Variability in the Control of Type 2 Diabetes in Primary Care and Its Association with Hospital Admissions for Vascular Events. The APNA Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Size
2.2. Study Variables
2.3. Statistical Analysis
2.4. Inclusion and Exclusion Criteria
2.5. Study Outcome
2.6. Ethical Aspects
3. Results
3.1. Descriptive Statistics
3.2. Multivariate Cluster Analysis of GPPs
3.3. Cox Regression
4. Discussion
4.1. Strengths and Limitations
4.2. Variability in Control Indicators
4.3. Variability: The Risk of Admission for a CVE
4.4. Implications for Research and Practice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rawshani, A.; Rawshani, A.; Franzén, S.; Sattar, N.; Eliasson, B.; Svensson, A.-M.; Zethelius, B.; Miftaraj, M.; McGuire, D.K.; Rosengren, A.; et al. Risk Factors, Mortality, and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2018, 379, 633–644. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-W.; Wang, C.-Y.; Ko, Y. Costs and Length of Stay of Hospitalizations due to Diabetes-Related Complications. J. Diabetes Res. 2019, 2019, 2363292. [Google Scholar] [CrossRef]
- American Diabetes Association. Cardiovascular disease and risk management: Standards of medical care in diabetesd 2021. Diabetes Care 2021, 44, S125–S150. [Google Scholar] [CrossRef] [PubMed]
- The Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef]
- Sattar, N.; Rawshani, A.; Franzén, S.; Rawshani, A.; Svensson, A.-M.; Rosengren, A.; McGuire, D.K.; Eliasson, B.; Gudbjörnsdottir, S. Age at Diagnosis of Type 2 Diabetes Mellitus and Associations With Cardiovascular and Mortality Risks. Circulation 2019, 139, 2228–2237. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, B.; Galbete, A.; Goñi, M.J.; Forga, L.; Arnedo, L.; Aizpuru, F.; Librero, J.; Lecea, O.; Cambra, K. Socioeconomic inequalities in cardiometabolic control in patients with type 2 diabetes. BMC Public Health 2018, 18, 408. [Google Scholar] [CrossRef]
- Walker, J.; Halbesma, N.; Lone, N.; McAllister, D.; Weir, C.J.; Wild, S.H. Socioeconomic status, comorbidity and mortality in patients with type 2 diabetes mellitus in Scotland 2004–2011: A cohort study. J. Epidemiol. Community Health 2016, 70, 596–601. [Google Scholar] [CrossRef]
- Saydah, S.H.; Imperatore, G.; Beckles, G.L. Socioeconomic Status and Mortality: Contribution of health care access and psychological distress among U.S. adults with diagnosed diabetes. Diabetes Care 2013, 36, 49–55. [Google Scholar] [CrossRef]
- Saydah, S.; Lochner, K. Socioeconomic Status and Risk of Diabetes-Related Mortality in the U.S. Public Health Rep. 2010, 125, 377–388. [Google Scholar] [CrossRef]
- Dalsgaard, E.-M.; Vestergaard, M.; Skriver, M.V.; Borch-Johnsen, K.; Lauritzen, T.; Sandbaek, A. Socioeconomic position and cardiovascular risk factors among people with screen-detected Type 2 DM: Six-year follow-up of the ADDITION-Denmark trial. Prim. Care Diabetes 2014, 8, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Dalsgaard, E.-M.; Skriver, M.V.; Sandbaek, A.; Vestergaard, M. Socioeconomic Position, Type 2 Diabetes and Long-Term Risk of Death. PLoS ONE 2015, 10, e0124829. [Google Scholar] [CrossRef] [PubMed]
- O’Kane, M.J.; McMenamin, M.; Bunting, B.P.; Moore, A.; Coates, V.E. The relationship between socioeconomic deprivation and metabolic/cardiovascular risk factors in a cohort of patients with type 2 diabetes mellitus. Prim. Care Diabetes 2010, 4, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Islam, S.; Anand, S.; Almahmeed, W.; Damasceno, A.; Dans, A.; Lang, C.C.; Luna, M.A.; McQueen, M.; Rangarajan, S.; et al. Dysglycaemia and the risk of acute myocardial infarction in multiple ethnic groups: An analysis of 15,780 patients from the INTERHEART study. Diabetologia 2010, 53, 2509–2517. [Google Scholar] [CrossRef][Green Version]
- Lehto, S.; Ronnemaa, T.; Haffher, S.M.; Pyorala, K.; Kallio, V.; Laakso, M. Dyslipidemia and Hyperglycemia Predict Coronary Heart Disease Events in Middle-Aged Patients With NIDDM. Diabetes 1997, 46, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Hoerger, T.J.; Segel, J.E.; Gregg, E.W.; Saaddine, J.B. Is Glycemic Control Improving in U.S. Adults? Diabetes Care 2008, 31, 81–86. [Google Scholar] [CrossRef]
- Casagrande, S.S.; Fradkin, J.E.; Saydah, S.H.; Rust, K.F.; Cowie, C.C. The Prevalence of Meeting A1C, Blood Pressure, and LDL Goals Among People With Diabetes, 1988–2010. Diabetes Care 2013, 36, 2271–2279. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2020; p. 2.
