Central Hemodynamic Adjustments during Post-Exercise Hypotension in Hypertensive Patients with Ischemic Heart Disease: Concurrent Circuit Exercise versus High-Intensity Interval Exercise. A Preliminary Study
Abstract
:1. Introduction
2. Methods
2.1. Experimental Protocol
2.2. Echocardiography
2.3. Statistical Analysis
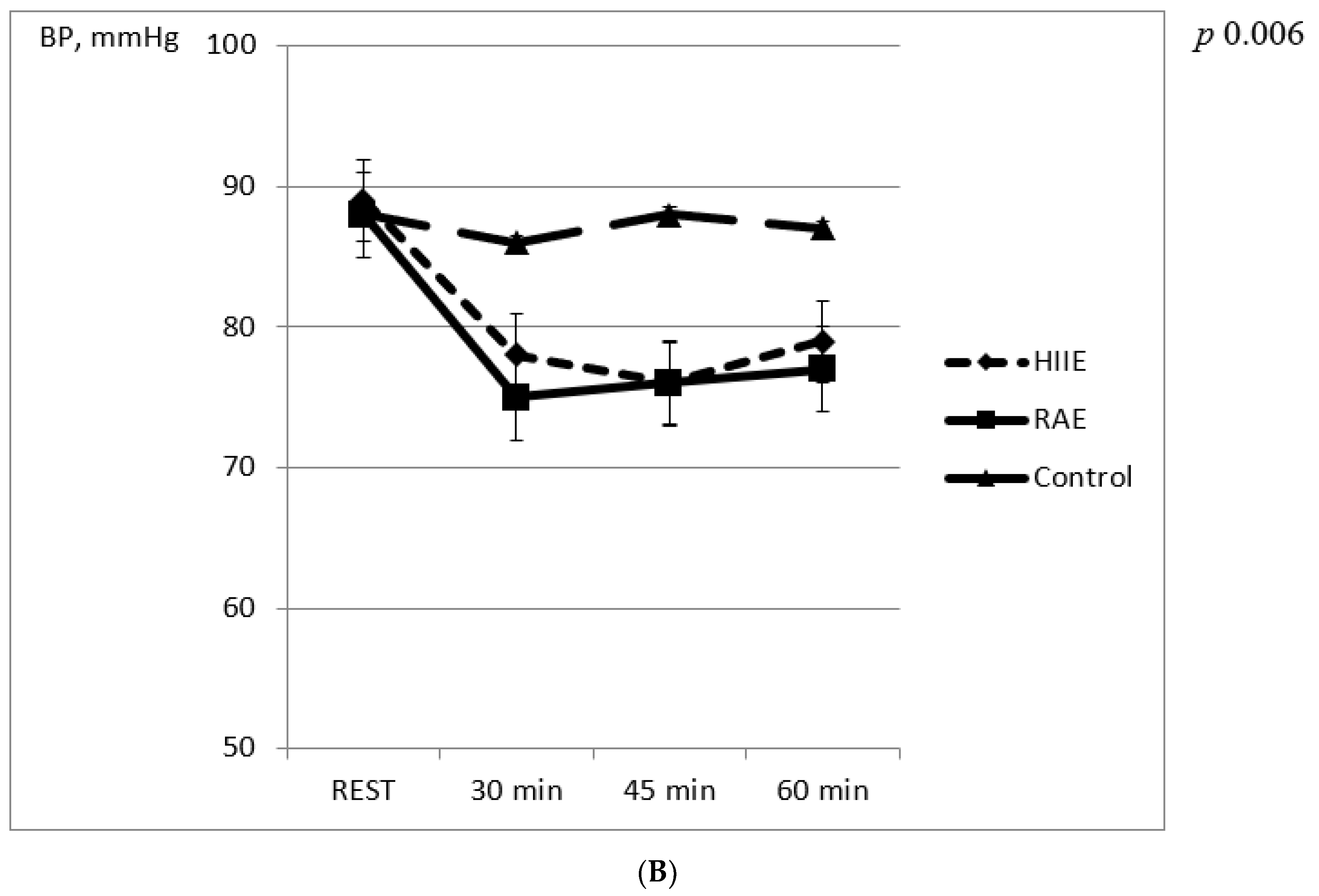
3. Results
3.1. LV Diastolic Function and LV Stiffness
3.2. Left Atrial Function and LA Stiffness
3.3. Left Ventricular Systolic Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Milanović, Z.; Sporiš, G.; Weston, M. Effectiveness of high-intensity interval training (HIT) and continuous endurance training for VO2max improvements: A systematic review and meta-analysis of controlled trials. Sports Med. 2015, 45, 1469–1481. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.G.; Bonardi, J.; Campos, G.O.; Bertani, R.F.; Scher, L.M.; Moriguti, J.C.; Ferriolli, E.; Lima, N.K. Combined aerobic andresistance training: Are there additional benefits for older hypertensiveadults? Clinics 2017, 72, 363–369. [Google Scholar] [CrossRef]
- Karstoft, K.; Winding, K.; Knudsen, S.H.; Nielsen, J.S.; Thomsen, C.; Pedersen, B.K.; Solomon, T.P. The effects of free-living interval-walking training on glycemic control, body composition, and physical fitness in type 2 diabetic patients: A randomized, controlled trial. Diabetes Care 2013, 36, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Caminiti, G.; Iellamo, F.; Manzi, V.; Fossati, C.; Cioffi, V.; Punzo, N.; Murugesan, J.; Volterrani, M.; Rosano, G. Anabolic hormonal response to different exercise training intensities in men with chronic heart failure. Int. J. Cardiol. 2014, 176, 1433–1434. [Google Scholar] [CrossRef]
- Schumann, M.; Rønnestad, B.R. Concurrent Aerobic and Strength Training: Scientific Basics and Practical Applications; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Luttrell, M.J.; Halliwill, J.R. Recovery from exercise: Vulnerable state, window of opportunity, or crystal ball? Front. Physiol. 2015, 6, 204. [Google Scholar] [CrossRef] [Green Version]
- Iellamo, F.; Caminiti, G.; Montano, M.; Manzi, V.; Franchini, A.; Mancuso, A.; Volterrani, M. Prolonged post-exercise hypotension: Effects of different exercise modalities and training statuses in elderly patients with hypertension. Int. J. Environ. Res. Public Health 2021, 18, 3229. [Google Scholar] [CrossRef]
- Ferrari, R.; Umpierre, D.; Vogel, G.; Vieira, P.J.C.; Santos, L.P.; de Mello, R.B.; Tanaka, H.; Fuchs, S.C. Effects of concurrent and aerobic exercises on postexercise hypotension in elderly hypertensive men. Exp. Gerontol. 2017, 98, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Grace, F.; Herbert, P.; Elliott, A.D.; Richards, J.; Beaumont, A.; Sculthorpe, N.F. High intensity interval training (HIIT) improves resting blood pressure, metabolic (MET) capacity and heart rate reserve without compromising cardiac function in sedentary aging men. Exp. Gereontol. 2018, 109, 75–81. [Google Scholar] [CrossRef]
