Ejaculations and Benign Prostatic Hyperplasia: An Impossible Compromise? A Comprehensive Review
Abstract
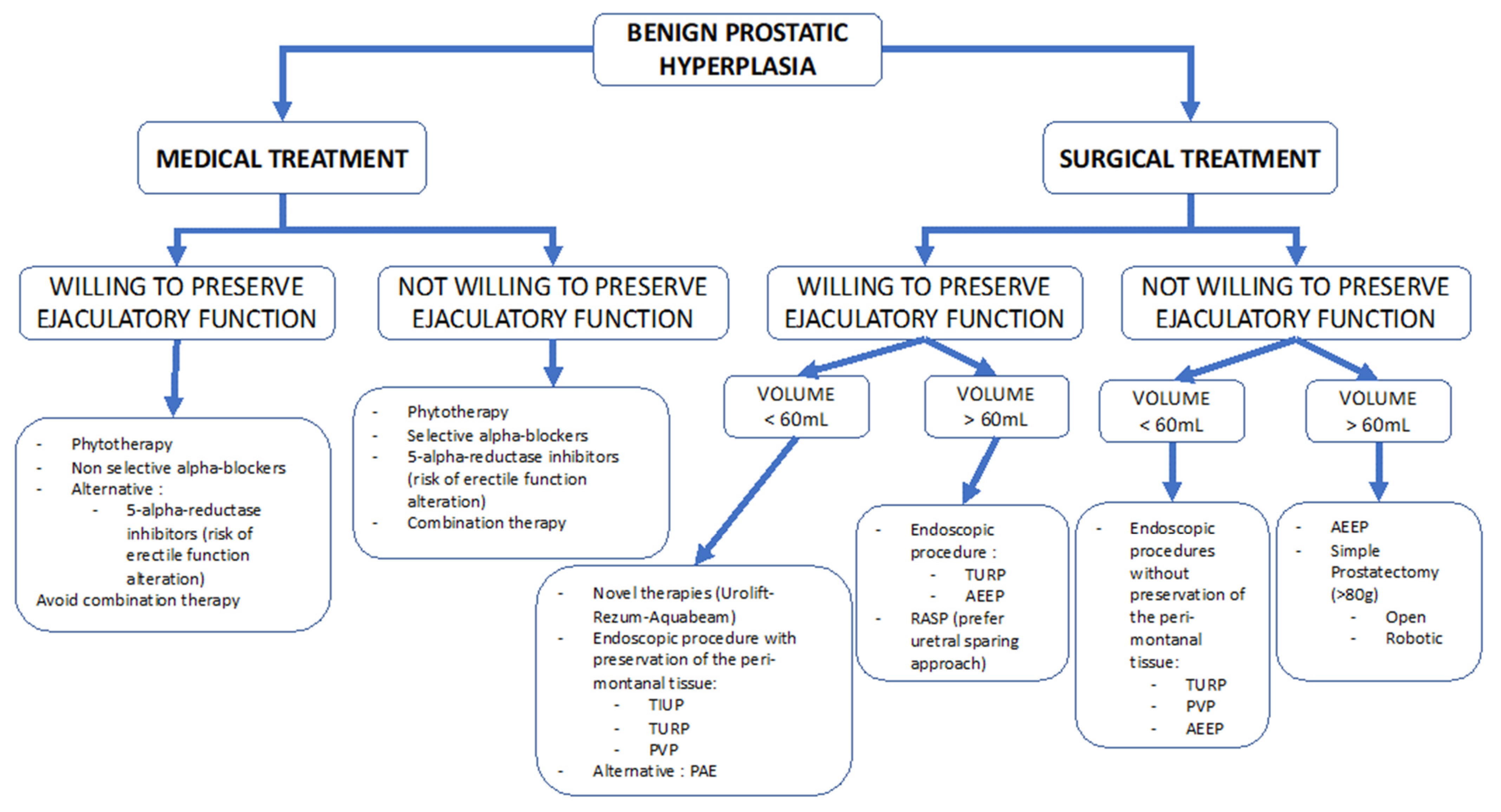
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Eligibility
2.3. Inclusion Criteria
2.4. Data Management
3. Results
3.1. Physiopathology of Ejaculatory Disorders in BPH
3.1.1. Alteration of the NO-GMPc Signaling Pathway
3.1.2. Hyperactivation of the RhoA-ROCK Signaling Channel
3.1.3. Hyperactivation of the Autonomic Nervous System
3.1.4. Pelvic Vascular Arteriosclerosis
3.2. Assessment Methods for Ejaculatory Disorders in BPH
3.2.1. Male Sexual Health Questionnaire (MSHQ)
3.2.2. Danish Prostatic Symptoms Score (DAN-PSS)
3.2.3. International Continence Society Sex (ICS-Sex)
3.2.4. Brief Male Sexual Function Inventory (BMFSI)
3.3. Epidemiology of Ejaculatory Disorders (EjD) in BPH
3.4. Anatomic Rational for Ejaculatory Disorders in BPH
3.5. Impact of Medical Treatments
3.5.1. Phytotherapy
3.5.2. Alpha-blockers
3.5.3. 5-Alpha Reductase Inhibitors (5ARI)
3.6. Impact of Surgical Treatments
3.6.1. Standard Endoscopic Procedures
3.6.2. Greenlight Laser Photo Vaporization of the Prostate (PVP)
3.6.3. Simple Prostatectomy (Open- and Robot-Assisted)
3.6.4. Anatomic Endoscopic Enucleation of the Prostate (AEEP)
3.7. Interventional Radiology: Prostate Artery Embolization (PAE)
3.8. New Surgical Therapies
3.8.1. Rezum©
3.8.2. Prostate Urethral Lift or Urolift©
3.8.3. Aquablation©
4. Discussion
4.1. Definition and Evaluation
4.2. Medical Therapies
4.3. Surgical Management
4.4. New Surgical Therapies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wein, A.J.; Coyne, K.S.; Tubaro, A.; Sexton, C.C.; Kopp, Z.S.; Aiyer, L.P. The impact of lower urinary tract symptoms on male sexual health: EpiLUTS. BJU Int. 2009, 103 (Suppl. 3), 33–41. [Google Scholar] [CrossRef]
- Gacci, M.; Eardley, I.; Giuliano, F.; Hatzichristou, D.; Kaplan, S.A.; Maggi, M.; McVary, K.T.; Mirone, V.; Porst, H.; Roehrborn, C.G. Critical analysis of the relationship between sexual dysfunctions and lower urinary tract symptoms due to benign prostatic hyperplasia. Eur. Urol. 2011, 60, 809–825. [Google Scholar] [CrossRef]
- Rosen, R.; Altwein, J.; Boyle, P.; Kirby, R.S.; Lukacs, B.; Meuleman, E.; O’Leary, M.P.; Puppo, P.; Robertson, C.; Giuliano, F. Lower urinary tract symptoms and male sexual dysfunction: The multinational survey of the aging male (MSAM-7). Eur. Urol. 2003, 44, 637–649. [Google Scholar] [CrossRef]
- Lowe, F.C. Treatment of lower urinary tract symptoms suggestive of benign prostatic hyperplasia: Sexual function. BJU Int. 2005, 95 (Suppl. 4), 12–18. [Google Scholar] [CrossRef]
- DeLay, K.J.; Nutt, M.; McVary, K.T. Ejaculatory dysfunction in the treatment of lower urinary tract symptoms. Transl. Androl. Urol. 2016, 5, 450–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoshany, O.; Abhyankar, N.; Elyaguov, J.; Niederberger, C. Efficacy of treatment with pseudoephedrine in men with retrograde ejaculation. Andrology 2017, 5, 744–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caremel, R.; Laccarier, E.; Sibert, L. Les inhibiteurs de la phosphodiestérase de type 5: Une révolution dans le traitement des symptômes du bas appareil urinaire? Basic Clin. Androl. 2012, 22, 80–91. [Google Scholar] [CrossRef] [Green Version]
- Hellstrom, W.J.G.; Giuliano, F.; Rosen, R.C. Ejaculatory dysfunction and its association with lower urinary tract symptoms of benign prostatic hyperplasia and BPH treatment. Urology 2009, 74, 15–21. [Google Scholar] [CrossRef]
- Giuliano, F. Questionnaires in sexual medicine. Progres En Urol. J. Assoc. Fr. Urol. Soc. Fr. Urol. 2013, 23, 811–821. [Google Scholar]
- Rosen, R.C.; Catania, J.A.; Althof, S.E.; Pollack, L.M.; O’Leary, M.; Seftel, A.D.; Coon, D.W. Development and validation of four-item version of Male Sexual Health Questionnaire to assess ejaculatory dysfunction. Urology 2007, 69, 805–809. [Google Scholar] [CrossRef]
- Donovan, J.L.; Abrams, P.; Peters, T.J.; Kay, H.E.; Reynard, J.; Chapple, C.; De La Rosette, J.J.; Kondo, A. The ICS-’BPH’ Study: The psychometric validity and reliability of the ICSmale questionnaire. Br. J. Urol. 1996, 77, 554–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- l’Urologie, M. De Proposition d’un Modèle de Représentation de la Sexualité de l’homme: Comparaison des Questionnaires de Sexualité. Available online: https://www.urofrance.org/base-bibliographique/proposition-dun-modele-de-representation-de-la-sexualite-de-lhomme-comparaison (accessed on 27 January 2020).
- O’Leary, M.P.; Fowler, F.J.; Lenderking, W.R.; Barber, B.; Sagnier, P.P.; Guess, H.A.; Barry, M.J. A brief male sexual function inventory for urology. Urology 1995, 46, 697–706. [Google Scholar] [CrossRef]
- Robert, G.; De La Taille, A.; Descazeaud, A. Epidemiology of benign prostatic hyperplasia. Progres En Urol. J. Assoc. Fr. Urol. Soc. Fr. Urol. 2018, 28, 803–812. [Google Scholar]
