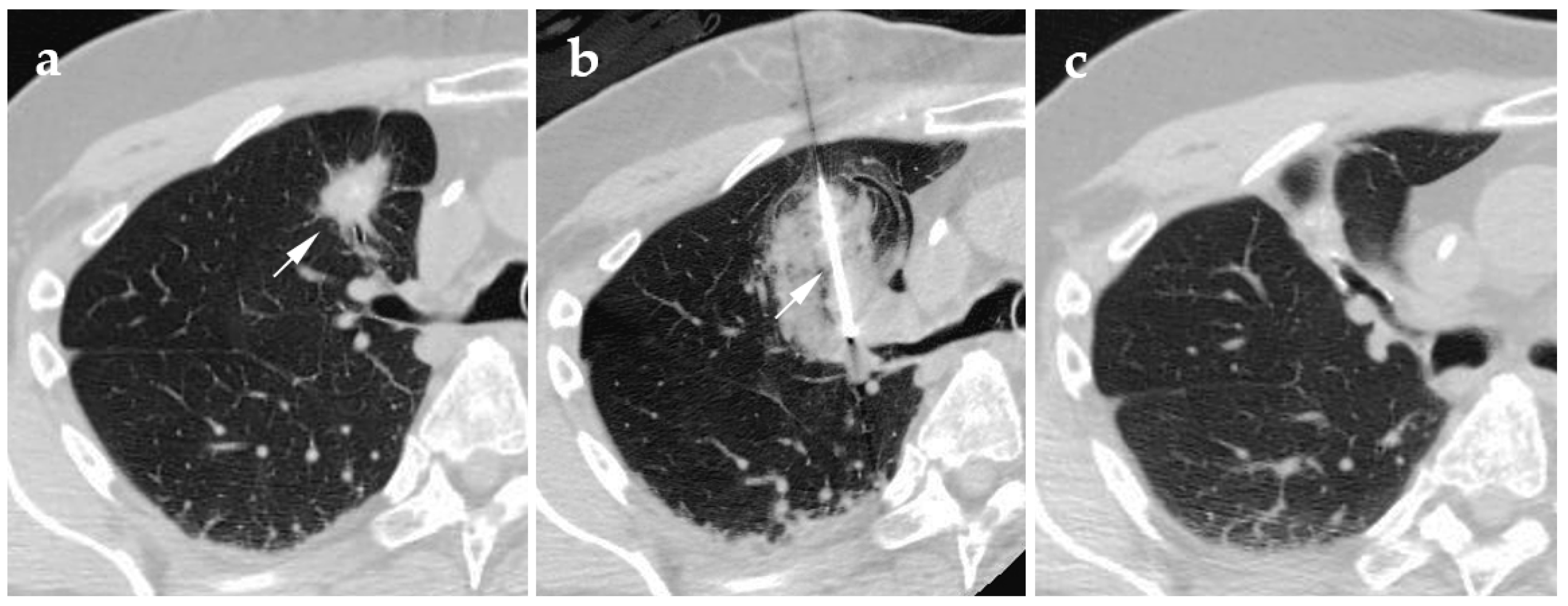
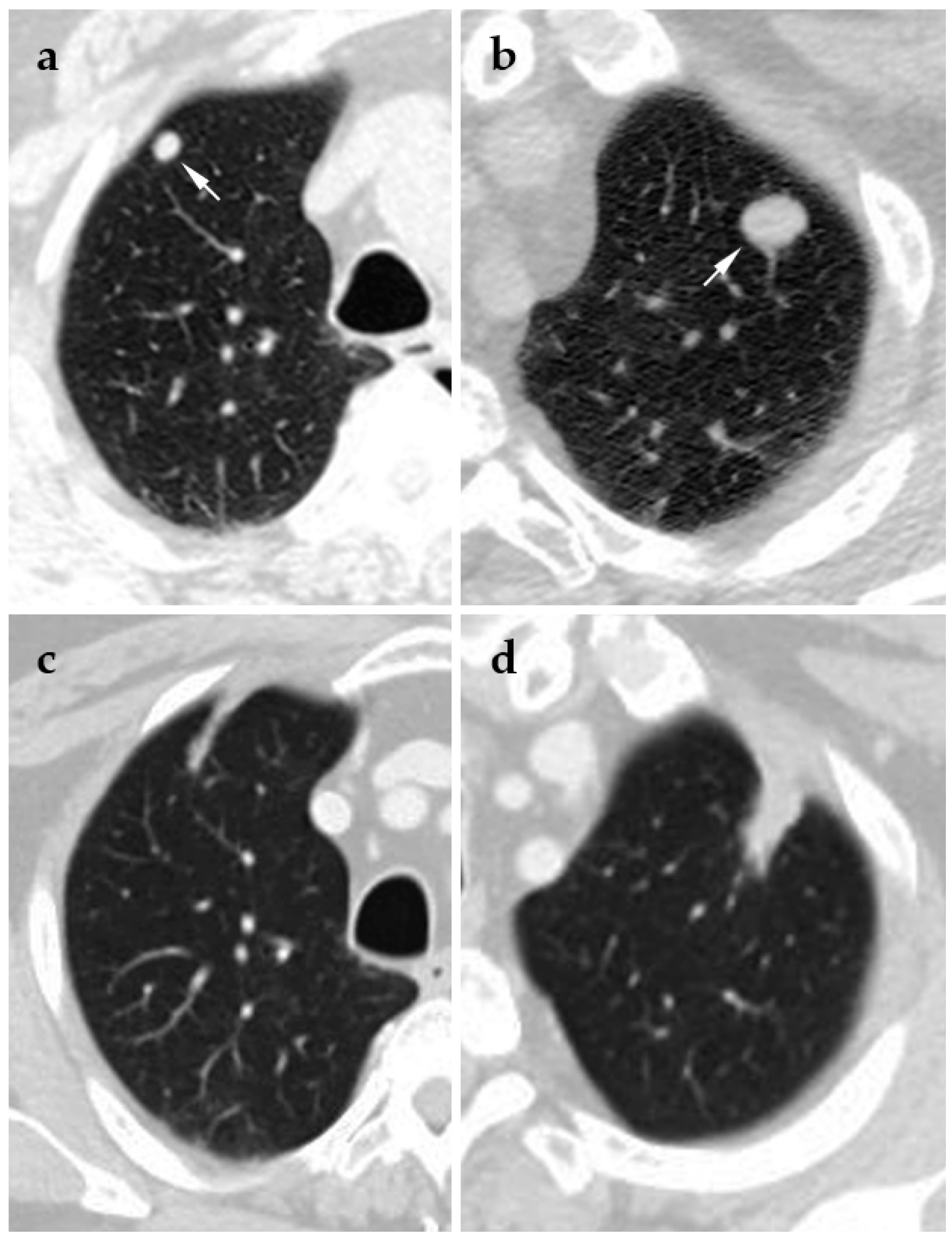
4.1. Cryoablation
4.2. Radiofrequency Ablation
4.3. Microwave Ablation
4.4. Laser Ablation
4.5. Irreversible Electroporation
5. Contraindications and Complications
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Jamil, A.; Kasi, A. Cancer, Metastasis to the Lung; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Pastorino, U.; Buyse, M.; Friedel, G.; Ginsberg, R.J.; Girard, P.; Goldstraw, P.; Johnston, M.; McCormack, P.; Pass, H.; Putnam, J.; et al. Long-term results of lung metastasectomy: Prognostic analyses based on 5206 cases. J. Thorac. Cardiovasc. Surg. 1997, 113, 37–49. [Google Scholar] [CrossRef] [Green Version]
- American Joint Committee on Cancer. AJCC Cancer Staging Manual, 6th ed.; Greene, F.L., Page, D.L., Fleming, I.D., Fritz, A.G., Balch, C.M., Haller, D.G., Morrow, M., Eds.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Chang, J.Y.; Li, Q.Q.; Xu, Q.Y.; Allen, P.K.; Rebueno, N.; Gomez, D.R.; Balter, P.; Komaki, R.; Mehran, R.; Swisher, S.G.; et al. Stereotactic ablative radiation therapy for centrally located early stage or isolated parenchymal recurrences of non-small cell lung cancer: How to fly in a “no fly zone”. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 1120–1128. [Google Scholar] [CrossRef]
- Rowell, N.P.; Williams, C. Radical radiotherapy for stage I/II non-small cell lung cancer in patients not sufficiently fit for or declining surgery (medically inoperable). In Cochrane Database of Systematic Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Dupuy, D.E.; Zagoria, R.J.; Akerley, W.; Mayo-Smith, W.W.; Kavanagh, P.V.; Safran, H. Technical innovation: Percutaneous radiofrequency ablation of malignancies in the lung. Am. J. Roentgenol. 2000, 174, 57–59. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Non-Small Cell Lung Cancer (Version 8.2020). Available online: https://www.nccn.org/ (accessed on 10 February 2021).
- Rose, S.C.; Dupuy, D.E.; Gervais, D.A.; Millward, S.F.; Brown, D.B.; Cardella, J.F.; Wallace, M.J. Research Reporting Standards for Percutaneous Thermal Ablation of Lung Neoplasms. J. Vasc. Interv. Radiol. 2009, 20, S474–S485. [Google Scholar] [CrossRef]
- Pereira, P.L.; Salvatore, M. Standards of practice: Guidelines for thermal ablation of primary and secondary lung tumors. Cardiovasc. Intervent. Radiol. 2012, 35, 247–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ketchedjian, A.; Daly, B.D.T.; Fernando, H.C.; Florin, L.; Hunter, C.J.; Morelli, D.M.; Shemin, R.J. Location as an important predictor of lymph node involvement for pulmonary adenocarcinoma. J. Thorac. Cardiovasc. Surg. 2006, 132, 544–548. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Zhang, B.; Zhang, H.; Li, W.; Wang, K.P.; Shen, H. Evaluation of lymph node metastasis in lung cancer: Who is the chief justice? J. Thorac. Dis. 2015, 7, S231–S237. [Google Scholar] [PubMed]
- Martin, J.T.; Durbin, E.B.; Chen, L.; Gal, T.; Mahan, A.; Ferraris, V.; Zwischenberger, J. Nodal upstaging during lung cancer resection is associated with surgical. In Proceedings of the Sixty-First Annual Meeting of the Southern Thoracic Surgical Association, Tucson, AZ, USA, 5–8 November 2014; Elsevier: Amsterdam, The Netherlands, 2016; Volume 101, pp. 238–245. [Google Scholar]