- Assmann, G.; Schulte, H. The Prospective Cardiovascular Münster (PROCAM) study: Prevalence of hyperlipidemia in persons with hypertension and/or diabetes mellitus and the relationship to coronary heart disease. Am. Heart J. 1988, 116, 1713–1724. [Google Scholar] [CrossRef]
- Cases, M.M.; Menéndez, S.A.; Espino, J.D.; Ezkurra, P. Actualización de 2020 del Algoritmo de Tratamiento de la Hiperglucemia en la Diabetes Mellitus Tipo 2 de la redGDPS. Diabetes Práctica 2020, 11, 41–76. [Google Scholar] [CrossRef]
- Casanova, L.; Bocquier, A.; Cortaredona, S.; Nauleau, S.; Sauze, L.; Sciortino, V.; Villani, P.; Verger, P. Membership in a diabetes-care network and adherence to clinical practice guidelines for treating type 2 diabetes among general practitioners: A four-year follow-up. Prim. Care Diabetes 2016, 10, 342–351. [Google Scholar] [CrossRef]
- Khunti, K.; Ganguli, S.; Baker, R.; Lowy, A. Features of primary care associated with variations in process and outcome of care of people with diabetes. Br. J. Gen. Pract. 2001, 51, 356–360. [Google Scholar]
- Nøkleby, K.; Berg, T.J.; Mdala, I.; Tran, A.T.; Bakke, Å.; Gjelsvik, B.; Claudi, T.; Cooper, J.G.; Løvaas, K.F.; Thue, G.; et al. Variation between general practitioners in type 2 diabetes processes of care. Prim. Care Diabetes 2021, 15, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.T.; Bakke, Å.; Berg, T.J.; Gjelsvik, B.; Mdala, I.; Nøkleby, K.; Rai, A.S.; Cooper, J.G.; Claudi, T.; Løvaas, K.; et al. Are general practitioners characteristics associated with the quality of type 2 diabetes care in general practice? Results from the Norwegian ROSA4 study from 2014. Scand. J. Prim. Health Care 2018, 36, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Nadal, J.F.; Menéndez, S.A.; Espino, J.D.; Cases, M.M. The evolution of quality care indicators of patients with type 2 diabetes in the Spanish primary care (1996–2007). The RedGEDAPS quality of care program. Med. Clin. 2010, 135, 600–607. [Google Scholar] [CrossRef]
- Mata-Cases, M.; Roura-Olmeda, P.; Berengué-Iglesias, M.; Birulés-Pons, M.; Mundet-Tuduri, X.; Franch-Nadal, J.A.; Benito-Badorrey, B.; Cano-Pérez, J.F.; Diabetes Study Group in Primary Health Care (GEDAPS: Grup d’Estudi de la Diabetis a l’Atenció Primària de Salut, Catalonian Society of Family and Community Medicine). Fifteen years of continuous improvement of quality care of type 2 diabetes mellitus in primary care in Catalonia, Spain. Int. J. Clin. Pract. 