- Aaron, L.; Franco, O.E.; Hayward, S.W. Review of Prostate Anatomy and Embryology and the Etiology of Benign Prostatic Hyperplasia. Urol. Clin. N. Am. 2016, 43, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Malalasekera, A.P.; Sivasuganthan, K.; Sarangan, S.; Thaneshan, K.; Weerakoon, D.N.; Mathangasinghe, Y.; Gunasekera, C.L.; Mallawaarachchi, S.; Nanayakkara, N.D.; Anthony, D.J.; et al. Morphological variations of the human ejaculatory ducts in relation to the prostatic urethra. Clin. Anat. N. Y. N 2018, 31, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Bauer, H.W.; Casarosa, C.; Cosci, M.; Fratta, M.; Blessmann, G. Saw palmetto fruit extract for treatment of benign prostatic hyperplasia. Results of a placebo-controlled double-blind study. MMW Fortschr. Med. 1999, 141, 62. [Google Scholar]
- MacDonald, R.; Tacklind, J.W.; Rutks, I.; Wilt, T.J. Serenoa repens monotherapy for benign prostatic hyperplasia (BPH): An updated Cochrane systematic review. BJU Int. 2012, 109, 1756–1761. [Google Scholar] [CrossRef]
- Debruyne, F.; Koch, G.; Boyle, P.; Da Silva, F.C.; Gillenwater, J.G.; Hamdy, F.C.; Perrin, P.; Teillac, P.; Vela-Navarrete, R.; Raynaud, J.-P. Comparison of a phytotherapeutic agent (Permixon) with an alpha-blocker (Tamsulosin) in the treatment of benign prostatic hyperplasia: A 1-year randomized international study. Eur. Urol. 2002, 41, 497–506, discussion 506–507. [Google Scholar] [CrossRef]
- Roehrborn, C.G.; Van Kerrebroeck, P.; Nordling, J. Safety and efficacy of alfuzosin 10 mg once-daily in the treatment of lower urinary tract symptoms and clinical benign prostatic hyperplasia: A pooled analysis of three double-blind, placebo-controlled studies. BJU Int. 2003, 92, 257–261. [Google Scholar] [CrossRef] [Green Version]
- van Moorselaar, R.J.A.; Hartung, R.; Emberton, M.; Harving, N.; Matzkin, H.; Elhilali, M.; Alcaraz, A.; Vallancien, G.; ALF-ONE Study Group. Alfuzosin 10 mg once daily improves sexual function in men with lower urinary tract symptoms and concomitant sexual dysfunction. BJU Int. 2005, 95, 603–608. [Google Scholar] [CrossRef]
- Elhilali, M.; Emberton, M.; Matzkin, H.; van Moorselaar, R.J.A.; Hartung, R.; Harving, N.; Alcaraz, A.; Vallancien, G.; ALF-ONE Study Group. Long-term efficacy and safety of alfuzosin 10 mg once daily: A 2-year experience in “real-life” practice. BJU Int. 2006, 97, 513–519. [Google Scholar] [CrossRef]
- Kobayashi, K.; Masumori, N.; Hisasue, S.; Kato, R.; Hashimoto, K.; Itoh, N.; Tsukamoto, T. Inhibition of Seminal emission is the main cause of anejaculation induced by a new highly selective alpha1A-blocker in normal volunteers. J. Sex. Med. 2008, 5, 2185–2190. [Google Scholar] [CrossRef]
- Bozkurt, O.; Demir, O.; Sen, V.; Esen, A. Silodosin causes impaired ejaculation and enlargement of seminal vesicles in sexually active men treated for lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Urology 2015, 85, 1085–1089. [Google Scholar] [CrossRef]
- Chapple, C.R.; Montorsi, F.; Tammela, T.L.J.; Wirth, M.; Koldewijn, E.; Fernández Fernández, E.; European Silodosin Study Group. Silodosin therapy for lower urinary tract symptoms in men with suspected benign prostatic hyperplasia: Results of an international, randomized, double-blind, placebo- and active-controlled clinical trial performed in Europe. Eur. Urol. 2011, 59, 342–352. [Google Scholar] [CrossRef]
- Fwu, C.-W.; Eggers, P.W.; Kirkali, Z.; McVary, K.T.; Burrows, P.K.; Kusek, J.W. Change in sexual function in men with lower urinary tract symptoms/benign prostatic hyperplasia associated with long-term treatment with doxazosin, finasteride and combined therapy. J. Urol. 2014, 191, 1828–1834. [Google Scholar] [CrossRef]
- McVary, K.T.; Roehrborn, C.G.; Avins, A.L.; Barry, M.J.; Bruskewitz, R.C.; Donnell, R.F.; Foster, H.E.; Gonzalez, C.M.; Kaplan, S.A.; Penson, D.F.; et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J. Urol. 2011, 185, 1793–1803. [Google Scholar] [CrossRef]
- McClellan, K.J.; Markham, A. Finasteride: A review of its use in male pattern hair loss. Drugs 1999, 57, 111–126. [Google Scholar] [CrossRef]
- Roehrborn, C.G.; Boyle, P.; Nickel, J.C.; Hoefner, K.; Andriole, G. ARIA3001 ARIA3002 and ARIA3003 Study Investigators Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology 2002, 60, 434–441. [Google Scholar] [CrossRef]
- Roehrborn, C.G.; Siami, P.; Barkin, J.; Damião, R.; Major-Walker, K.; Morrill, B.; Montorsi, F. CombAT Study Group The effects of dutasteride, tamsulosin and combination therapy on lower urinary tract symptoms in men with benign prostatic hyperplasia and prostatic enlargement: 2-year results from the CombAT study. J. Urol. 2008, 179, 616–621, discussion 621. [Google Scholar] [CrossRef]
- Welliver, C.; Essa, A. Sexual Side Effects of Medical and Surgical Benign Prostatic Hyperplasia Treatments. Urol. Clin. North Am. 2016, 43, 393–404. [Google Scholar] [CrossRef]
