Left Ventricular Lead Placement Guided by Reduction in QRS Area
Abstract
:1. Introduction
2. Methods
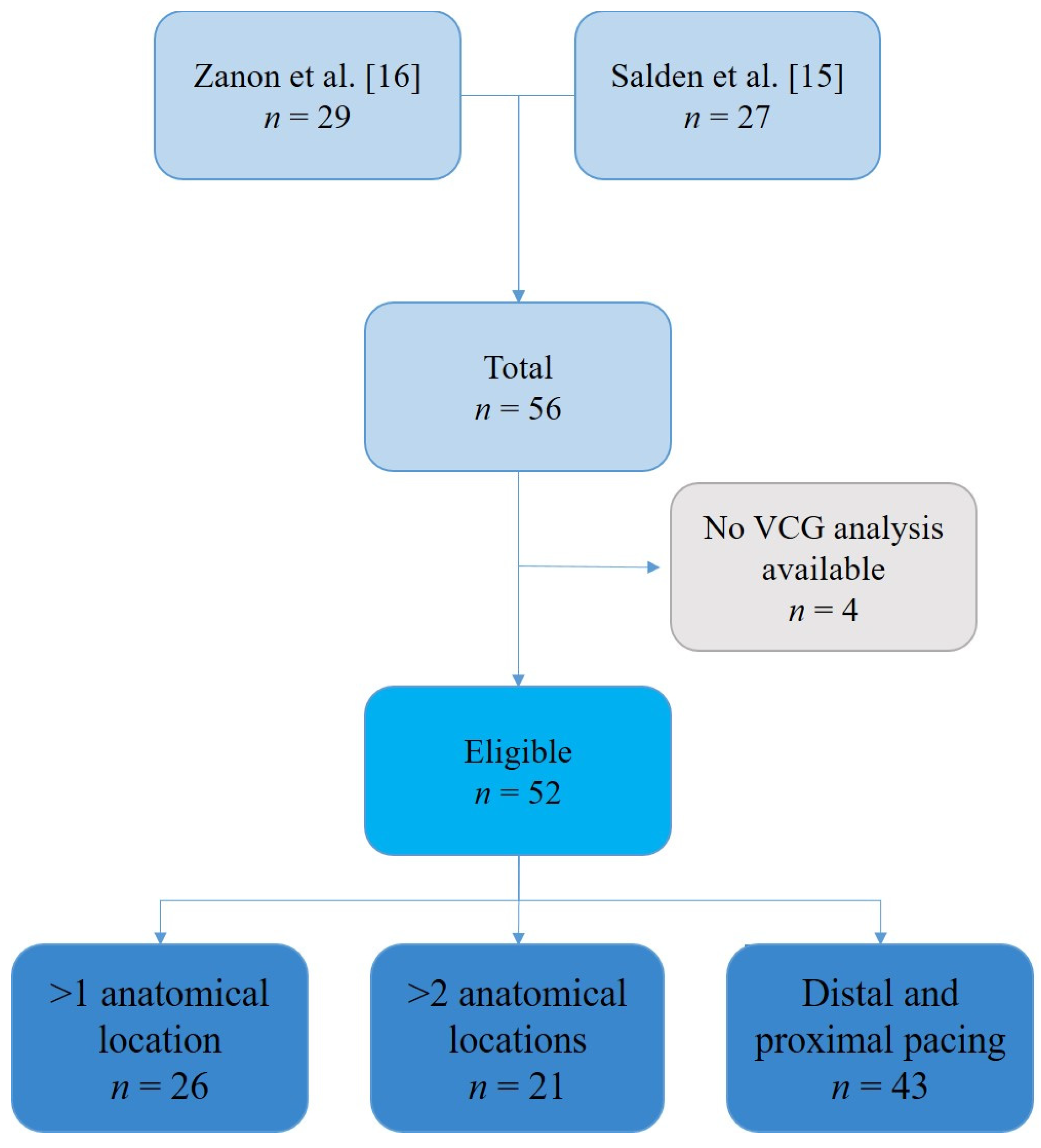
2.1. Study Population
2.2. Study Protocol and Hemodynamic Measurements
2.3. Electrocardiographic Data
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
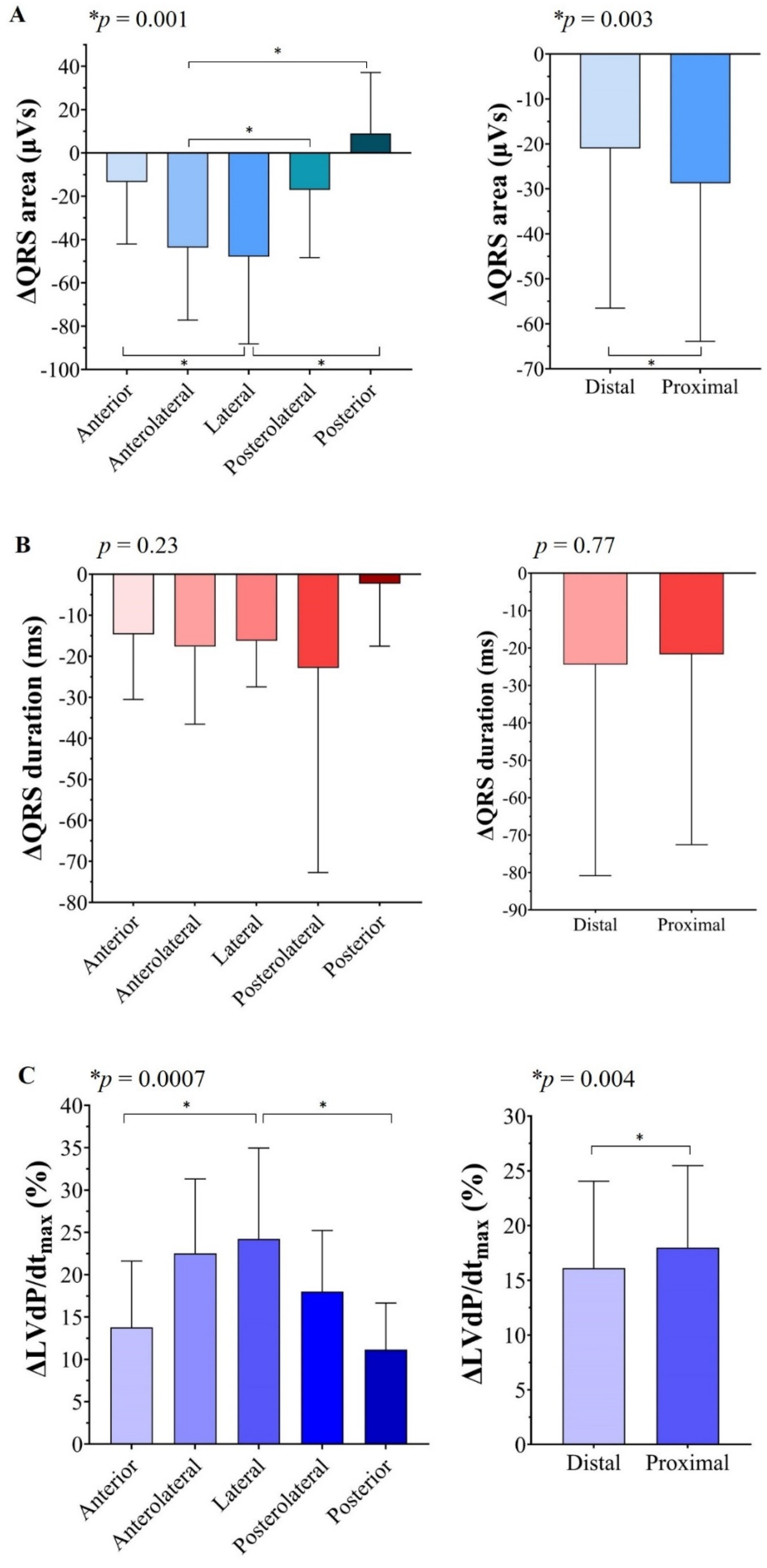
3.2. ∆QRS Area, ∆QRS Duration, and %∆LVdP/dtmax at Various LV Lead Positions
3.3. Relationship between Anatomical LV Lead Position and Largest QRS Area Reduction and Relative LVdP/dtmax Increase
3.4. ∆QRS Area and %∆LVdP/dtmax with Pacing from the Distal and Proximal LV Lead
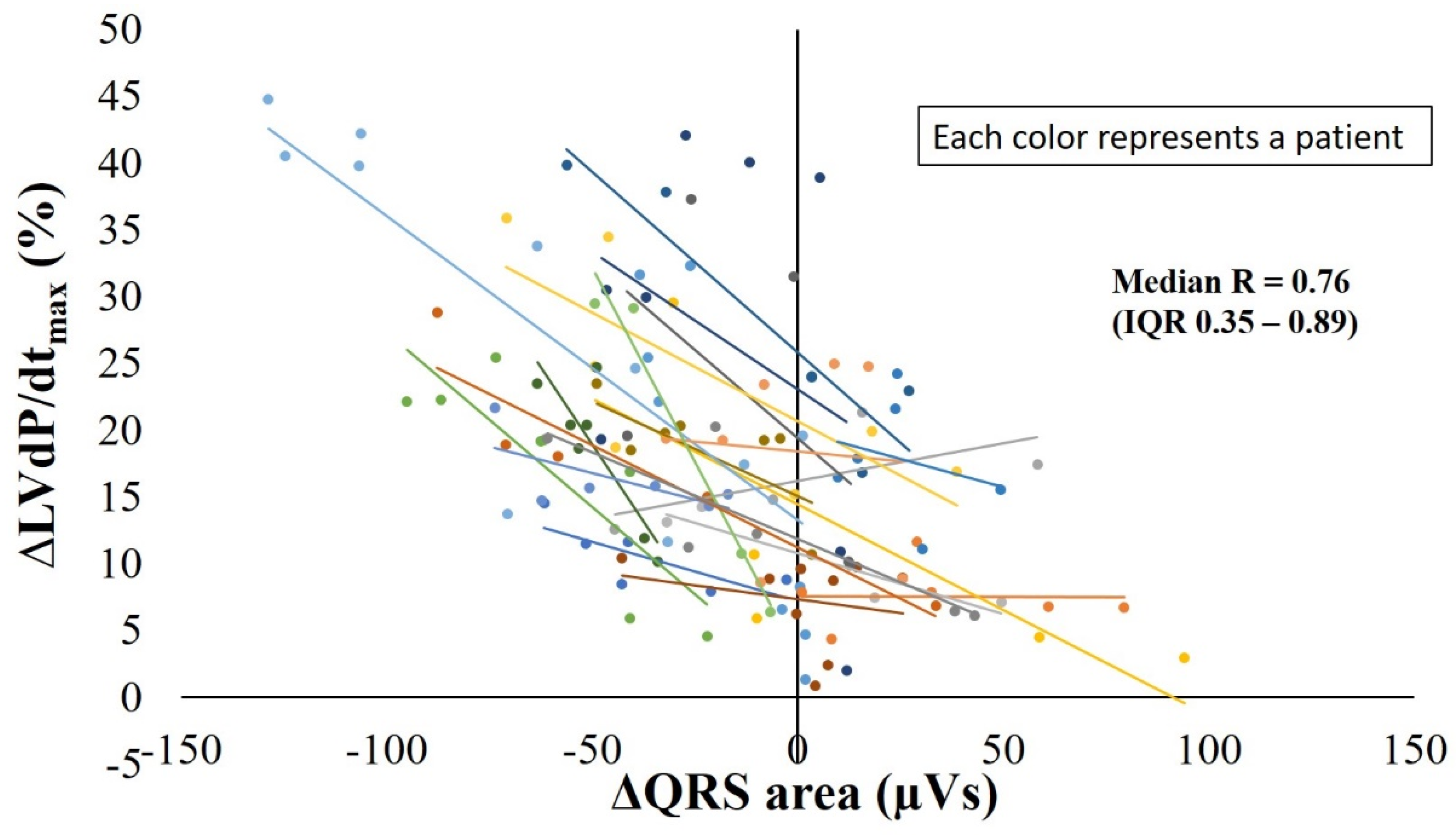
3.5. Relation between ∆QRS Area and ∆LVdP/dtmax
4. Discussion
4.1. Main Findings
4.2. QRS Area Reduction Represents Resynchronization Better Than QRS Duration or Morphology Change
4.3. The Relevance of the LV Lead Position
4.4. Clinical Implications and Future Perspectives