- Scott, W.J.; Howington, J.; Feigenberg, S.; Movsas, B.; Pisters, K. Treatment of non-small cell lung cancer stage I and stage II: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007, 132, 234S–242S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mery, C.M.; Pappas, A.N.; Bueno, R.; Colson, Y.L.; Linden, P.; Sugarbaker, D.J.; Jaklitsch, M.T. Similar long-term survival of elderly patients with non-small cell lung cancer treated with lobectomy or wedge resection within the surveillance, epidemiology, and end results database. Chest 2005, 128, 237–245. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Sujendran, V.; Alexiou, C.; Beggs, L.; Beggs, D. Long term results of surgery versus continuous hyperfractionated accelerated radiotherapy (CHART) in patients aged >70 years with stage 1 non-small cell lung cancer. Eur. J. Cardiothorac. Surg. 2003, 24, 1002–1007. [Google Scholar] [CrossRef]
- Grutters, J.P.C.; Kessels, A.G.H.; Pijls-Johannesma, M.; De Ruysscher, D.; Joore, M.A.; Lambin, P. Comparison of the effectiveness of radiotherapy with photons, protons and carbon-ions for non-small cell lung cancer: A meta-analysis. Radiother. Oncol. 2010, 95, 32–40. [Google Scholar] [CrossRef]
- Zheng, X.; Schipper, M.; Kidwell, K.; Lin, J.; Reddy, R.; Ren, Y.; Chang, A.; Lv, F.; Orringer, M.; Spring Kong, F.M. Survival outcome after stereotactic body radiation therapy and surgery for stage i non-small cell lung cancer: A meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 603–611. [Google Scholar] [CrossRef]
- Klapper, J.A.; Hittinger, S.A.; Denlinger, C.E. Alternatives to Lobectomy for High-Risk Patients with Early-Stage Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2017, 103, 1330–1339. [Google Scholar] [CrossRef]
- Gauden, S.; Ramsay, J.; Tripcony, L. The curative treatment by radiotherapy alone of stage I non-small cell carcinoma of the lung. Chest 1995, 108, 1278–1282. [Google Scholar] [CrossRef]
- Simon, C.J.; Dupuy, D.E.; DiPetrillo, T.A.; Safran, H.P.; Grieco, C.A.; Ng, T.; Mayo-Smith, W.W. Pulmonary radiofrequency ablation: Long-term safety and efficacy in 153 patients. Radiology 2007, 243, 268–275. [Google Scholar] [CrossRef]
- Kodama, H.; Yamakado, K.; Takaki, H.; Kashima, M.; Uraki, J.; Nakatsuka, A.; Takao, M.; Taguchi, O.; Yamada, T.; Takeda, K. Lung radiofrequency ablation for the treatment of unresectable recurrent non-small-cell lung cancer after surgical intervention. Cardiovasc. Intervent. Radiol. 2012, 35, 563–569. [Google Scholar] [CrossRef]
- Moore, W.; Talati, R.; Bhattacharji, P.; Bilfinger, T. Five-year survival after cryoablation of stage I non-small cell lung cancer in medically inoperable patients. J. Vasc. Interv. Radiol. 2015, 26, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Izumi, Y.; Hashimoto, K.; Yashiro, H.; Inoue, M.; Nakatsuka, S.; Goto, T.; Anraku, M.; Ohtsuka, T.; Kohno, M.; et al. Percutaneous Cryoablation for the Treatment of Medically Inoperable Stage I Non-Small Cell Lung Cancer. PLoS ONE 2012, 7, e33223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Din, O.S.; Harden, S.V.; Hudson, E.; Mohammed, N.; Pemberton, L.S.; Lester, J.F.; Biswas, D.; Magee, L.; Tufail, A.; Carruthers, R.; et al. Accelerated hypo-fractionated radiotherapy for non small cell lung cancer: Results from 4 UK centres. Radiother. Oncol. 2013, 109, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Ambrogi, M.C.; Fanucchi, O.; Cioni, R.; Dini, P.; De Liperi, A.; Cappelli, C.; Davini, F.; Bartolozzi, C.; Mussi, A. Long-term results of radiofrequency ablation treatment of stage i non-small cell lung cancer: A prospective intention-to-treat study. J. Thorac. Oncol. 2011, 6, 2044–2051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Ye, X.; Zheng, A.; Huang, G.; Ni, X.; Wang, J.; Han, X.; Li, W.; Wei, Z. Percutaneous microwave ablation of stage I medically inoperable non-small cell lung cancer: Clinical evaluation of 47 cases. J. Surg. Oncol. 2014, 110, 758–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ming, X.; Feng, Y.; Yang, C.; Wang, W.; Wang, P.; Deng, J. Radiation-induced heart disease in lung cancer radiotherapy: A dosimetric update. Medicine 2016, 95, e5051. [Google Scholar] [CrossRef] [PubMed]