- Muntener, M.; Aellig, S.; Kuettel, R.; Gehrlach, C.; Sulser, T.; Strebel, R.T. Sexual function after transurethral resection of the prostate (TURP): Results of an independent prospective multicentre assessment of outcome. Eur. Urol. 2007, 52, 510–515. [Google Scholar] [CrossRef]
- Marra, G.; Sturch, P.; Oderda, M.; Tabatabaei, S.; Muir, G.; Gontero, P. Systematic review of lower urinary tract symptoms/benign prostatic hyperplasia surgical treatments on men’s ejaculatory function: Time for a bespoke approach? Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2016, 23, 22–35. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, L.; Fan, Q.-L.; Zhou, J.; Peng, Y.-B.; Wang, Z. Bipolar transurethral resection in saline vs traditional monopolar resection of the prostate: Results of a randomized trial with a 2-year follow-up. BJU Int. 2010, 106, 1339–1343. [Google Scholar] [CrossRef]
- Riehmann, M.; Knes, J.M.; Heisey, D.; Madsen, P.O.; Bruskewitz, R.C. Transurethral resection versus incision of the prostate: A randomized, prospective study. Urology 1995, 45, 768–775. [Google Scholar] [CrossRef]
- Bachmann, A.; Tubaro, A.; Barber, N.; d’Ancona, F.; Muir, G.; Witzsch, U.; Grimm, M.-O.; Benejam, J.; Stolzenburg, J.-U.; Riddick, A.; et al. 180-W XPS GreenLight laser vaporisation versus transurethral resection of the prostate for the treatment of benign prostatic obstruction: 6-month safety and efficacy results of a European Multicentre Randomised Trial—The GOLIATH study. Eur. Urol. 2014, 65, 931–942. [Google Scholar] [CrossRef]
- Gacci, M.; Bartoletti, R.; Figlioli, S.; Sarti, E.; Eisner, B.; Boddi, V.; Rizzo, M. Urinary symptoms, quality of life and sexual function in patients with benign prostatic hypertrophy before and after prostatectomy: A prospective study. BJU Int. 2003, 91, 196–200. [Google Scholar] [CrossRef] [Green Version]
- Dixon, A.R.; Lord, P.H.; Madigan, M.R. The Madigan prostatectomy. J. Urol. 1990, 144, 1401–1403. [Google Scholar] [CrossRef]
- Porpiglia, F.; Checcucci, E.; Amparore, D.; Niculescu, G.; Volpi, G.; Piramide, F.; De Cillis, S.; Manfredi, M.; Autorino, R.; Fiori, C. Urethral-sparing Robot-assisted Simple Prostatectomy: An Innovative Technique to Preserve Ejaculatory Function Overcoming the Limitation of the Standard Millin Approach. Eur. Urol. 2020, 80, 222–233. [Google Scholar] [CrossRef]
- Wilson, L.C.; Gilling, P.J.; Williams, A.; Kennett, K.M.; Frampton, C.M.; Westenberg, A.M.; Fraundorfer, M.R. A randomised trial comparing holmium laser enucleation versus transurethral resection in the treatment of prostates larger than 40 grams: Results at 2 years. Eur. Urol. 2006, 50, 569–573. [Google Scholar] [CrossRef]
- Kim, M.; Song, S.H.; Ku, J.H.; Kim, H.-J.; Paick, J.-S. Pilot study of the clinical efficacy of ejaculatory hood sparing technique for ejaculation preservation in Holmium laser enucleation of the prostate. Int. J. Impot. Res. 2015, 27, 20–24. [Google Scholar] [CrossRef]
- Huet, R.; Peyronnet, B.; Khene, Z.-E.; Freton, L.; Verhoest, G.; Manunta, A.; Bensalah, K.; Vincendeau, S.; Mathieu, R. Prospective Assessment of the Sexual Function After Greenlight Endoscopic Enucleation and Greenlight 180W XPS Photoselective Vaporization of the Prostate. Urology 2019, 131, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Bajic, P.; Noriega, N.; Gorbonos, A.; Karpman, E. GreenLight Laser Enucleation of the Prostate (GreenLEP): Initial Experience with a Simplified Technique. Urology 2019, 131, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Saredi, G.; Pacchetti, A.; Pirola, G.M.; Martorana, E.; Berti, L.; Scroppo, F.I.; Marconi, A.M. Impact of Thulium Laser Enucleation of the Prostate on Erectile, Ejaculatory and Urinary Functions. Urol. Int. 2016, 97, 397–401. [Google Scholar] [CrossRef]
- Morozov, A.; Taratkin, M.; Kozlov, V.; Tarasov, A.; Bezrukov, E.; Enikeev, M.; Afyouni, A.S.; Okhunov, Z.; Glybochko, P.; Enikeev, D. Retrospective Assessment of Endoscopic Enucleation of Prostate Complications: A Single-Center Experience of More Than 1400 Patients. J. Endourol. 2020, 34, 192–197. [Google Scholar] [CrossRef]
- Enikeev, D.; Glybochko, P.; Rapoport, L.; Okhunov, Z.; O’Leary, M.; Potoldykova, N.; Sukhanov, R.; Enikeev, M.; Laukhtina, E.; Taratkin, M. Impact of endoscopic enucleation of the prostate with thulium fiber laser on the erectile function. BMC Urol. 2018, 18, 87. [Google Scholar] [CrossRef] [PubMed]
- Enikeev, D.; Taratkin, M.; Laukhtina, E.; Alekseeva, T.; Snurnitsyna, O.; Potoldykova, N.; Gerasimov, A.; Glybochko, P. En bloc and two-lobe techniques for laser endoscopic enucleation of the prostate: Retrospective comparative analysis of peri- and postoperative outcomes. Int. Urol. Nephrol. 2019, 51, 1969–1974. [Google Scholar] [CrossRef]
- Herrmann, T.R.W.; Gravas, S.; de la Rosette, J.J.; Wolters, M.; Anastasiadis, A.G.; Giannakis, I. Lasers in Transurethral Enucleation of the Prostate-Do We Really Need Them. J. Clin. Med. 2020, 9, 1412. [Google Scholar] [CrossRef]