2012, 66, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Cambra, K.; Galbete, A.; Forga, L.; Lecea, O.; Ariz, M.J.; Moreno-Iribas, C.; Aizpuru, F.; Ibañez, B. Sex and age differences in the achievement of control targets in patients with type 2 diabetes: Results from a population-based study in a South European region. BMC Fam. Pract. 2016, 17, 144. [Google Scholar] [CrossRef] [PubMed]
- Galbete, A.; Cambra, K.; Forga, L.; Baquedano, F.J.; Aizpuru, F.; Lecea, O.; Librero, J.; Ibáñez, B. Achievement of cardiovascular risk factor targets according to sex and previous history of cardiovascular disease in type 2 diabetes: A population-based study. J. Diabetes Complicat. 2019, 33, 107445. [Google Scholar] [CrossRef]
- Brugos-Larumbe, A.; Aldaz-Herce, P.; Guillen-Grima, F.; Garjón-Parra, F.J.; Bartolomé-Resano, F.J.; Arizaleta-Beloqui, M.T.; Pérez-Ciordia, I.; Fernández-Navascués, A.M.; Lerena-Rivas, M.J.; Berjón-Reyero, J.; et al. Assessing variability in compliance with recommendations given by the International Diabetes Federation (IDF) for patients with type 2 diabetes in primary care using electronic records. The APNA study. Prim. Care Diabetes 2018, 12, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Martin-Rodriguez, E.; Guillen-Grima, F.; Martí, A.; Brugos-Larumbe, A. Comorbidity associated with obesity in a large population: The APNA study. Obes. Res. Clin. Pract. 2015, 9, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Aragón, L.F.; Ariz, M.J.; Berraondo, M.R.; Díez, J.; Goñi, M.J.; Lafita, J.; Marí, G.M.; MA, P.N. Diabetes Tipo 2. Guía de Actuación en Atención Primaria, 3rd ed.; Servicio Navarro de Salud-Osasunbidea: Pamplona, Spain, 2012. [Google Scholar]
- D’Hoore, W.; Sicotte, C.; Tilquin, C. Risk adjustment in outcome assessment: The Charlson comorbidity index. Methods Inf. Med. 1993, 32, 382–387. [Google Scholar]
- Goldstein, L.B.; Samsa, G.P.; Matchar, D.B.; Horner, R.D. Charlson Index Comorbidity Adjustment for Ischemic Stroke Outcome Studies. Stroke 2004, 35, 1941–1945. [Google Scholar] [CrossRef] [PubMed]
- D’Hoore, W.; Bouckaert, A.; Tilquin, C. Practical considerations on the use of the Charlson comorbidity index with administrative data bases. J. Clin. Epidemiol. 1996, 49, 1429–1433. [Google Scholar] [CrossRef]