- Stam, B.; Peulen, H.; Guckenberger, M.; Mantel, F.; Hope, A.; Werner-Wasik, M.; Belderbos, J.; Grills, I.; O’Connell, N.; Sonke, J.J. Dose to heart substructures is associated with non-cancer death after SBRT in stage I–II NSCLC patients. Radiother. Oncol. 2017, 123, 370–375. [Google Scholar] [CrossRef]
- Ochiai, S.; Yamakado, K.; Kodama, H.; Nomoto, Y.; Ii, N.; Takaki, H.; Sakuma, H. Comparison of therapeutic results from radiofrequency ablation and stereotactic body radiotherapy in solitary lung tumors measuring 5 cm or smaller. Int. J. Clin. Oncol. 2015, 20, 499–507. [Google Scholar] [CrossRef]
- Uhlig, J.; Ludwig, J.M.; Goldberg, S.B.; Chiang, A.; Blasberg, J.D.; Kim, H.S. Survival Rates after Thermal Ablation versus Stereotactic Radiation Therapy for Stage 1 Non–Small Cell Lung Cancer: A National Cancer Database Study. Radiology 2018, 289, 862–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baine, M.J.; Sleightholm, R.; Neilsen, B.K.; Oupický, D.; Smith, L.M.; Verma, V.; Lin, C. Stereotactic body radiation therapy versus nonradiotherapeutic ablative procedures (laser/cryoablation and electrocautery) for early-stage non–small cell lung cancer. JNCCN J. Natl. Compr. Cancer Netw. 2019, 17, 450–458. [Google Scholar] [CrossRef]
- Stella, G.M.; Kolling, S.; Benvenuti, S.; Bortolotto, C. Lung-seeking metastases. Cancers (Basel) 2019, 11, 1010. [Google Scholar] [CrossRef] [Green Version]
- Chudgar, N.P.; Brennan, M.F.; Munhoz, R.R.; Bucciarelli, P.R.; Tan, K.S.; D’Angelo, S.P.; Bains, M.S.; Bott, M.; Huang, J.; Park, B.J.; et al. Pulmonary metastasectomy with therapeutic intent for soft-tissue sarcoma. J. Thorac. Cardiovasc. Surg. 2017, 154, 319–330.e1. [Google Scholar] [CrossRef] [Green Version]
- Hornbech, K.; Ravn, J.; Steinbrüchel, D.A. Outcome after pulmonary metastasectomy: Analysis of 5 years consecutive surgical resections 2002–2006. J. Thorac. Oncol. 2011, 6, 1733–1740. [Google Scholar] [CrossRef] [Green Version]
- Handy, J.R.; Bremner, R.M.; Crocenzi, T.S.; Detterbeck, F.C.; Fernando, H.C.; Fidias, P.M.; Firestone, S.; Johnstone, C.A.; Lanuti, M.; Litle, V.R.; et al. Expert Consensus Document on Pulmonary Metastasectomy. Ann. Thorac. Surg. 2019, 107, 631–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, X.; Fan, W.; Wang, H.; Wang, J.; Wang, Z.; Gu, S.; Feng, W.; Zhuang, Y.; Liu, B.; Li, X.; et al. Expert consensus workshop report: Guidelines for thermal ablation of primary and metastatic lung tumors (2018 edition). J. Cancer Res. Ther. 2018, 14, 730–744. [Google Scholar] [CrossRef]
- Gonzalez, M.; Poncet, A.; Combescure, C.; Robert, J.; Ris, H.B.; Gervaz, P. Risk factors for survival after lung metastasectomy in colorectal cancer patients: A systematic review and meta-analysis. Ann. Surg. Oncol. 2013, 20, 572–579. [Google Scholar] [CrossRef]
- De Baère, T.; Aupérin, A.; Deschamps, F.; Chevallier, P.; Gaubert, Y.; Boige, V.; Fonck, M.; Escudier, B.; Palussiére, J. Radiofrequency ablation is a valid treatment option for lung metastases: Experience in 566 patients with 1037 metastases. Ann. Oncol. 2015, 26, 987–991. [Google Scholar] [CrossRef]
- Callstrom, M.R.; Woodrum, D.A.; Nichols, F.C.; Palussiere, J.; Buy, X.; Suh, R.D.; Abtin, F.G.; Pua, B.B.; Madoff, D.C.; Bagla, S.L.; et al. Multicenter Study of Metastatic Lung Tumors Targeted by Interventional Cryoablation Evaluation (SOLSTICE). J. Thorac. Oncol. 2020, 15, 1200–1209. [Google Scholar] [CrossRef]