- Amouyal, G.; Thiounn, N.; Pellerin, O.; Yen-Ting, L.; Del Giudice, C.; Dean, C.; Pereira, H.; Chatellier, G.; Sapoval, M. Clinical Results After Prostatic Artery Embolization Using the PErFecTED Technique: A Single-Center Study. Cardiovasc. Intervent. Radiol. 2016, 39, 367–375. [Google Scholar] [CrossRef]
- Carnevale, F.C.; Iscaife, A.; Yoshinaga, E.M.; Moreira, A.M.; Antunes, A.A.; Srougi, M. Transurethral Resection of the Prostate (TURP) Versus Original and PErFecTED Prostate Artery Embolization (PAE) Due to Benign Prostatic Hyperplasia (BPH): Preliminary Results of a Single Center, Prospective, Urodynamic-Controlled Analysis. Cardiovasc. Intervent. Radiol. 2016, 39, 44–52. [Google Scholar] [CrossRef]
- Salem, R.; Hairston, J.; Hohlastos, E.; Riaz, A.; Kallini, J.; Gabr, A.; Ali, R.; Jenkins, K.; Karp, J.; Desai, K.; et al. Prostate Artery Embolization for Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia: Results From a Prospective FDA-Approved Investigational Device Exemption Study. Urology 2018, 120, 205–210. [Google Scholar] [CrossRef]
- Ray, A.F.; Powell, J.; Speakman, M.J.; Longford, N.T.; DasGupta, R.; Bryant, T.; Modi, S.; Dyer, J.; Harris, M.; Carolan-Rees, G.; et al. Efficacy and safety of prostate artery embolization for benign prostatic hyperplasia: An observational study and propensity-matched comparison with transurethral resection of the prostate (the UK-ROPE study). BJU Int. 2018, 122, 270–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abt, D.; Müllhaupt, G.; Hechelhammer, L.; Markart, S.; Güsewell, S.; Schmid, H.-P.; Mordasini, L.; Engeler, D.S. Prostatic Artery Embolisation Versus Transurethral Resection of the Prostate for Benign Prostatic Hyperplasia: 2-yr Outcomes of a Randomised, Open-label, Single-centre Trial. Eur. Urol. 2021, 80, 34–42. [Google Scholar] [CrossRef] [PubMed]
- McVary, K.T.; Gange, S.N.; Gittelman, M.C.; Goldberg, K.A.; Patel, K.; Shore, N.D.; Levin, R.M.; Rousseau, M.; Beahrs, J.R.; Kaminetsky, J.; et al. Minimally Invasive Prostate Convective Water Vapor Energy Ablation: A Multicenter, Randomized, Controlled Study for the Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia. J. Urol. 2016, 195, 1529–1538. [Google Scholar] [CrossRef]
- McVary, K.T.; Gange, S.N.; Gittelman, M.C.; Goldberg, K.A.; Patel, K.; Shore, N.D.; Levin, R.M.; Rousseau, M.; Beahrs, J.R.; Kaminetsky, J.; et al. Erectile and Ejaculatory Function Preserved with Convective Water Vapor Energy Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia: Randomized Controlled Study. J. Sex. Med. 2016, 13, 924–933. [Google Scholar] [CrossRef] [PubMed]
- McVary, K.T.; Rogers, T.; Roehrborn, C.G. Rezūm Water Vapor Thermal Therapy for Lower Urinary Tract Symptoms Associated With Benign Prostatic Hyperplasia: 4-Year Results From Randomized Controlled Study. Urology 2019, 126, 171–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roehrborn, C.G.; Rukstalis, D.B.; Barkin, J.; Gange, S.N.; Shore, N.D.; Giddens, J.L.; Bolton, D.M.; Cowan, B.E.; Cantwell, A.L.; McVary, K.T.; et al. Three year results of the prostatic urethral L.I.F.T. study. Can. J. Urol. 2015, 22, 7772–7782. [Google Scholar] [PubMed]
- Roehrborn, C.G.; Barkin, J.; Gange, S.N.; Shore, N.D.; Giddens, J.L.; Bolton, D.M.; Cowan, B.E.; Cantwell, A.L.; McVary, K.T.; Te, A.E.; et al. Five year results of the prospective randomized controlled prostatic urethral L.I.F.T. study. Can. J. Urol. 2017, 24, 8802–8813. [Google Scholar]
- Beurrier, S.; Peyromaure, M.; Belas, O.; Barry Delongchamps, N. Are the UroLift(®) implants an alternative for the treatment of benign prostatic hyperplasia? Short-term results and predictive factors of failure. Progres En Urol. J. Assoc. Fr. Urol. Soc. Fr. Urol. 2015, 25, 523–529. [Google Scholar]
- Userovici, M.; Ochoa, A.; Anract, J.; Beurrier, S.; Peyromaure, M.; Barry Delongchamps, N. Prostatic urethral lift using Urolift® system for benign prostatic hyperplasia: 7years experience. Progres En Urol. J. Assoc. Fr. Urol. Soc. Fr. Urol. 2020, 30, 147–154. [Google Scholar]
- Plante, M.; Gilling, P.; Barber, N.; Bidair, M.; Anderson, P.; Sutton, M.; Aho, T.; Kramolowsky, E.; Thomas, A.; Cowan, B.; et al. Symptom relief and anejaculation after aquablation or transurethral resection of the prostate: Subgroup analysis from a blinded randomized trial. BJU Int. 2019, 123, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Gilling, P.; Barber, N.; Bidair, M.; Anderson, P.; Sutton, M.; Aho, T.; Kramolowsky, E.; Thomas, A.; Cowan, B.; Kaufman, R.P.; et al. Two-Year Outcomes After Aquablation Compared to TURP: Efficacy and Ejaculatory Improvements Sustained. Adv. Ther. 2019, 36, 1326–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, E.C.; Jung, J.H.; Borofsky, M.; Kim, M.H.; Dahm, P. Aquablation of the prostate for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database Syst. Rev. 2019, 2, CD013143. [Google Scholar] [CrossRef] [PubMed]
