Anti-SARS-CoV-2 S-RBD IgG Antibody Responses after COVID-19 mRNA Vaccine in the Chronic Disorder of Consciousness: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Enrollment
2.2. Ethics
2.3. Procedure
2.4. Biochemical Analysis
2.5. Statistical Analysis
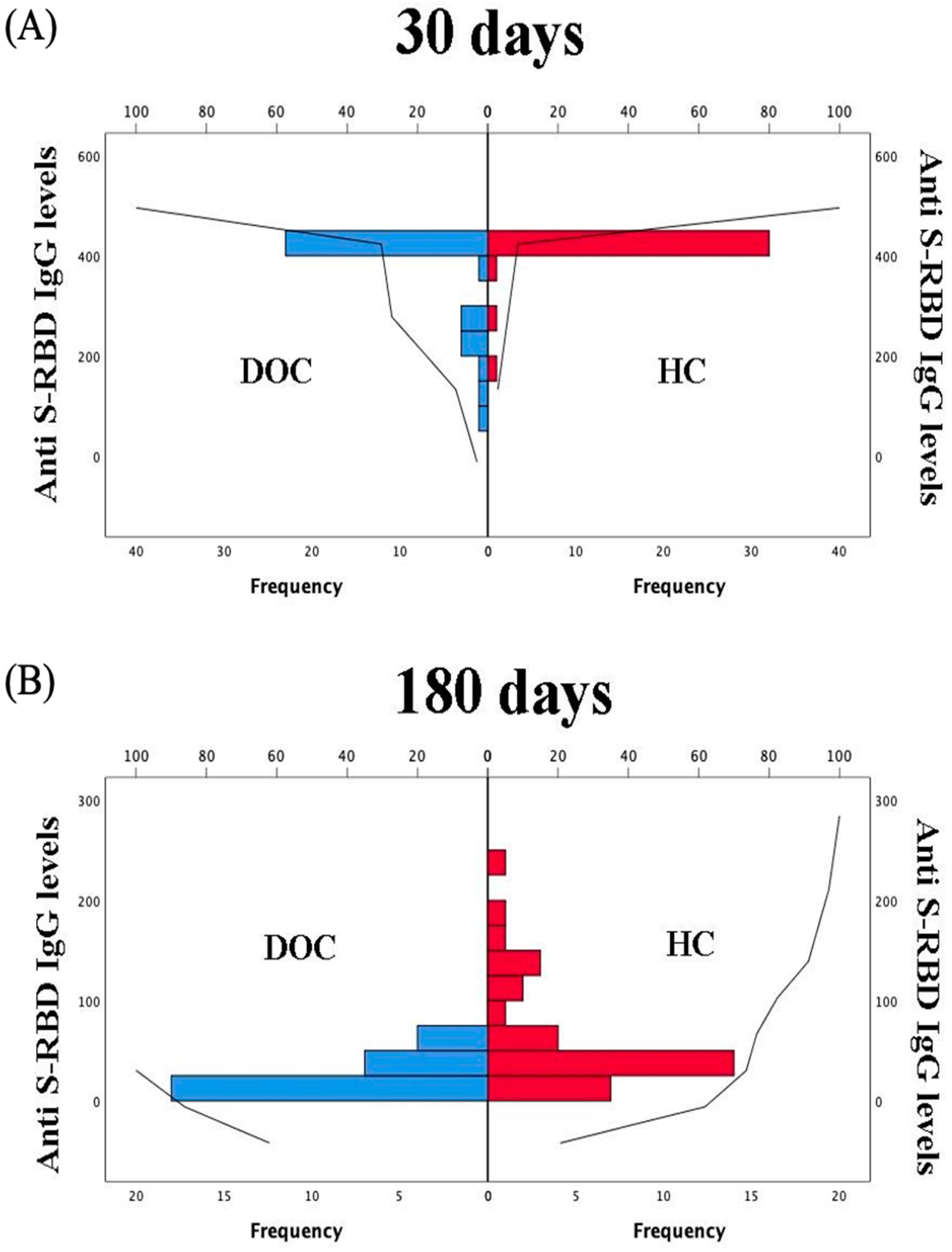
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Novel Coronavirus (2019-nCoV) Situation Report—1 21 January 2020; WHO: Geneva, Switzerland, 2020; Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf (accessed on 29 January 2020).