- Morrow, C.E.; Vassilopoulos, P.P.; Grage, T.B. Surgical resection for metastatic neoplasms of the lung: Experience at the university of minnesota hospitals. Cancer 1980, 45, 2981–2985. [Google Scholar] [CrossRef]
- Wright, J.O.; Brandt, B.; Ehrenhaft, J.L. Results of pulmonary resection for metastatic lesions. J. Thorac. Cardiovasc. Surg. 1982, 83, 94–99. [Google Scholar] [CrossRef]
- McCormack, P.M.; Burt, M.E.; Bains, M.S.; Martini, N.; Rusch, V.W.; Ginsberg, R.J. Lung Resection for Colorectal Metastases: 10-Year Results. Arch. Surg. 1992, 127, 1403–1406. [Google Scholar] [CrossRef] [PubMed]
- Eiken, P.W.; Welch, B.T. Cryoablation of Lung Metastases: Review of Recent Literature and Ablation Technique. Semin. Intervent. Radiol. 2019, 36, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Louis Hinshaw, J.; Lubner, M.G.; Ziemlewicz, T.J.; Lee, F.T.; Brace, C.L. Percutaneous tumor ablation tools: Microwave, radiofrequency, or cryoablation-what should you use and why? Radiographics 2014, 34, 1344–1362. [Google Scholar] [CrossRef]
- Lee, K.S.; Erinjeri, J.P. Decision Making in Interventional Oncology: Ablative Options in the Lung. Semin. Intervent. Radiol. 2017, 34, 176–181. [Google Scholar] [CrossRef] [Green Version]
- Schlijper, R.C.J.; Grutters, J.P.C.; Houben, R.; Dingemans, A.M.C.; Wildberger, J.E.; Van Raemdonck, D.; Van Cutsem, E.; Haustermans, K.; Lammering, G.; Lambin, P.; et al. What to choose as radical local treatment for lung metastases from colo-rectal cancer: Surgery or radiofrequency ablation? Cancer Treat. Rev. 2014, 40, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Kvale, P.A.; Selecky, P.A.; Prakash, U.B.S. Palliative care in lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007, 132, 368S–403S. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Zhi, X.; Zhang, J. Radiofrequency ablation (RFA) for palliative treatment of painful non-small cell lung cancer (NSCLC) rib metastasis: Experience in 12 patients. Thorac. Cancer 2015, 6, 761–764. [Google Scholar] [CrossRef]
- Soga, N.; Yamakado, K.; Gohara, H.; Takaki, H.; Hiraki, T.; Yamada, T.; Arima, K.; Takeda, K.; Kanazawa, S.; Sugimura, Y. Percutaneous radiofrequency ablation for unresectable pulmonary metastases from renal cell carcinoma. BJU Int. 2009, 104, 790–794. [Google Scholar] [CrossRef]
- Baisi, A.; Raveglia, F.; De Simone, M.; Cioffi, U. Palliative role of percutaneous radiofrequency ablation for severe hemoptysis in an elderly patient with inoperable lung cancer. J. Thorac. Cardiovasc. Surg. 2010, 140, 1196–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.M.; Jin, G.Y.; Goldberg, S.N.; Lee, Y.C.; Chung, G.H.; Han, Y.M.; Lee, S.Y.; Kim, C.S. Percutaneous Radiofrequency Ablation for Inoperable Non-Small Cell Lung Cancer and Metastases: Preliminary Report. Radiology 2004, 230, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.C.; Kim, K.; Rice, T.W.; Rajeswaran, J.; Bukowski, R.; Decamp, M.M.; Blackstone, E.H. Can we predict long-term survival after pulmonary metastasectomy for renal cell carcinoma? Ann. Thorac. Surg. 2005, 79, 996–1003. [Google Scholar] [CrossRef]
- Kawashima, A.; Nakayama, M.; Oka, D.; Sato, M.; Hatano, K.; Mukai, M.; Nagahara, A.; Nakai, Y.; Takayama, H.; Inoue, M.; et al. Pulmonary metastasectomy in patients with renal cell carcinoma: A single-institution experience. Int. J. Clin. Oncol. 2011, 16, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Chen, J.; Yao, F.; Zhou, L.; Zhang, C.; Wen, W.; Bi, X.; Hu, Y.; Piao, X.; Jiang, F.; et al. Percutaneous cryoablation for stage IV lung cancer: A retrospective analysis. Cryobiology 2013, 67, 151–155. [Google Scholar] [CrossRef]