- Wisløff, U.; Støylen, A.; Loennechen, J.P.; Bruvold, M.; Rognmo, Ø.; Haram, P.M.; Tjønna, A.E.; Helgerud, J.; Slørdahl, S.A.; Lee, S.J.; et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: A randomized study. Circulation 2007, 115, 3086–3094. [Google Scholar] [CrossRef] [Green Version]
- Schmid, J.P.; Anderegg, M.; Romanens, M.; Morger, C.; Noveanu, M.; Hellige, G.; Saner, H. Combined endurance/resistance training early on, after a first myocardial infarction, does not induce negative left ventricular remodelling. Eur. J. Cardiov. Prev. Rehab. 2008, 15, 341–346. [Google Scholar] [CrossRef]
- Lund, J.S.; Aksetøy, I.L.A.; Dalen, H.; Amundsen, B.H.; Støylen, A. Left ventricular diastolic function: Effects of high-intensity exercise after acute myocardial infarction. Echocardiography 2020, 37, 858–866. [Google Scholar] [CrossRef]
- Guirado, G.N.; Damatto, R.L.; Matsubara, B.B.; Roscani, M.G.; Fusco, D.R.; Seki, M.M.; Teixeira, A.S.; Okoshi, K.; Okoshi, M.P. Combined exercise training in asymptomatic elderly with controlled hypertension: Effects on functional capacity and cardiac diastolic function. Med Sci Monit. 2012, 18, CR461–CR465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalande, S.; Johnson, B.D. Diastolic dysfunction: A link between hypertension and heart failure. Drugs Today 2008, 4, 503–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadruz, W.; Shah, A.M.; Solomon, S.D. Diastolic dysfunction and hypertension. Med. Clin. N. Am. 2017, 101, 7–17. [Google Scholar] [CrossRef]
- Kasner, M.; Sinning, D.; Burkhoff, D.; Tschöpe, C. Diastolic pressure-volume quotient (DPVQ) as a novel echocardiographic index for estimation of LV stiffness in HFpEF. Clin Res Cardiol. 2015, 104, 955–963. [Google Scholar] [CrossRef]
- Machino-Ohtsuka, T.; Seo, Y.; Tada, H.; Ishizu, T.; Machino, T.; Yamasaki, H.; Igarashi, M.; Xu, D.; Sekiguchi, Y.; Aonuma, K. Left atrial stiffness relates to left ventricular diastolic dysfunction and recurrence after pulmonary vein isolation for atrial fibrillation. J Cardiovasc. Electrophysiol. 2011, 22, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the american society of echocardiography and the European association of cardiovascular imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobushi, T.; Nakano, M.; Hosokawa, K.; Koga, H.; Yamada, A. Improved Diastolic Function Is Associated With Higher Cardiac Output in Patients With Heart Failure Irrespective of Left Ventricular Ejection Fraction. J. Am. Heart Assoc. 2017, 28, e003389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontes-Carvalho, R.; Azevedo, A.I.; Sampaio, F.; Teixeira, M.; Bettencourt, N.; Campos, L.; Roca Concalves, F.R.; Ribeiro, V.G.; Azevedo, A.; Leite–Moreira, A. The effect of exercise training on diastolic and systolic function after acute myocardial infarction: A randomized study. Medicine 2015, 94, e1450. [Google Scholar] [CrossRef]