- Lederer, D.J.; Bell, S.C.; Branson, R.D.; Chalmers, J.D.; Marshall, R.; Maslove, D.M.; Ost, D.E.; Punjabi, N.M.; Schatz, M.; Smyth, A.R.; et al. Control of Confounding and Reporting of Results in Causal Inference Studies. Guidance for Authors from Editors of Respiratory, Sleep, and Critical Care Journals. Ann. Am. Thorac. Soc. 2019, 16, 22–28. [Google Scholar] [CrossRef]
- Textor, J.; van der Zander, B.; Gilthorpe, M.S.; Liśkiewicz, M.; Ellison, G.T.H. Robust causal inference using directed acyclic graphs: The R package ‘dagitty’. Int. J. Epidemiol. 2017, 45, 1887–1894. [Google Scholar] [CrossRef]
- García-Albéniz, X.; Hsu, J.; Bretthauer, M.; Hernán, M.A. Estimating the Effect of Preventive Services with Databases of Administrative Claims: Reasons to Be Concerned. Am. J. Epidemiol. 2019, 188, 1764–1767. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, S.C.; Hernán, M.A.; McElrath, T.F.; Hernández-Díaz, S. Assessment of recording bias in pregnancy studies using health care databases: An application to neurologic conditions. Paediatr. Perinat. Epidemiol. 2018, 32, 281–286. [Google Scholar] [CrossRef]
- Vinagre, I.; Mata-Cases, M.; Hermosilla, E.; Morros, R.; Fina, F.; Rosell, M.; Castell, C.; Franch-Nadal, J.; Bolíbar, B.; Mauricio, D. Control of glycemia and cardiovascular risk factors in patients with type 2 diabetes in primary care in Catalonia (Spain). Diabetes Care 2012, 35, 774–779. [Google Scholar] [CrossRef]
- Soriguer, F.; Goday, A.; Bosch-Comas, A.; Bordiú, E.; Calle-Pascual, A.; Carmena, R.; Casamitjana, R.; Castaño, L.; Castell, C.; Catalá, M.; et al. Prevalence of diabetes mellitus and impaired glucose regulation in Spain: The Di@bet.es Study. Diabetologia 2012, 55, 88–93. [Google Scholar] [CrossRef]
- Larrañaga, I.; Arteagoitia, J.M.; Rodriguez, J.L.; Gonzalez, F.; Esnaola, S.; Piniés, J.A.; Sentinel Practice Network of the Basque Country. Socio-economic inequalities in the prevalence of Type 2 diabetes, cardiovascular risk factors and chronic diabetic complications in the Basque Country, Spain. Diabet. Med. 2005, 22, 1047–1053. [Google Scholar] [CrossRef]
- Navarro-Pérez, J.; Franch-Nadal, J.; Artola-Menéndez, S.; Diez-Espino, J.; Garcia-Soidan, J. La historia clínica electrónica y los registros sobre diabetes en España. Av. Diabetol. 2011, 27, 128–136. [Google Scholar] [CrossRef]