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481, Erratum in 2020, 8, e26. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.F.; Yuan, S.; Kok, K.H.; To, K.K.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.-Y.; Poon, R.W.-S.; et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef] [Green Version]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Ehmsen, S.; Asmussen, A.; Jeppesen, S.S.; Nilsson, A.C.; Østerlev, S.; Vestergaard, H.; Justesen, U.S.; Johansen, I.S.; Frederiksen, H.; Ditzel, H.J. Antibody and T cell immune responses following mRNA COVID-19 vaccination in patients with cancer. Cancer Cell 2021, 39, 1034–1036. [Google Scholar] [CrossRef] [PubMed]
- Maneikis, K.; Šablauskas, K.; Ringelevičiūtė, U.; Vaitekėnaitė, V.; Čekauskienė, R.; Kryžauskaitė, L.; Naumovas, D.; Banys, V.; Pečeliūnas, V.; Beinortas, T.; et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: A national prospective cohort study. Lancet Haematol. 2021, 8, e583–e592. [Google Scholar] [CrossRef]
- Grupper, A.; Sharon, N.; Finn, T.; Cohen, R.; Israel, M.; Agbaria, A.; Rechavi, Y.; Schwartz, I.F.; Schwartz, D.; Lellouch, Y.; et al. Humoral Response to the Pfizer BNT162b2 Vaccine in Patients Undergoing Maintenance Hemodialysis. Clin. J. Am. Soc. Nephrol. 2021, 16, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA 2021, 325, 2204–2206. [Google Scholar] [CrossRef]
- Braun-Moscovici, Y.; Kaplan, M.; Braun, M.; Markovits, D.; Giryes, S.; Toledano, K.; Tavor, Y.; Dolnikov, K.; Balbir-Gurman, A. Disease activity and humoral response in patients with inflammatory rheumatic diseases after two doses of the Pfizer mRNA vaccine against SARS-CoV-2. Ann. Rheum. Dis. 2021, 80, 1317–1321. [Google Scholar] [CrossRef]
- Apostolidis, S.A.; Kakara, M.; Painter, M.M.; Goel, R.R.; Mathew, D.; Lenzi, K.; Rezk, A.; Patterson, K.R.; Espinoza, D.A.; Kadri, J.C.; et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021, 27, 1990–2001. [Google Scholar] [CrossRef]
- Ritzel, R.M.; Doran, S.J.; Barrett, J.P.; Henry, R.J.; Ma, E.L.; Faden, A.I.; Loane, D.J. Chronic Alterations in Systemic Immune Function after Traumatic Brain Injury. J. Neurotrauma 2018, 35, 1419–1436. [Google Scholar] [CrossRef] [PubMed]
- Munno, I.; Damiani, S.; Lacedra, G.; Mastropasqua, V.; Megna, G.F. Impairment of non-specific immunity in patients in persistent vegetative state. Immunopharmacol. Immunotoxicol. 1996, 18, 549–569. [Google Scholar] [CrossRef] [PubMed]
- Wolach, B.; Sazbon, L.; Gavrieli, R.; Ben-Tovim, T.; Zagreba, F.; Schlesinger, M. Some aspects of the humoral and neutrophil functions in post-comatose unawareness patients. Brain Inj. 1993, 7, 401–410. [Google Scholar] [CrossRef]
- Wolach, B.; Sazbon, L.; Gavrieli, R.; Broda, A.; Schlesinger, M. Early immunological defects in comatose patients after acute brain injury. J. Neurosurg. 2001, 94, 706–711, Erratum in 2001, 95, 170. [Google Scholar] [CrossRef] [PubMed]
- Giacino, J.T.; Ashwal, S.; Childs, N.; Cranford, R.; Jennett, B.; Katz, D.I.; Kelly, J.P.; Rosenberg, J.H.; Whyte, J.; Zafonte, R.D.; et al. The minimally conscious state: Definition and diagnostic criteria. Neurology 2002, 58, 349–353. [Google Scholar] [CrossRef]
- Müller, L.; Andrée, M.; Moskorz, W.; Drexler, I.; Walotka, L.; Grothmann, R.; Ptok, J.; Hillebrandt, J.; Ritchie, A.; Rabl, D.; et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. Clin. Infect. Dis. 2021, 381. [Google Scholar] [CrossRef]