- Korzeniowska-Kubacka, I.; Bilińska, M.; Michalak, E.; Kuśmierczyk-Droszcz, B.; Dobraszkiewicz-Wasilewska, B.; Piotrowicz, R. Influence of exercise training on left ventricular diastolic function and its relationship to exercise capacity in patients after myocardial infarction. Cardiol. J. 2010, 17, 136–142. [Google Scholar] [PubMed]
- George, K.; Oxborough, D.; Forster, J.; Whyte, G.; Shave, R.; Dawson, E.; Stephenson, C.; Dugdill, L.; Edwards, B.; Gaze, D. Mitral annular myocardial velocity assessment of segmental left ventricular diastolic function after prolonged exercise in humans. J. Physiol. 2005, 569, 305–313. [Google Scholar] [CrossRef] [Green Version]
- Shave, R.; Dawson, E.; Whyte, G.; George, K.; Gaze, D.; Collinson, P. Altered cardiac function and minimal cardiac damage during prolonged exercise. Med. Sci. Sports Exerc. 2004, 36, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.; Ritti-Dias, R.M.; Tinucci, T.; MionJúnior, D.; Forjaz, C.L. Post-concurrent exercise hemodynamics and cardiac autonomic modulation. Eur. J. Appl. Physiol. 2011, 111, 2069–2078. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, R.; Mira, P.A.; Monteiro, W.; Cunha, F.; Laterza, M.C.; Pescatello, L.S.; Martinez, D.G.; Farinatti, P. Hemodynamics and cardiac autonomic modulation after an acute concurrent exercise circuit in older individuals with pre- to established hypertension. Clinics 2021, 76, e1971. [Google Scholar] [CrossRef] [PubMed]
- Tomcazak, C.R.; Thompson, R.B.; Paterson, I.; Schulte, F.; Cheng-Baron, J.; Haenned, R.G.; Haykowsky, M.J. Effect of acute high-intensity interval exercise on post-exercise biventricular function in mild heart failure. J. Appl. Physiol. 2011, 110, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.C.; Queiroz, A.C.C.; Forjaz, C.L.M. Influence of population and exercise protocol characteristics on hemodynamic determinants of post-aerobic exercise hypotension. Braz. J. Med. Biol. Res. 2014, 47, 626–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borovac, J.A.; D’Amario, D.; Bozic, J.; Glavas, D. Sympathetic nervous system activation and heart failure: Current state of evidence and the pathophysiology in the light of novel biomarkers. World J. Cardiol. 2020, 26, 373–408. [Google Scholar] [CrossRef]
- Piña, I.L.; Apstein, C.S.; Balady, G.J.; Belardinelli, R.; Chaitman, B.R.; Duscha, B.D.; Fletcher, B.J.; Fleg, J.L.; Myers, J.N.; Sullivan, M.J. American Heart Association Committee on exercise, rehabilitation, and prevention. Exercise and heart failure: A statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation 2003, 107, 1210–1225. [Google Scholar] [CrossRef]
- Brito, L.C.; Azevêdo, L.; Peçanha, T.; Fecchio, R.Y.; Rezende, R.A.; da Silva, G.V.; Pio-Abreu, A.; Mion, D.; Halliwill, J.R.; Frojaz, C.L.M. Effects of ACEi and ARB on post-exercise hypotension induced by exercises conducted at different times of day in hypertensive men. Clin. Exp. Hypertens. 2020, 42, 722–727. [Google Scholar] [CrossRef]