- Herrero, A.; Garzón, G.; Gil, A.; García, I.; Vargas, E.; Torres, N. Control of cardiovascular risk factors among patients with diabetes with and without cardiovascular disease. Semergen 2015, 41, 354–361. [Google Scholar] [CrossRef]
- Arroyo, J.; Badía, X.; de la Calle, H.; Díez, J.; Estmatjes, E.; Fernández, I.; Filozof, C.; Franch, J.; Gambús, G.; Gomis, R.; et al. Tratamiento de los pacientes con diabetes mellitus tipo 2 en atención primaria en España. Med. Clin. (Barc) 2005, 125, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, F.; Piñera, M.; Iglesias, P.; Nogales, P.; Salinero-Fort, M.A.; Abanades, J.C.; Botella-Carretero, J.I.; Calañas, A.; Balsa, J.A.; Zamarrón, I.; et al. Metabolic control and chronic complications during a 3-year follow-up period in a cohort of type 2 diabetic patients attended in primary care in the Community of Madrid (Spain). Endocrinol. Nutr. 2014, 61, 11–17. [Google Scholar] [CrossRef]
- López, P.B.; García-Mayor, R.V.; Domingo, M.P.; Manteca, J.M.; Sánchez, L.F.P.; Nogueras, E.F.; Mateu, R.R.; Ferragud, M.A.; López, J.S. Pathological characteristics of patients with diabetes mellitus type 2, in Spanish Primary Care. Rev. Clin. Esp. 2004, 204, 18–24. [Google Scholar] [CrossRef]
- Orozco-Beltrán, D.; Gil-Guillen, V.F.; Quirce, F.; Navarro-Perez, J.; Pineda, M.; de la Camara, A.G.; Pita, S.; Diez-Espino, J.; Mateos, J.; Merino, J.; et al. Control of diabetes and cardiovascular risk factors in patients with type 2 diabetes in primary care. The gap between guidelines and reality in Spain. Int. J. Clin. Pract. 2007, 61, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Birtwhistle, R.; Green, M.E.; Frymire, E.; Dahrouge, S.; Whitehead, M.; Khan, S.; Greiver, M.; Glazier, R.H. Hospital Admission Rates and Emergency Department Use in Relation to Glycated Hemoglobin in People with Diabetes Mellitus: A Linkage Study Using Electronic Medical Record and Administrative Data in Ontario. CMAJ Open 2017, 5, E557–E564. [Google Scholar] [CrossRef][Green Version]
- Morrish, N.J.; Stevens, L.K.; Fuller, J.H.; Keen, H.; Jarrett, R.J. Incidence of macrovascular disease in diabetes mellitus: The London cohort of the WHO Multinational Study of Vascular Disease in Diabetics. Diabetologia 1991, 34, 584–589. [Google Scholar] [CrossRef]
- Stamler, J.; Vaccaro, O.; Neaton, J.D.; Wentworth, D. Diabetes, Other Risk Factors, and 12-Yr Cardiovascular Mortality for Men Screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 1993, 16, 434–444. [Google Scholar] [CrossRef]