- Bhojani, N.; Bidair, M.; Zorn, K.C.; Trainer, A.; Arther, A.; Kramolowsky, E.; Doumanian, L.; Elterman, D.; Kaufman, R.P.; Lingeman, J.; et al. Aquablation for Benign Prostatic Hyperplasia in Large Prostates (80-150 cc): 1-Year Results. Urology 2019, 129, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieken, M.; Antunes-Lopes, T.; Geavlete, B.; Marcelissen, T. EAU Young Academic Urologists Functional Urology and BPH Group What Is New with Sexual Side Effects after Transurethral Male Lower Urinary Tract Symptom Surgery? Eur. Urol. Focus 2018, 4, 43–45. [Google Scholar] [CrossRef]
| Reference | Aim | Study Design | Main Results | EjD Results |
|---|---|---|---|---|
| MacDonald R, et al., BJU Int. June 2012 [18] | To estimate the effectiveness and harms of Serenoa repens monotherapy in the treatment of lower urinary tract symptoms (LUTS) consistent with benign prostatic hyperplasia | Systematic review 17 randomised study, phytotherapy vs. placebo | Serenoa repens therapy does not improve LUTS or Q (max) compared with placebo | Not studied |
| Bauer HW et al., MMW Fortschr Med. 24 June 1999 [17] | To evaluate the efficacy of Saw palmetto fruit on urinary function | placebo-controlled double-blind study. Moderate-term study (6 months) | Statistically significant improvement of IPSS with Serenoa repens therapy (37% improvement) vs. placebo (14%) | No ejaculatory changes under phytotherapy |
| Debruyne et al., Eur Urol. May 2002 [19] | To assess the equivalent efficacy of Permixon and tamsulosin. | Prospective, double-blind randomized trial | no differences were observed in either irritative or obstructive symptom improvements after 1-year follow-up | ejaculation disorders occurred more frequently in the tamsulosin group (4.2% vs. 0.6% in Permixon group p = 0.001). |
| Reference | Aim | Study Design | Main Results | EjD Results |
|---|---|---|---|---|
| Roehrborn CG et al., BJU Int. August 2003 [20] | To examine the efficacy and safety of a once-daily formulation of alfuzosin | Prospective randomized, double-blind, placebo-controlled 3-month study | Significant improve of IPPS score with alfuzosin vs. placebo −6.0 (5.1) vs. −4.2 (5.7) with placebo (p < 0.005) and the PFR, by + 2.3 (3.8) vs. + 1.1 (3.1) mL/s with placebo (p < 0.001) | Rare sexual adverse events with alfuzosin (impotence, 1.5%; ejaculation failure, 0.6%) |
| Van Moorselaar et al., BJU Int. March 2005 [21] | To assess the effect on sexual function of alfuzosin 10 mg once daily | Prospective, observationnal, real life practice study | alfuzosin significantly improved the total IPSS (−6.1, −32%) | Significant improvements in weighted scores related to reduced rigidity of erection (−0.5), reduced amount of ejaculate (−0.4) and pain/discomfort on ejaculation (−1.2, all p < 0.001) over baseline |
| Elhilali et al., BJU Int. March 2007 [22] | To assess the 2-year efficacy and safety of alfuzosin 10 mg once daily | Prospective, observationnal, real life practice study | total IPSS improved by 7 points (−38.5%) from baseline (p < 0.001) | Ejaculatory disorders were uncommon (0.3%) |
| Kobayashi et al., J Sex Med. September 2008 [23] | To evaluate the effect of silodosin on ejaculatory function of normal volunteers. | double-blind, placebo- controlled, randomized, crossover design N:15 | 100% anejaculation 0% retrograde ejaculation | 100% anejaculation 0% retrograde ejaculation |
| Bozkurt et al., Urology. May 2015 [24] | To evaluate the sexual side effects including ejaculation after silodosin treatment in potent men with regular sexual activity | Prospective cohort N:30 | Na | 90% of impaired ejaculation |
| Chapple et al., Eur Urol. March 2011 [25] | To test silodosin’s superiority to placebo and noninferiority to tamsulosin | multicenter double-blind, placebo- and active-controlled parallel group study | IPSS total score with silodosin and tamsulosin was significantly superior to that with placebo (p < 0.001) | 14% Anejaculation |
| Reference | Aim | Study Design | Main Results | EjD Results | |
|---|---|---|---|---|---|
| 5ARI | Fwu et al., J Urol. June 2014 [26] | To examine the effects of doxazosin, finasteride and combined therapy on sexual function | Multicenter, randomized, double-blind, placebo controlled | Slight worsening of ejaculatory function with finasteride and combined therapy compared with men on placebo. no significant difference in men assigned to doxazosin alone compared to placebo. | Non evaluated |
| McVary et al., J Urol. May 2011 [27] | To revise the 2003 version of the American Urological Association’s (AUA) Guideline on the management of benign prostatic hyperplasia | Systematic review | Ejaculatory dysfunction of 4% (against 1% for the placebo) with finasteride | ||
| McClellan et al., Drugs. 1999 [28] | Review of finasteride use in male pattern hair loss | Phase III | 3.8% sexual function disorders (p < 0.041) −1.8% discreased libido −1.2% ejaculation disorder −1.3% erectile dyfunction | ||