- Abreu, R.M.; Rehder-Santos, P.; Simões, R.P.; Catai, A.M. Can high-intensity interval training change cardiac autonomic control? A systematic review. Braz. J. Phys. Ther. 2019, 23, 279–289. [Google Scholar] [CrossRef]
- O’Driscoll, J.M.; Wright, S.M.; Taylor, K.A.; Coleman, D.A.; Sharma, R.; Wiles, J.D. Cardiac autonomic and left ventricular mechanics following high intensity interval training: A randomized crossover controlled study. J. Appl. Physiol. 2018, 125, 1030–1040. [Google Scholar] [CrossRef] [Green Version]
- Trevizani, G.A.; Peçanha, T.; Nasario-Junior, O.; Vianna, J.M.; Silva, L.P.; Nadal, J. Cardiac autonomic responses after resistance exercise in treated hypertensive subjects. Front. Physiol. 2015, 6, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishikawa, Y.; Roberts, J.P.; Tan, P.; Klopfenstein, C.E.; Klopfenstein, H.S. Effect of dynamic exercise on left atrial function in conscious dogs. J. Physiol. 1994, 481, 457–468. [Google Scholar] [CrossRef] [Green Version]
- Cuspidi, C.; Tadic, M.; Sala, C.; Gherbesi, E.; Grassi, G.; Mancia, G. Left atrial function in elite athletes: A meta-analysis of two-dimensional speckle tracking echocardiographic studies. Clin. Cardiol. 2019, 42, 579–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugimoto, T.; Bandera, F.; Alfonzetti, E.; Bussadori, C.; Guazzi, M. Left atrial function dynamics during exercise in heart failure pathophysiological implications on the right heart and exercise ventilation inefficiency. JACC Cardiovasc. Imaging 2017, 10, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Deniz Acar, R.; Bulut, M.; Ergün, S.; Yesin, M.; Alıcı, G.; Akçakoyun, M. Effect of cardiac rehabilitation on left atrial functions in patients with acute myocardial infarction. Ann. Phys. Rehabil. Med. 2014, 57, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Sahin, A.A.; Ozben, B.; Sunbul, M.; Sayar, N.; Cincin, A.; Gurel, E.; Tigen, K.; Basaran, Y. The effect of cardiac rehabilitation on blood pressure, and on left atrial and ventricular functions in hypertensive patients. J. Clin. Ultrasound 2021, 49, 456–465. [Google Scholar] [CrossRef]

| Pre-RAE | Post-RAE | Pre-HIIE | Post-HIIE | Controls (T0) | Controls (T1) | Between-Group p | |
|---|---|---|---|---|---|---|---|
| LV Function | |||||||
| HR, bpm | 62.8 ± 13.4 | 67.5 ± 11.2 | 57.2 ± 20.6 | 55.9 ± 19.3 | 60.8 ± 15.2 | 60.4 ± 17.7 | 0.238 |
| EDV, mL | 164.3 ± 37.3 | 154.7 ± 48.5 | 162.6 ± 44.2 | 148.6 ± 39.7 | 160.3 ± 46.2 | 162.3 ± 40.4 | 0.142 |
| ESV, mL | 74.1 ± 18.5 | 73.2 ± 13.9 | 72.9 ± 19.4 | 62.5 ± 17.3 | 74.5 ± 21.2 | 72.5 ± 15.8 | 0.190 |
| SV, mL | 80.1 ± 20.6 | 82.4 ± 26.2 | 81.7 ± 21.5 | 74.1 ± 28.2 | 80.7 ± 17.5 | 79.3 ± 19.4 | 0.117 |
| CO, L/min | 5.3 ± 1.8 | 5.5 ± 1.3 | 5.3 ± 2.5 | 5.1 ± 1.8 | 5.3 ± 2.1 | 5.2 ± 1.4 | 0.092 |