- Cavero-Redondo, I.; Peleteiro, B.; Álvarez-Bueno, C.; Rodriguez-Artalejo, F.; Martínez-Vizcaíno, V. Glycated haemoglobin A1c as a risk factor of cardiovascular outcomes and all-cause mortality in diabetic and non-diabetic populations: A systematic review and meta-analysis. BMJ Open 2017, 7, e015949. [Google Scholar] [CrossRef]
- Khaw, K.-T.; Wareham, N.; Bingham, S.; Luben, R.; Welch, A.; Day, N. Association of Hemoglobin A 1c with Cardiovascular Disease and Mortality in Adults: The European Prospective Investigation into Cancer in Norfolk. Ann. Intern. Med. 2004, 141, 413–420. [Google Scholar] [CrossRef]
- Eeg-Olofsson, K.; Cederholm, J.; Nilsson, P.M.; Zethelius, B.; Svensson, A.-M.; Gudbjörnsdóttir, S.; Eliasson, B. New aspects of HbA1c as a risk factor for cardiovascular diseases in type 2 diabetes: An observational study from the Swedish National Diabetes Register (NDR). J. Intern. Med. 2010, 268, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Leleu, H.; Minvielle, E. Relationship between longitudinal continuity of primary care and likelihood of death: Analysis of national insurance data. PLoS ONE 2013, 8, e71669. [Google Scholar] [CrossRef] [PubMed]
- Hoertel, N.; Limosin, F.; Leleu, H. Poor longitudinal continuity of care is associated with an increased mortality rate among patients with mental disorders: Results from the French National Health Insurance Reimbursement Database. Eur. Psychiatry 2014, 29, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Hermans, M.P.; Elisaf, M.; Michel, G.; Muls, E.; Nobels, F.; Vandenberghe, H.; Brotons, C. Benchmarking is associated with improved quality of care in type 2 diabetes: The OPTIMISE randomized, controlled trial. Diabetes Care 2013, 36, 3388–3395. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; Colagiuri, S.; Flack, J.R. Successful implementation of diabetes audits in Australia: The Australian National Diabetes Information Audit and Benchmarking (ANDIAB) initiative. Diabet. Med. 2018, 35, 929–936. [Google Scholar] [CrossRef]
 , ancestors of outcome
, ancestors of outcome  , ancestors of exposure
, ancestors of exposure  , exposure
, exposure  , and outcome
, and outcome  . Based on DAGitty version 3.0.
. Based on DAGitty version 3.0.
 , ancestors of outcome
, ancestors of outcome  , ancestors of exposure