- Lo Sasso, B.; Giglio, R.V.; Vidali, M.; Scazzone, C.; Bivona, G.; Gambino, C.M.; Ciaccio, A.M.; Agnello, L.; Ciaccio, M. Evaluation of Anti-SARS-Cov-2 S-RBD IgG Antibodies after COVID-19 mRNA BNT162b2 Vaccine. Diagnostics 2021, 11, 1135. [Google Scholar] [CrossRef]
- Terpos, E.; Trougakos, I.P.; Apostolakou, F.; Charitaki, I.; Sklirou, A.D.; Mavrianou, N.; Papanagnou, E.-D.; Liacos, C.-I.; Gumeni, S.; Rentziou, G.; et al. Age-dependent and gender-dependent antibody responses against SARS-CoV-2 in health workers and octogenarians after vaccination with the BNT162b2 mRNA vaccine. Am. J. Hematol. 2021, 96, E257–E259. [Google Scholar] [CrossRef]
- Hazeldine, J.; Lord, J.M.; Belli, A. Traumatic brain injury and peripheral immune suppression: Primer and prospectus. Front. Neurol. 2015, 6, 235. [Google Scholar] [CrossRef] [Green Version]
- Alharfi, I.M.; Charyk Stewart, T.; Al Helali, I.; Daoud, H.; Fraser, D.D. Infection rates, fevers, and associated factors in pediatric severe traumatic brain injury. J. Neurotrauma 2014, 31, 452–458. [Google Scholar] [CrossRef]
- Scott, B.N.; Roberts, D.J.; Robertson, H.L.; Kramer, A.H.; Laupland, K.B.; Ousman, S.S.; Kubes, P.; Zygun, D.A. Incidence, prevalence, and occurrence rate of infection among adults hospitalized after traumatic brain injury: Study protocol for a systematic review and meta-analysis. Syst. Rev. 2013, 2, 68. [Google Scholar] [CrossRef] [Green Version]
- Meisel, C.; Schwab, J.M.; Prass, K.; Meisel, A.; Dirnagl, U. Central nervous system injury-induced immune deficiency syndrome. Nat. Rev. Neurosci. 2005, 6, 775–786. [Google Scholar] [CrossRef]
- Woiciechowsky, C.; Asadullah, K.; Nestler, D.; Eberhardt, B.; Platzer, C.; Schöning, B.; Glöckner, F.; Lanksch, W.R.; Volk, H.-D.; Döcke, W.-D. Sympathetic activation triggers systemic interleukin-10 release in immunodepression induced by brain injury. Nat. Med. 1998, 4, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef]
- Feuerstein, G.Z.; Liu, T.; Barone, F.C. Cytokines, inflammation, and brain injury: Role of tumor necrosis factor-α. Cerebrovasc. Brain Metab. Rev. 1994, 6, 341–360. [Google Scholar]
- Johansson, A.; Olsson, T.; Carlberg, B.; Karlsson, K.; Fagerlund, M. Hypercortisolism after stroke—Partly cytokine-mediated? J. Neurol. Sci. 1997, 147, 43–47. [Google Scholar] [CrossRef]
- Szczudlik, A.; Dziedzic, T.; Bartus, S.; Slowik, A.; Kieltyka, A. Serum interleukin-6 predicts cortisol release in acute stroke patients. J. Endocrinol. Investig. 2004, 27, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Woiciechowsky, C.; Schöning, B.; Cobanov, J.; Lanksch, W.R.; Volk, H.D.; Döcke, W.D. Early IL-6 plasma concentrations correlate with severity of brain injury and pneumonia in brain-injured patients. J. Trauma Acute Care Surg. 2002, 52, 339–345. [Google Scholar] [CrossRef]
- Singer, J.; Le, N.S.; Mattes, D.; Klamminger, V.; Hackner, K.; Kolinsky, N.; Scherb, M.; Errhalt, P.; Kreye, G.; Pecherstorfer, M.; et al. Evaluation of Antibody Responses to COVID-19 Vaccines among Solid Tumor and Hematologic Patients. Cancers 2021, 13, 4312. [Google Scholar] [CrossRef]

| DOC (n = 32) | HC (n = 34) | p-Level | |
|---|---|---|---|