- Abtin, F.; De Baere, T.; Dupuy, D.E.; Genshaft, S.; Healey, T.; Khan, S.; Suh, R. Updates on Current Role and Practice of Lung Ablation. J. Thorac. Imaging 2019, 34, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Tsao, A.S.; Wistuba, I.; Roth, J.A.; Kindler, H.L. Malignant pleural mesothelioma. J. Clin. Oncol. 2009, 27, 2081–2090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldini, E.H.; Richards, W.G.; Gill, R.R.; Goodman, B.M.; Winfrey, O.K.; Eisen, H.M.; Mak, R.H.; Chen, A.B.; Kozono, D.E.; Bueno, R.; et al. Updated patterns of failure after multimodality therapy for malignant pleural mesothelioma. J. Thorac. Cardiovasc. Surg. 2015, 149, 1374–1381. [Google Scholar] [CrossRef] [Green Version]
- Federico, R.; Adolfo, F.; Giuseppe, M.; Lorenzo, S.; Martino, D.P.T.; Anna, C.; Adriano, P.; Gino, C.; Francesca, R.; Matteo, C.; et al. Phase II trial of neoadjuvant pemetrexed plus cisplatin followed by surgery and radiation in the treatment of pleural mesothelioma. BMC Cancer 2013, 13, 22. [Google Scholar] [CrossRef] [Green Version]
- Minatel, E.; Trovo, M.; Polesel, J.; Baresic, T.; Bearz, A.; Franchin, G.; Gobitti, C.; Rumeileh, I.A.; Drigo, A.; Fontana, P.; et al. Radical pleurectomy/decortication followed by high dose of radiation therapy for malignant pleural mesothelioma. Final results with long-term follow-up. Lung Cancer 2014, 83, 78–82. [Google Scholar] [CrossRef]
- Cao, C.; Tian, D.; Manganas, C.; Matthews, P.; Yan, T.D. Systematic review of trimodality therapy for patients with malignant pleural mesothelioma. Ann. Cardiothorac. Surg. 2012, 1, 428–42837. [Google Scholar] [CrossRef] [PubMed]
- Abtin, F.; Quirk, M.T.; Suh, R.D.; Hsu, W.; Han, S.X.; Kim, G.H.J.; Genshaft, S.; Sandberg, J.K.; Olevsky, O.; Cameron, R.B. Percutaneous Cryoablation for the Treatment of Recurrent Malignant Pleural Mesothelioma: Safety, Early-Term Efficacy, and Predictors of Local Recurrence. J. Vasc. Interv. Radiol. 2017, 28, 213–221. [Google Scholar] [CrossRef]
- Lucchi, M.; Basolo, F.; Mussi, A. Surgical treatment of pleural recurrence from thymoma. Eur. J. Cardio-Thorac. Surg. 2008, 33, 707–711. [Google Scholar] [CrossRef] [Green Version]
- Okumura, M.; Shiono, H.; Inoue, M.; Tanaka, H.; Yoon, H.E.; Nakagawa, K.; Matsumura, A.; Ohta, M.; Iuchi, K.; Matsuda, H. Outcome of surgical treatment for recurrent thymic epithelial tumors with reference to World Health Organization histologic classification system. J. Surg. Oncol. 2007, 95, 40–44. [Google Scholar] [CrossRef]
- Monden, Y.; Nakahara, K.; Iioka, S.; Nanjo, S.; Ohno, K.; Fujii, Y.; Hashimoto, J.; Kitagawa, Y.; Masaoka, A.; Kawashima, Y. Recurrence of Thymoma: Clinicopathological Features, Therapy, and Prognosis. Ann. Thorac. Surg. 1985, 39, 165–169. [Google Scholar] [CrossRef]
- Abtin, F.; Suh, R.D.; Nasehi, L.; Han, S.X.; Hsu, W.; Quirk, M.; Genshaft, S.; Gutierrez, A.J.; Cameron, R.B. Percutaneous cryoablation for the treatment of recurrent thymoma: Preliminary safety and efficacy. J. Vasc. Interv. Radiol. 2015, 26, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Skye, H.M.; Pfotenhauer, J.M. Joule Thomson Cryocoolers and Cryoablation; Springer: Cham, Switzerland, 2020; pp. 47–63. [Google Scholar]
- Song, K.D. Percutaneous cryoablation for hepatocellular carcinoma. Clin. Mol. Hepatol. 2016, 22, 509–515. [Google Scholar] [CrossRef] [Green Version]
- Mazur, P. Freezing of living cells: Mechanisms and implications. Am. J. Physiol. 1984, 247, C125–C142. [Google Scholar] [CrossRef] [PubMed]