| Roehrborn et al., Urology. Sept 2002 [29] | To study the efficacy and safety of dutasteride | Randomized, double-blind, placebo controlled | Decrease in AUA-SI of 4.5 point at 24 months (p < 0.01) | 2.2% ejaculation disorder (p < 0.01) | |
| Associations | Roehrborn et al., J Urol. February 2008 [30] | To evaluate if combination therapy with dutasteride and tamsulosin is more effective than either monotherapy alone for improving symptoms and long-term outcomes in men with moderate to severe lower urinary tract symptoms and prostatic enlargement | Prospective, multicenter, randomized, double-blind, parallel group study | Significantly greater improvements in urinary symptoms with combinaison versus single therapy | Significant increase in drug related adverse events with combination therapy vs. monotherapies (×4) |
| Reference | Aim | Study Design | Main Results | EjD Results |
|---|---|---|---|---|
| Riehmann et al., Urology. May 1995 [35] | To evaluate longer term effects of transurethral resection (TURP) and incision (TUIP) of the prostate in randomized patients. | Randomized, prospective study. Prostate < 20 cc | Decrease in obstructive symptoms in both groups (p < 0.034), no significant difference between the 2 groups. | 68% retrograde ejaculation after TURP vs. 35% after TUIP (p = 0.02). |
| Marra et al., Int. J Urol. January 2013 [33] | To evaluate ejaculatory dysfunction in relation to benign prostatic hyperplasia surgery. | Systematic review; 42 randomized controlled trials comprising a total of 3857 patients were included. | 66% retrograde ejaculation after TURP 21% after TUIP 41.9 after PVP 76.3% after HOLEP. | 66% retrograde ejaculation after TURP 21% after TUIP 41.9 after PVP 76.3% after HOLEP. |
| Muntener et al., Eur Urol. August 2007 [32] | To evaluate the influence of TURP on erectile and ejaculatory function. | Prospective, multicenter, observational N 1014. | Significant decrease in ejaculatory function (p < 0.001) No significant difference of erectile function. | Significant decrease in ejaculatory function (p < 0.001) No significant difference in erectile function. |
| Chen et al., BJU Int. November 2010 [34] | To present 2-year follow-up data of a randomized clinical trial comparing bipolar transurethral resection in saline (TURIS) with monopolar transurethral resection of the prostate (TURP). | 100 consecutive patients were randomized to TURIS or TURP. | Operative duration and resected tissue weight were similar between the groups significant improvements in IPSS and maximum urinary flow rates in both group. | 50% retrograde ejaculation after TURP vs. 36% after TURIS (p = 0.52). |
| Reference | Aim | Study Design | Main Results | EjD Results |
|---|---|---|---|---|
| Bachmann et al., Eur Urol. May 2014 [36] | To evaluate the noninferiority of 180-W GL XPS (XPS) to TURP for International Prostate Symptom Score (IPSS) and maximum flow rate (Qmax) at 6 mo and the proportion of patients who were complication free. | Multicenter, Prospective randomised controlled trial. N 281. | Noninferiority of XPS to TURP for IPSS, Qmax, and complication-free proportion. | 63% retrograde ejaculation after TURP vs. 65% after PVP. |
| Reference | Aim | Study Design | Main Results | EjD Results |
|---|---|---|---|---|
| Gacci et al., BJU Int. February 2003 [37] | To evaluate urinary symptoms, sexual dysfunction and quality of life in patients with benign prostatic hypertrophy (BPH) before and after open prostatectomy | Monocentric Prospective N 60 | Significant improvement in obstructive (mean 9.68–3.38) and irritative symptom (6.70–3.06), and quality-of-life scores (3.41–1.34) | No significant difference before and after SP concerning erectile and orgasm function |
| Porpiglia et al., Eur Urol. September 2020 [39] | To evaluate the efficacy of urethral-sparing robotic-assisted simple prostatectomy technique (usRASP) in obtaining effective deobstruction and maintaining anterograde ejaculation | Monocentric Prospective (retrospective control group) N 92. | Same perioperative and urinary functional outcomess in both groups | 81% antegrade ejaculation in usRASP vs. 8.8% in RASP group |
| Reference | Aim | Study Design | Main Results | EjD Results |
|---|---|---|---|---|
| Welliver et al., Urol Clin North Am. August 2016 [31] | To consider potential pathophysiologic causes of dysfunction with treatment of LUTS due to BPH and attempts to critically review the available data to assess sexually related AEs. | Literature review | 75% retrograde ejaculation | |
| Wilson et al., Eur Urol. September 2006 [40] | To compare holmium laser enucleation of the prostate (HoLEP) with transurethral resection of the prostate (TURP) for treatment of men with bladder outflow obstruction (BOO) secondary to benign prostatic hyperplasia with a minimum of 24-month follow-up. | Randomized prospective trial N 61 | HoLEP group: shorter catheter times and hospital stays; more prostate tissue retrieved; At six months, HoLEP was urodynamically superior to TURP in relieving BOO. No difference à 24 months (AUA, Qmax) | 75% retrograde ejaculation in HOLEP; 62% in TURP |