| Age | 53.5 ± 15.8 | 52.9 ± 12.5 | 0.819 § |
| Sex, (%) male | 53% | 53% | 1.00 * |
| Hypertension (Yes; %) | 43.7% | 14.7% | 0.02 * |
| Diabetes mellitus (Yes; %) | 0% | 2.9% | 0.97 * |
| Heart disease (Yes; %) | 18.7% | 8.9% | 0.41 * |
| Renal insufficiency (Yes; %) | 0% | 2.9% | 0.97 * |
| Obstructive pulmonary disease (Yes; %) | 9.3% | 5.8% | 0.94 * |
| Liver disease (Yes; %) | 6.2% | 0% | 0.47 * |
| Endocrinopathies (Yes; %) | 9.3% | 8.8% | 0.72 * |
| Tumor (Yes; %) | 3.1% | 0% | 0.97 * |
| CRS-r at enrolment | 8.3 ± 3.6 | ||
| Time from injury (years) | 4.1 ± 3.6 | ||
Etiology n (%)
| |||
| 12 (37.5%) | |||
| 10 (31.25%) | ||
| 6 (18.7%) | ||
| 4 (12.5%) | ||
Diagnosis n (%)
| |||
| 12 (37.5%) | |||
| 20 (62.5%) |
| DOC Patients | Healthy Controls | |||
|---|---|---|---|---|
| After First Dose | After Second Dose | After First Dose | After Second Dose | |
| Local pain | 0% | 0% | 73.5% | 64.7% |
| Fever | 3.1% | 6.25% | 11.7% | 14.7% |
| Diffuse muscle pain, headache, fatigue | 0% | 0% | 47% | 55.8% |
| Severe reactions | 0% | 0% | 0% | 0% |
| Univariate Analysis (Mann–Whitney U) | Statistic | p-Level |
|---|---|---|
| Group (DOC vs. HC) | 263 | < 0.001 |
| Sex | 510 | 0.699 |
| Hypertension | 333 | 0.110 |
| Diabetes mellitus | 27.0 | 0.793 |
| Heart disease | 222 | 0.519 |
| Renal insufficiency | 22.0 | 0.600 |
| Obstructive pulmonary disease | 130 | 0.586 |
| Liver disease | 53.0 | 0.694 |
| Endocrinopathies | 162 | 0.696 |
| Tumor | 6.0 | 0.172 |
| Multivariate Analysis (Omnibus ANOVA Test) | Statistic | p-Level |
| Age | 6.6660 | 0.012 |
| Group (DOC vs. HC) | 11.1537 | 0.001 |
| Sex | 0.9258 | 0.34 |
| Diabetes mellitus | 0.1570 | 0.693 |
| Heart disease | 0.9867 | 0.325 |
| Tumors | 1.1026 | 0.298 |
| Chronic obstructive pulmonary disease | 0.0940 | 0.768 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pugliese, M.E.; Battaglia, R.; Cerasa, A.; Raso, M.G.; Coschignano, F.; Pagliuso, A.; Bruschetta, R.; Pugliese, G.; Scola, P.; Tonin, P. Anti-SARS-CoV-2 S-RBD IgG Antibody Responses after COVID-19 mRNA Vaccine in the Chronic Disorder of Consciousness: A Pilot Study. J. Clin. Med. 2021, 10, 5830. https://doi.org/10.3390/jcm10245830
Pugliese ME, Battaglia R, Cerasa A, Raso MG, Coschignano F, Pagliuso A, Bruschetta R, Pugliese G, Scola P, Tonin P. Anti-SARS-CoV-2 S-RBD IgG Antibody Responses after COVID-19 mRNA Vaccine in the Chronic Disorder of Consciousness: A Pilot Study. Journal of Clinical Medicine. 2021; 10(24):5830. https://doi.org/10.3390/jcm10245830
Chicago/Turabian StylePugliese, Maria Elena, Riccardo Battaglia, Antonio Cerasa, Maria Girolama Raso, Francesco Coschignano, Angela Pagliuso, Roberta Bruschetta, Giovanni Pugliese, Paolo Scola, and Paolo Tonin. 2021. "Anti-SARS-CoV-2 S-RBD IgG Antibody Responses after COVID-19 mRNA Vaccine in the Chronic Disorder of Consciousness: A Pilot Study" Journal of Clinical Medicine 10, no. 24: 5830. https://doi.org/10.3390/jcm10245830
APA StylePugliese, M. E., Battaglia, R., Cerasa, A., Raso, M. G., Coschignano, F., Pagliuso, A., Bruschetta, R., Pugliese, G., Scola, P., & Tonin, P. (2021). Anti-SARS-CoV-2 S-RBD IgG Antibody Responses after COVID-19 mRNA Vaccine in the Chronic Disorder of Consciousness: A Pilot Study. Journal of Clinical Medicine, 10(24), 5830. https://doi.org/10.3390/jcm10245830