- Staren, E.D.; Sabel, M.S.; Gianakakis, L.M.; Wiener, G.A.; Hart, V.M.; Gorski, M.; Dowlatshahi, K.; Corning, B.F.; Haklin, M.F.; Koukoulis, G. Cryosurgery of breast cancer. Arch. Surg. 1997, 132, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Mazur, P.; Rall, W.F.; Leibo, S.P. Kinetics of water loss and the likelihood of intracellular freezing in mouse ova—Influence of the method of calculating the temperature dependence of water permeability. Cell Biophys. 1984, 6, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.M.; Lee, F.T.; Chinn, D.O.; Warner, T.; Chosy, S.G.; Mahvi, D.M. Perivascular and intralesional tissue necrosis after hepatic cryoablation: Results in a porcine model. Surgery 1997, 122, 742–747. [Google Scholar] [CrossRef]
- Elliott, B.A.; Curry, T.B.; Atwell, T.D.; Brown, M.J.; Rose, S.H. Lung isolation, one-lung ventilation, and continuous positive airway pressure with air for radiofrequency ablation of neoplastic pulmonary lesions. Anesth. Analg. 2006, 103, 463–464. [Google Scholar] [CrossRef]
- Littrup, P.J.; Jallad, B.; Vorugu, V.; Littrup, G.; Currier, B.; George, M.; Herring, D. Lethal Isotherms of Cryoablation in a Phantom Study: Effects of Heat Load, Probe Size, and Number. J. Vasc. Interv. Radiol. 2009, 20, 1343–1351. [Google Scholar] [CrossRef] [Green Version]
- Pan, P.J.; Bansal, A.K.; Genshaft, S.J.; Kim, G.H.; Suh, R.D.; Abtin, F. Comparison of Double-Freeze versus Modified Triple-Freeze Pulmonary Cryoablation and Hemorrhage Volume Using Different Probe Sizes in an In Vivo Porcine Lung. J. Vasc. Interv. Radiol. 2018, 29, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Bansal, A.; Genshaft, S.; Kim, G.; Wallace, W.; Suh, R.; Abtin, F. Double- versus modified triple-freeze pulmonary cryoablation protocols: Comparison of ablation and hemorrhage volume with different probe types in an in vivo porcine lung model. J. Vasc. Interv. Radiol. 2017, 28, S103–S104. [Google Scholar] [CrossRef] [Green Version]
- Hinshaw, J.L.; Littrup, P.J.; Durick, N.; Leung, W.; Lee, F.T.; Sampson, L.; Brace, C.L. Optimizing the protocol for pulmonary cryoablation: A comparison of a dual- and triple-freeze protocol. Cardiovasc. Intervent. Radiol. 2010, 33, 1180–1185. [Google Scholar] [CrossRef] [Green Version]
- Littrup, P.J.; Mody, A.; Sparschu, R.; Prchevski, P.; Montie, J.; Zingas, A.P.; Grignon, D. Prostatic cryotherapy: Ultrasonographicand pathologic correlation in the canine model. Urology 1994, 44, 175–183. [Google Scholar] [CrossRef]
- Kodama, H.; Yamakado, K.; Murashima, S.; Takaki, H.; Uraki, J.; Nakatsuka, A.; Shoumura, S.; Tarukawa, T.; Shimamoto, A.; Takao, M.; et al. Intractable bronchopleural fistula caused by radiofrequency ablation: Endoscopic bronchial occlusion with silicone embolic material. Br. J. Radiol. 2009, 82, e225–e227. [Google Scholar] [CrossRef]
- Zheng, A.; Yang, X.; Ye, X.; Huang, G.; Wei, Z.; Wang, J.; Han, X.; Ni, X.; Meng, M. Bronchopleural fistula after lung ablation: Experience in two cases and literature review. Indian J. Cancer 2015, 52, e41–e46. [Google Scholar] [CrossRef]
- Allaf, M.E.; Varkarakis, I.M.; Bhayani, S.B.; Inagaki, T.; Kavoussi, L.R.; Solomon, S.B. Pain control requirements for percutaneous ablation of renal tumors: Cryoablation versus radiofrequency ablation—Initial observations. Radiology 2005, 237, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wallace, A.N.; Waqar, S.N.; Morgensztern, D.; Madaelil, T.P.; Tomasian, A.; Jennings, J.W. Percutaneous Image-Guided Ablation in the Treatment of Osseous Metastases from Non-small Cell Lung Cancer. Cardiovasc. Intervent. Radiol. 2018, 41, 726–733. [Google Scholar] [CrossRef]
- Chheang, S.; Abtin, F.; Guteirrez, A.; Genshaft, S.; Suh, R. Imaging features following thermal ablation of lung malignancies. Semin. Intervent. Radiol. 2013, 30, 157–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, K.; Georgiades, C. Radiofrequency ablation: Mechanism of action and devices. J. Vasc. Interv. Radiol. 2010, 21, S179–S186. [Google Scholar] [CrossRef] [PubMed]