| Kim et al., Int J Impot Res. February 2015 [41] | To explore the effectiveness of ejaculatory hood sparing technique to Holmium laser enucleation of the prostate (HoLEP) for ejaculation preservation | Prospective, controlled | Ejaculation preservation was 46.2% in the EH-HoLEP group and 26.9% in the conventional-HoLEP group (p = 0.249) | Ejaculation preservation was 46.2% in the EH-HoLEP group and 26.9% in the conventional-HoLEP group (p = 0.249) |
| Huet et al., Urology. Sept 2019 [42] | To evaluate the impact of Greenlight 180W photoselective vaporization of the prostate (PVP) and endoscopic enucleation of the prostate (GreenLEP) on ejaculatory and erectile functions. | Prospective, monocentric N 440 | Antegrade ejaculation in 26.9% in the PVP group vs. 1.2% in the GreenLEP group at 12 months (p < 0.001) | Antegrade ejaculation in 26.9% in the PVP group vs. 1.2% in the GreenLEP group at 12 months (p < 0.001) |
| Bajic et al., Urology. Sept 2019 [43] | To present outcomes of a simplified GreenLight laser enucleation of the prostate (GreenLEP) technique and to inform urologists considering incorporation of enucleation into their practice. | Monocentric, prospective consecutive GreenLEPs by a single surgeon N 108 | Significant improvements at 3 months in Qmax (237%, p < 0.01), in IPSS (−64%, p < 0.01), in postvoid residual (−83%, p < 0.01) | 100% of retrograde ejaculation in patient with sexual activity (36%) |
| Saredi et al., Urol Int. 2016 [44] | To test the impact of Thulium laser enucleation of the prostate (ThuLEP) on erectile and ejaculatory functions, on lower urinary tract symptoms and on quality of life (QoL). | Monocentric, prospective N 177 | Decrease in IPSS (p < 0.0001) | No difference in erectile function (IIEF) before and after surgery Reduction in ejaculation (p < 0.0001) 11.86% of antegrade ejaculation at 8 months |
| Enikeev et al., Int Urol Nephrol. November 2019 [47] | To perform a comparative analysis of en bloc and two-lobe techniques for holmium laser enucleation of the prostate (HoLEP) and thulium fiber laser enucleation of the prostate (ThuFLEP). | Retrospective N 1115 | Mean surgery times (68.8 ± 30.6 min vs. 67.4 ± 30.1 min; p = 0.604) and enucleation rates (1.9 ± 0.74 g/min vs. 1.9 ± 0.69 g/min; p = 0.217) were comparable | No evaluation of ejaculatory function |
| Reference | Aim | Study Design | Main Results | EjD Results |
|---|---|---|---|---|
| Amouyal et al., Cardiovasc Intervent Radiol. Mar 2016 [49] | To report experience and clinical results on patients suffering from symptomatic BPH, who underwent PAE aiming at using the PErFecTED technique. | Single-center retrospective open label N 32 | Mean IPSS decreased from 15.3 to 4.2 (p = 0.03), mean QoL from 5.4 to 2 (p = 0.03), mean Qmax increased from 9.2 to 19.2 (p = 0.25) | No retrograde ejaculation |
| Salem et al., Urology 2018 [51] | To evaluate the safety and efficacy of prostate artery embolization (PAE) for lower urinary tract symptoms (LUTS) attributed to benign prostatic hyperplasia | Prospective, single-center, open-label N 45 | At 1 month, improvements in IPSS (23.6 ± 6.1 to 12.0 ± 5.9, p < 0.0001), QoL (4.8 ± 0.9 to 2.6 ± 1.6, p < 0.0001), Qmax (5.8 ± 1.0 to 12.4 ± 6.8, p < 0.0001). At 3 months, there were improvements in IPSS (10.2 ± 6.0, p < 0.0001), QoL (2.4 ± 1.6, p < 0.0001) and Qmax (15.3 ± 12.3, p < 0.0001). At 6 months, there were improvements in IPSS (11.0 ± 7.6, p < 0.0001) and QoL (2.3 ± 1.7, p < 0.0001). At 1 year, there were improvements in IPSS (12.4 ± 8.4, p < 0.0001) and QoL (2.6 ± 1.6, p < 0.0001). | No adverse effects on erectile function or sexual health |
| Ray et al., BJU Int. August 2018 [52] | To assess the efficacy and safety of prostate artery embolization (PAE) for lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH) and to conduct an indirect comparison of PAE with transurethral resection of the prostate (TURP) | Multicenter N 305 | Median 10-point IPSS improvement from baseline at 12 months post-procedure | 24.1% retrograde ejaculation rate for EAP against 47.5% for RTUP |
| Reference | Aim | Study Design | Main Results | EjD Results |
|---|---|---|---|---|
| McVary et al., J Urol. May 2016 [54] | To evaluate the efficacy ok REZUM versus placebo | Multicenter, randomized, controlled study N 197 | IPSS was reduced by 11.2 ± 7.6 in REZUM and 4.3 ± 6.9 in control (p < 0.0001) | No substantial decrements to erectil or ejaculatory function |
| McVary et al., J Sex Med. 2016 [55] | To determine whether water vapor thermal therapy would significantly improve lower urinary tract symptoms secondary to benign prostatic hyperplasia and urinary flow rate while preserving erectile and ejaculatory functions. | Multicenter, randomized, controlled study | IPSS and peak flow rate were significantly superior to controls at 3 months and throughout 1 year (p < 0.0001).a | 0 de novo erectile dysfunction after REZUM IIEF was not differente between baseline and at 1 year. Ejaculatory bother score improved 31% over baseline (p = 0.0011). |