| GLS, % | −15.6 ± 3.7 | −15.0 ± 2.9 | −15.7 ± 3.1 | −15.2 ± 4.4 | −15.5 ± 5.0 | −15.3 ± 3.6 | 0.277 |
| EF, % | 52.4 ± 6.6 | 53.1 ± 8.1 | 51.5 ± 7.8 | 52.1 ± 8.3 | 52.4 ± 6.9 | 51.9 ± 9.0 | 0.314 |
| E, cm/s | 69.0 ± 21.3 | 64.3 ± 24.1 | 69.5 ± 18.7 | 61.8 ± 15.6 | 69 ± 16.0 | 68 ± 17.1 | 0.289 |
| A, cm/s | 68.5 ± 16.8 | 68.3 ± 18.3 | 70.8 ± 19.5 | 71.1 ± 16.2 | 70.6 ± 19.2 | 69.4 ± 21.0 | 0.332 |
| E’, cm/s | 9.1 ± 1.5 | 8.7 ± 2.2 | 9.5 ± 1.9 | 5.5 ± 1.4 * | 9.4 ± 2.0 | 9.3 ± 1.8 | 0.085 |
| E/E’ | 7.5 ± 1.7 | 8.1 ± 2.4 | 7.6 ± 1.1 | 12.2 ± 1.6 * | 7.6 ± 2.2 | 7.8 ± 1.9 | 0.002 |
| LV stiffness | 0.045 ± 0.7 | 0.052 ± 0.9 | 0.046 ± 0.6 | 0.082 ± 0.4 * | 0.047 ± 0.2 | 0.048 ± 0.8 | 0.013 |
| LA Function | |||||||
| PALS, % | 37.8 ± 11.0 | 39.4 ± 6.7 | 35.8 ± 9.3 | 31.2 ± 10.5 | 37.8 ± 12.5 | 37.2 ± 11.3 | 0.032 |
| PACS, % | 18.6 ± 2.1 | 20.9 ± 2.6 | 15.6 ± 2.3 | 15.5 ± 1.8 | 18.6 ± 2.6 | 18.9 ± 2.8 | 0.096 |
| LA stiffness | 0.20 ± 0.08 | 0.22 ± 0.04 | 0.21 ± 0.07 | 0.38 ± 0.06 * | 0.20 ± 0.04 | 0.20 ± 0.05 | 0.083 |
| LAVI, mL/m2 | 32.4 ± 3.6 | 33.0 ± 4.1 | 32.7 ± 4.0 | 33.5 ± 3.6 | 32.0 ± 5.9 | 32.3 ± 4.6 | 0.302 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caminiti, G.; Iellamo, F.; Perrone, M.A.; D’Antoni, V.; Catena, M.; Manzi, V.; Morsella, V.; Franchini, A.; Volterrani, M. Central Hemodynamic Adjustments during Post-Exercise Hypotension in Hypertensive Patients with Ischemic Heart Disease: Concurrent Circuit Exercise versus High-Intensity Interval Exercise. A Preliminary Study. J. Clin. Med. 2021, 10, 5881. https://doi.org/10.3390/jcm10245881
Caminiti G, Iellamo F, Perrone MA, D’Antoni V, Catena M, Manzi V, Morsella V, Franchini A, Volterrani M. Central Hemodynamic Adjustments during Post-Exercise Hypotension in Hypertensive Patients with Ischemic Heart Disease: Concurrent Circuit Exercise versus High-Intensity Interval Exercise. A Preliminary Study. Journal of Clinical Medicine. 2021; 10(24):5881. https://doi.org/10.3390/jcm10245881
Chicago/Turabian StyleCaminiti, Giuseppe, Ferdinando Iellamo, Marco Alfonso Perrone, Valentino D’Antoni, Matteo Catena, Vincenzo Manzi, Valentina Morsella, Alessio Franchini, and Maurizio Volterrani. 2021. "Central Hemodynamic Adjustments during Post-Exercise Hypotension in Hypertensive Patients with Ischemic Heart Disease: Concurrent Circuit Exercise versus High-Intensity Interval Exercise. A Preliminary Study" Journal of Clinical Medicine 10, no. 24: 5881. https://doi.org/10.3390/jcm10245881
APA StyleCaminiti, G., Iellamo, F., Perrone, M. A., D’Antoni, V., Catena, M., Manzi, V., Morsella, V., Franchini, A., & Volterrani, M. (2021). Central Hemodynamic Adjustments during Post-Exercise Hypotension in Hypertensive Patients with Ischemic Heart Disease: Concurrent Circuit Exercise versus High-Intensity Interval Exercise. A Preliminary Study. Journal of Clinical Medicine, 10(24), 5881. https://doi.org/10.3390/jcm10245881