, ancestors of exposure  , exposure
, exposure  , and outcome
, and outcome  . Based on DAGitty version 3.0.
. Based on DAGitty version 3.0.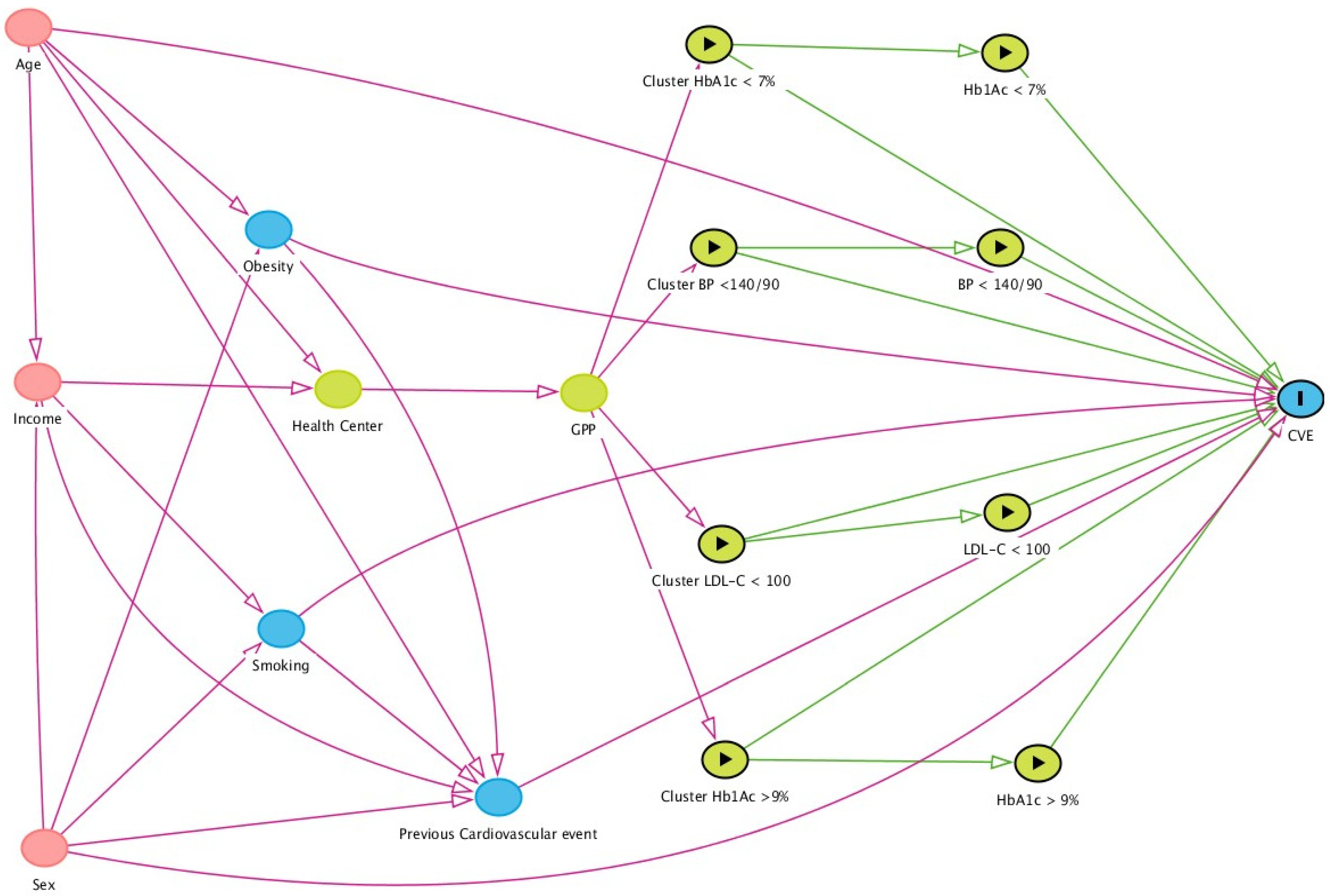
 , ancestors of exposure
, ancestors of exposure  , exposure
, exposure  , and outcome
, and outcome  . Based on DAGitty version 3.0.
. Based on DAGitty version 3.0.
 , ancestors of exposure
, ancestors of exposure  , exposure
, exposure  , and outcome
, and outcome  . Based on DAGitty version 3.0.
. Based on DAGitty version 3.0.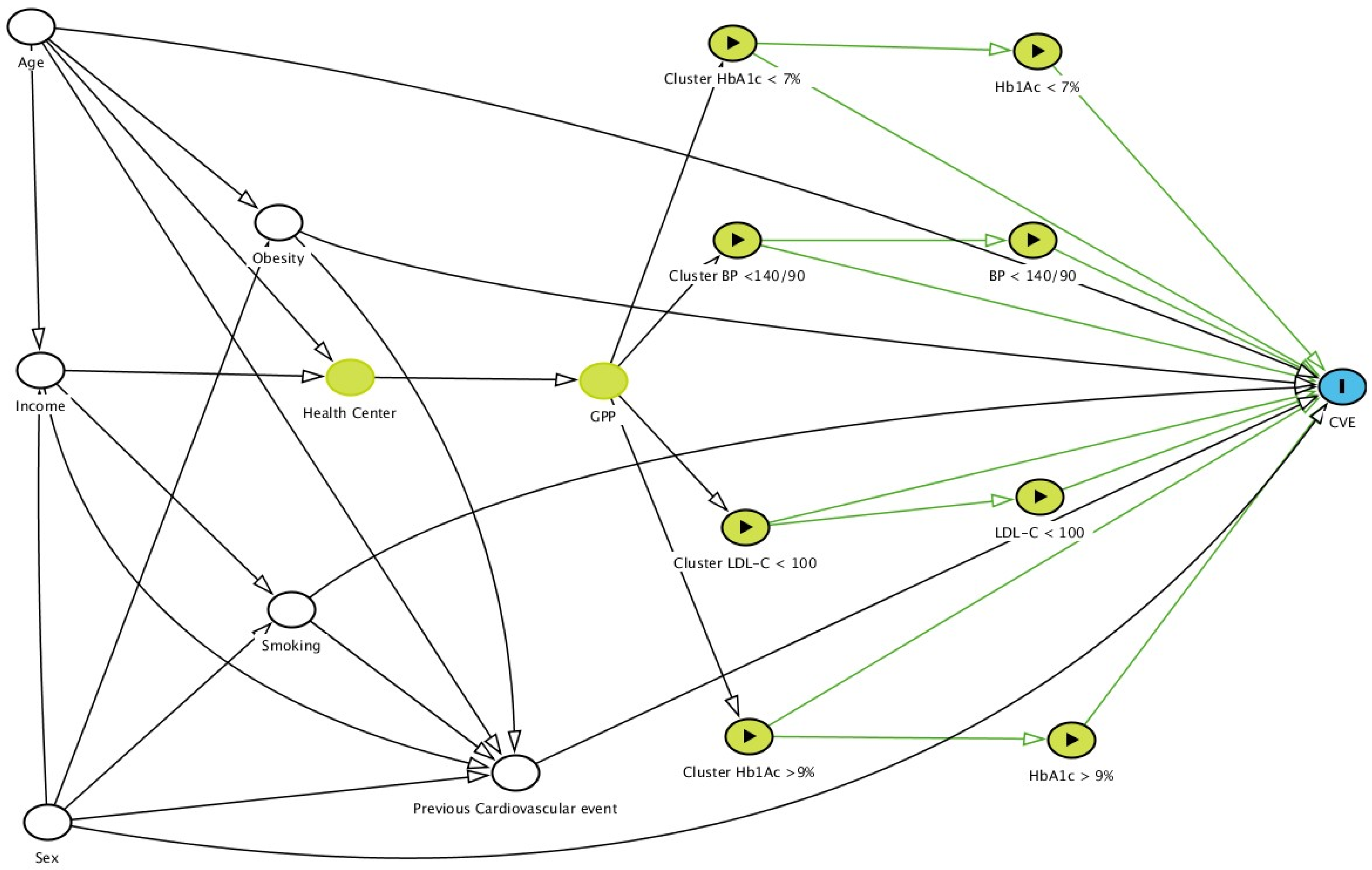
| Variable | HRa * | 95% CI | p | |
|---|---|---|---|---|
| HbA1c ≥ 9% ** | 1.339 | 1.070–1.676 | 0.010 | |
| HbA1c < 7% ** | 0.736 | 0.632–0.856 | <0.001 | |
| BP < 140/90 mmHg ** | 0.732 | 0.628–0.853 | <0.001 | |
| LDL-C < 100 mg/dL † or <70 mg/dL ‡ | 0.240 | 0.196–0.294 | <0.001 | |
| Cluster inadequate control HbA1c ≥ 9% ** | 1.119 | 1.003–1.250 | 0.045 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillen-Aguinaga, S.; Forga, L.; Brugos-Larumbe, A.; Guillen-Grima, F.; Guillen-Aguinaga, L.; Aguinaga-Ontoso, I. Variability in the Control of Type 2 Diabetes in Primary Care and Its Association with Hospital Admissions for Vascular Events. The APNA Study. J. Clin. Med. 2021, 10, 5854. https://doi.org/10.3390/jcm10245854
Guillen-Aguinaga S, Forga L, Brugos-Larumbe A, Guillen-Grima F, Guillen-Aguinaga L, Aguinaga-Ontoso I. Variability in the Control of Type 2 Diabetes in Primary Care and Its Association with Hospital Admissions for Vascular Events. The APNA Study. Journal of Clinical Medicine. 2021; 10(24):5854. https://doi.org/10.3390/jcm10245854
Chicago/Turabian StyleGuillen-Aguinaga, Sara, Luis Forga, Antonio Brugos-Larumbe, Francisco Guillen-Grima, Laura Guillen-Aguinaga, and Ines Aguinaga-Ontoso. 2021. "Variability in the Control of Type 2 Diabetes in Primary Care and Its Association with Hospital Admissions for Vascular Events. The APNA Study" Journal of Clinical Medicine 10, no. 24: 5854. https://doi.org/10.3390/jcm10245854
APA StyleGuillen-Aguinaga, S., Forga, L., Brugos-Larumbe, A., Guillen-Grima, F., Guillen-Aguinaga, L., & Aguinaga-Ontoso, I. (2021). Variability in the Control of Type 2 Diabetes in Primary Care and Its Association with Hospital Admissions for Vascular Events. The APNA Study. Journal of Clinical Medicine, 10(24), 5854. https://doi.org/10.3390/jcm10245854






_MD__MPH_PhD.png)