- Organ, L.W. Electrophysiologic principles of radiofrequency lesion making. Appl. Neurophysiol. 1976, 32, 69–76. [Google Scholar] [CrossRef]
- Schramm, W.; Yang, D.; Haemmerich, D. Contribution of direct heating, thermal conduction and perfusion during radiofrequency and microwave ablation. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. 2006, 2006, 5013–5016. [Google Scholar]
- Skonieczki, B.D.; Wells, C.; Wasser, E.J.; Dupuy, D.E. Radiofrequency and microwave tumor ablation in patients with implanted cardiac devices: Is it safe? Eur. J. Radiol. 2011, 79, 343–346. [Google Scholar] [CrossRef]
- Lubner, M.G.; Brace, C.L.; Hinshaw, J.L.; Lee, F.T. Microwave tumor ablation: Mechanism of action, clinical results, and devices. J. Vasc. Interv. Radiol. 2010, 21, S192–S203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupuy, D.E. Image-guided thermal ablation of lung malignancies. Radiology 2011, 260, 633–655. [Google Scholar] [CrossRef] [PubMed]
- Andreano, A.; Huang, Y.; Meloni, M.F.; Lee, F.T.; Brace, C. Microwaves create larger ablations than radiofrequency when controlled for power in ex vivo tissue. Med. Phys. 2010, 37, 2967–2973. [Google Scholar] [CrossRef] [Green Version]
- Sawabata, N.; Nezu, K.; Tojo, T.; Kitamura, S. In vitro study of ablated lung tissue in Nd:YAG laser irradiation. Ann. Thorac. Surg. 1996, 61, 164–169. [Google Scholar] [CrossRef]
- Rosenberg, C.; Puls, R.; Hegenscheid, K.; Kuehn, J.; Bollman, T.; Westerholt, A.; Weigel, C.; Hosten, N. Laser Ablation of Metastatic Lesions of the Lung: Long-Term Outcome. Am. J. Roentgenol. 2009, 192, 785–792. [Google Scholar] [CrossRef]
- Zhao, Q.; Tian, G.; Chen, F.; Zhong, L.; Jiang, T. CT-guided percutaneous laser ablation of metastatic lung cancer: Three cases report and literature review. Oncotarget 2017, 8, 2187–2196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubinsky, B.; Onik, G.; Mikus, P. Irreversible electroporation: A new ablation modality—Clinical implications. Technol. Cancer Res. Treat. 2007, 6, 37–48. [Google Scholar] [CrossRef]
- Maor, E.; Ivorra, A.; Leor, J.; Rubinsky, B. The effect of irreversible electroporation on blood vessels. Technol. Cancer Res. Treat. 2007, 6, 307–312. [Google Scholar] [CrossRef]
- Thomson, K.R.; Cheung, W.; Ellis, S.J.; Federman, D.; Kavnoudias, H.; Loader-Oliver, D.; Roberts, S.; Evans, P.; Ball, C.; Haydon, A. Investigation of the safety of irreversible electroporation in humans. J. Vasc. Interv. Radiol. 2011, 22, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Ricke, J.; Jürgens, J.H.W.; Deschamps, F.; Tselikas, L.; Uhde, K.; Kosiek, O.; De Baere, T. Irreversible Electroporation (IRE) Fails to Demonstrate Efficacy in a Prospective Multicenter Phase II Trial on Lung Malignancies: The ALICE Trial. Cardiovasc. Intervent. Radiol. 2015, 38, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Welch, B.T.; Brinjikji, W.; Schmit, G.D.; Callstrom, M.R.; Kurup, A.N.; Cloft, H.J.; Woodrum, D.A.; Nichols, F.C.; Atwell, T.D. A national analysis of the complications, cost, and mortality of percutaneous lung ablation. J. Vasc. Interv. Radiol. 2015, 26, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, B.; Bie, Z.; Li, Y.; Li, X. Synchronous core-needle biopsy and microwave ablation for highly suspicious malignant pulmonary nodule via a coaxial cannula. J. Cancer Res. Ther. 2019, 15, 1484–1489. [Google Scholar] [CrossRef]
- Steinke, K.; King, J.; Glenn, D.; Morris, D.L. Pulmonary hemorrhage during percutaneous radiofrequency ablation: A more frequent complication than assumed? Interact. Cardiovasc. Thorac. Surg. 2003, 2, 462–465. [Google Scholar] [CrossRef]