| McVary et al., Urology. 2019 [56] | To report 4-year outcomes of the randomized controlled trial of water vapor thermal therapy for treatment of moderate to severe lower urinary tract symptoms due to benign prostatic hyperplasia. | Lower urinary tract symptoms were significantly improved within ≤3 months after thermal therapy and remained consistently durable (International Prostate Symptom Score 47%, quality of life 43%, Qmax 50%, Benign Prostatic Hyperplasia Impact Index 52%) throughout 4 years (p < 0.0001) | No disturbances in sexual function were reported. |
| Reference | Aim | Study Design | Main Results | EjD Results |
|---|---|---|---|---|
| Roehrborn et al., Can J Urol. Jun 2015 [57] | To report the three year results of use of the Prostatic Urethral Lift | Prospective, multi-center, randomized, blinded, sham control | IPSS improvement of 88% at three month 41.1% at 3 years | No de novo erectile or ejaculation dysfunction |
| Roehrborn et al., Can J Urol. Jun 2017 [58] | To report the five year results of use of the Prostatic Urethral Lift | Prospective, multi-center, randomized, blinded, sham control | IPSS improvement of 88% 41.1% at 3 years 36% at 5 years Surgical retreatment: 13.6% over 5 years | No de novo erectile or ejaculation dysfunction |
| Beurrier et al., Prog Urol. Jul 2015 [59] | To report the results of UroLift implants after a 2-year experience in the technique | Prospective monocentric N 23 | Median IPSS and IPSS-QoL were improved significantly (11 [1–27] and 2 [0–6], p < 0.0001) No significant improved in Qmax | No patient reported retrograde ejaculation or worsened erectile function |
| Userovici et al., Prog Urol. Mar 2020 [60] | To report the results of Urolift® system in our center after 7years experience. | N 40 | At 3 months IPSS and IPSS-QdV were significantly improved (8 [4–11] vs. 20 [17–24]; p < 0.0001 and 2 [1,2] vs. 5 [4–6]; p < 0.0001). | MSHQ-EjD and IIEF5 were not modified (respectively 13 [11–14] vs. 12 [9–13]; p = 0.69 and 21 [18–23]; p = 0.13) |
| Reference | Aim | Study Design | Main Results | EjD Results |
|---|---|---|---|---|
| Plante et al., BJU Int. Apr 2019 [61] | To test the hypothesis that aquablation would have a more pronounced benefit in certain patient subgroups | Double-blind, multicentre prospective randomized controlled trial | Anejaculation 2% with aquablation vs. 41 with RTUP at 6 months (p < 0.0001) | |
| Gilling et al., Adv Ther. Jun 2019 [62] | To compare 2-year safety and efficacy outcomes after Aquablation or transurethral resection of the prostate (TURP) for the treatment of lower urinary tract symptoms related to benign prostate hyperplasia | Prospective, randomised Blinded follow up N 181 | IPSS simproved by 14.7 in Aquablation and 14.9 in TURP (p = 0.8304, 95% CI for difference—2.1–2.6 points) | Anejaculation 10% with aquablation vs. 36% with RTUP (p = 0.0003). No change in ejaculatory function on the MSHQ self-questionnaire with aquablation |
| Hwang et al., Cochrane Database Syst Rev. 2019 [63] | To assess the effects of Aquablation for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia | Systematic review | Similar improvement in urologic symptom scores to TURP (mean difference (MD) −0.06, 95% confidence interval (CI) −2.51 to 2.39 | No difference in IIEF before and after aquablation less ejaculatory dysfunction than TURP (MSHQED) |
| Bhojani et al., Urology. 2019 [64] | To report 12-month safety and effectiveness outcomes of the Aquablation procedure for the treatment of men with symptomatic benign prostatic hyperplasia (BPH) and large-volume prostates. | N 101 | IPSS improved from 23.2 at baseline to 6.2 at 12 months (p < 0.0001) | Antegrade ejaculation was maintained in 81% of sexually active men |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Couteau, N.; Duquesne, I.; Frédéric, P.; Thiounn, N.; Timsit, M.-O.; Mejean, A.; Pinar, U.; Audenet, F. Ejaculations and Benign Prostatic Hyperplasia: An Impossible Compromise? A Comprehensive Review. J. Clin. Med. 2021, 10, 5788. https://doi.org/10.3390/jcm10245788
Couteau N, Duquesne I, Frédéric P, Thiounn N, Timsit M-O, Mejean A, Pinar U, Audenet F. Ejaculations and Benign Prostatic Hyperplasia: An Impossible Compromise? A Comprehensive Review. Journal of Clinical Medicine. 2021; 10(24):5788. https://doi.org/10.3390/jcm10245788
Chicago/Turabian StyleCouteau, Nicolas, Igor Duquesne, Panthier Frédéric, Nicolas Thiounn, Marc-Olivier Timsit, Arnaud Mejean, Ugo Pinar, and François Audenet. 2021. "Ejaculations and Benign Prostatic Hyperplasia: An Impossible Compromise? A Comprehensive Review" Journal of Clinical Medicine 10, no. 24: 5788. https://doi.org/10.3390/jcm10245788