- Nour-Eldin, N.E.A.; Naguib, N.N.N.; MacK, M.; Abskharon, J.E.; Vogl, T.J. Pulmonary hemorrhage complicating radiofrequency ablation, from mild hemoptysis to life-threatening pattern. Eur. Radiol. 2011, 21, 197–204. [Google Scholar] [CrossRef]
- Hiraki, T.; Gobara, H.; Fujiwara, H.; Ishii, H.; Tomita, K.; Uka, M.; Makimoto, S.; Kanazawa, S. Lung cancer ablation: Complications. Semin. Intervent. Radiol. 2013, 30, 169–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghaye, B.; Bruyère, P.J.; Dondelinger, R.F. Nonfatal systemic air embolism during percutaneous radiofrequency ablation of a pulmonary metastasis. AJR. Am. J. Roentgenol. 2006, 187, W327–W328. [Google Scholar] [CrossRef]
- Okuma, T.; Matsuoka, T.; Tutumi, S.; Nakmura, K.; Inoue, Y. Air Embolism during Needle Placement for CT-guided Radiofrequency Ablation of an Unresectable Metastatic Lung Lesion. J. Vasc. Interv. Radiol. 2007, 18, 1592–1594. [Google Scholar] [CrossRef]
- Jeannin, A.; Saignac, P.; Palussière, J.; Gékière, J.P.; Descat, E.; Lakdja, F. Massive systemic air embolism during percutaneous radiofrequency ablation of a primary lung tumor. Anesth. Analg. 2009, 109, 484–486. [Google Scholar] [CrossRef]
- Okuma, T.; Matsuoka, T.; Yamamoto, A.; Oyama, Y.; Toyoshima, M.; Nakamura, K.; Inoue, Y. Frequency and risk factors of various complications after computed tomography-guided radiofrequency ablation of lung tumors. Cardiovasc. Intervent. Radiol. 2008, 31, 122–130. [Google Scholar] [CrossRef]
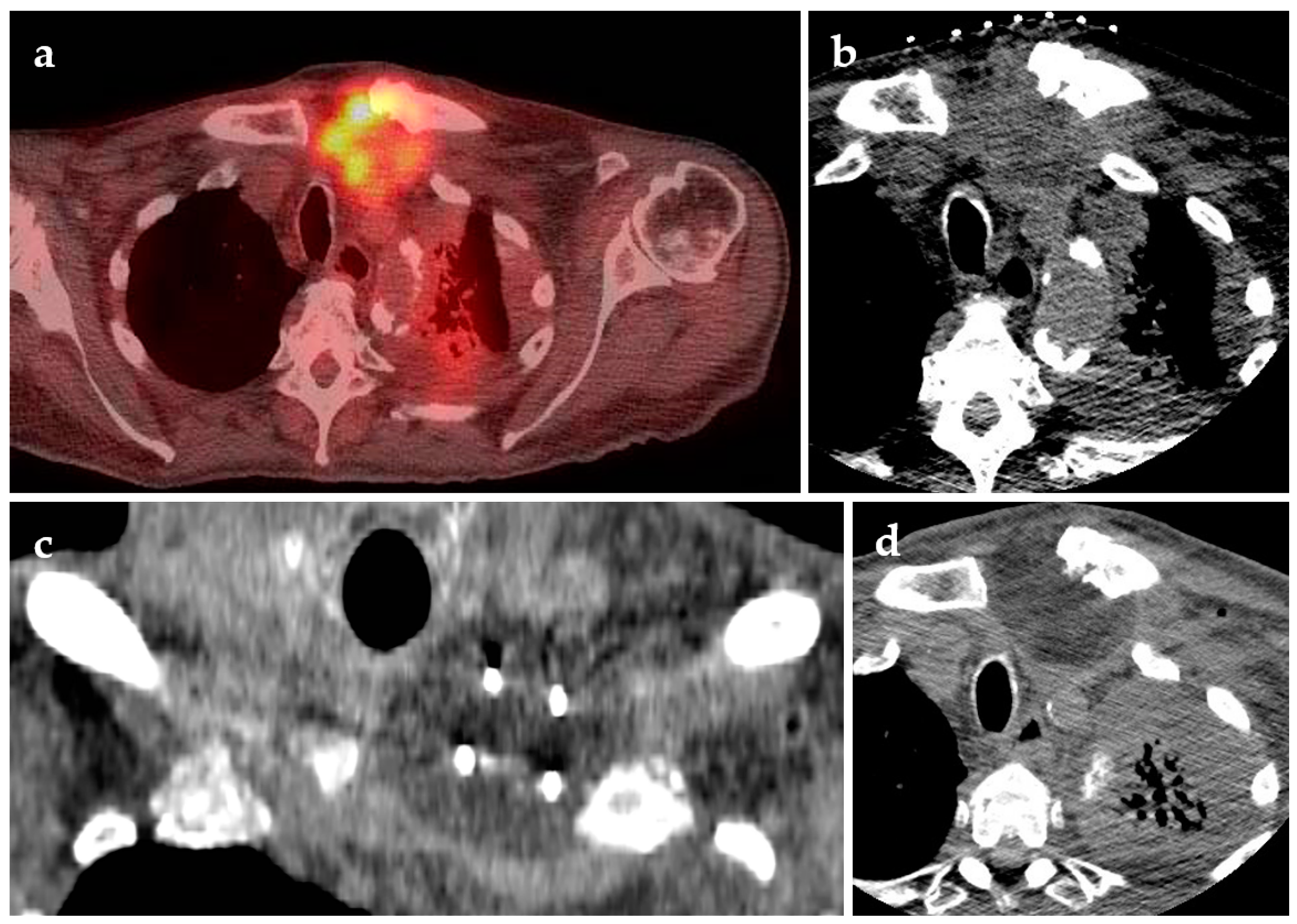
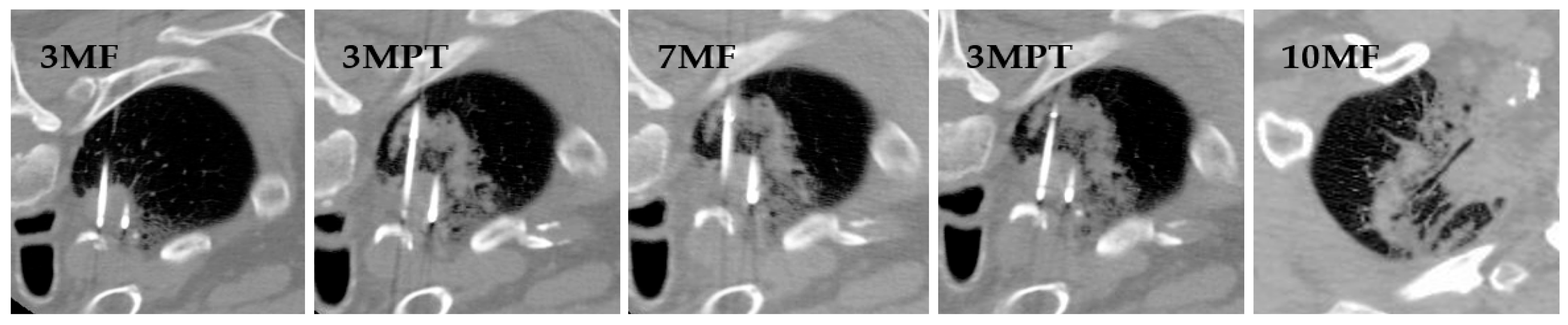
| Modality | Description | 2-Year Survival | 5-Year Survival | Median Survival | Sample Age (Mean, Median, Range) |
|---|---|---|---|---|---|
| Surgical metastasectomy [39] | The surgical removal of visible and palpable cancerous tissue | NA | 27–68% | 18.5–72 months | 55–65, NA, NA |
| Radiofrequency ablation [38] | A probe delivers high-energy radio waves to the tumor, heating the tumor and destroying cancerous cells | 79.4% | 51.5% | 62 months | 62.7, NA, 17–92 |
| Cryoablation [40] | A cryoprobe delivers gas to the tumor, freezing the tumor and killing any cancerous cells | 77.2% | NA | NA | NA, 65, 12–85 |
| Contraindications | Complications |
|---|---|
| Poorly controlled infection | Pneumothorax |
| Severe pulmonary fibrosis | Pneumonia |
| Severe hemorrhage tendency | Effusion |
| Severe organ dysfunction | In-hospital mortality |
| Severe anemia | Conversion to thoracoscopy/thoracotomy |
| Severe metabolic disorders | Hemorrhage |
| Extensive extrapulmonary metastases with an expected survival of less than 3 months | Systemic air embolism |
| Eastern Cooperative Oncology Group (ECOG) score greater than 3 | |
| Pacemaker (radiofrequency ablation) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzubaidi, S.J.; Liou, H.; Saini, G.; Segaran, N.; Scott Kriegshauser, J.; Naidu, S.G.; Patel, I.J.; Oklu, R. Percutaneous Image-Guided Ablation of Lung Tumors. J. Clin. Med. 2021, 10, 5783. https://doi.org/10.3390/jcm10245783
Alzubaidi SJ, Liou H, Saini G, Segaran N, Scott Kriegshauser J, Naidu SG, Patel IJ, Oklu R. Percutaneous Image-Guided Ablation of Lung Tumors. Journal of Clinical Medicine. 2021; 10(24):5783. https://doi.org/10.3390/jcm10245783
Chicago/Turabian StyleAlzubaidi, Sadeer J., Harris Liou, Gia Saini, Nicole Segaran, J. Scott Kriegshauser, Sailendra G. Naidu, Indravadan J. Patel, and Rahmi Oklu. 2021. "Percutaneous Image-Guided Ablation of Lung Tumors" Journal of Clinical Medicine 10, no. 24: 5783. https://doi.org/10.3390/jcm10245783