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef] [PubMed]
- Tracy, C.M.; Epstein, A.E.; Darbar, D.; Dimarco, J.P.; Dunbar, S.B.; Estes, N.A.M.; Ferguson, T.B.; Hammill, S.C.; Karasik, P.E.; Link, M.S.; et al. 2012 ACCF/AHA/HRS Focused Update of the 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Heart Rhythm. 2012, 9, 1737–1753. [Google Scholar] [CrossRef] [PubMed]
- Rad, M.M.; Wijntjens, G.W.; Engels, E.B.; Blaauw, Y.; Luermans, J.G.; Pison, L.; Crijns, H.J.; Prinzen, F.W.; Vernooy, K. Vectorcardiographic QRS area identifies delayed left ventricular lateral wall activation determined by electroanatomic mapping in candidates for cardiac resynchronization therapy. Heart Rhythm. 2016, 13, 217–225. [Google Scholar] [CrossRef] [Green Version]
- Maass, A.H.; Vernooy, K.; Cramer, M.J.; Vos, M.A.; Rienstra, M.; Van Gelder, I.C.; MARC Investigators. Refining success of cardiac resynchronization therapy using a simple score predicting the amount of reverse ventricular remodelling: Results from the MARC study—Authors reply. Europace 2018, 20, 393. [Google Scholar] [CrossRef]
- van Stipdonk, A.M.; ter Horst, I.; Kloosterman, M.; Engels, E.B.; Rienstra, M.; Crijns, H.J.; Vos, M.A.; van Gelder, I.C.; Prinzen, F.W.; Meine, M.; et al. QRS Area Is a Strong Determinant of Outcome in Cardiac Resynchronization Therapy. Circ. Arrhythm. Electrophysiol. 2018, 11, e006497. [Google Scholar] [CrossRef] [PubMed]
- Okafor, O.; Zegard, A.; van Dam, P.; Stegemann, B.; Qiu, T.; Marshall, H.; Leyva, F. Changes in QRS Area and QRS Duration After Cardiac Resynchronization Therapy Predict Cardiac Mortality, Heart Failure Hospitalizations, and Ventricular Arrhythmias. J. Am. Heart Assoc. 2019, 8, e013539. [Google Scholar] [CrossRef]
- Emerek, K.; Friedman, D.J.; Sørensen, P.L.; Hansen, S.M.; Larsen, J.M.; Risum, N.; Thøgersen, A.M.; Graff, C.; Kisslo, J.; Søgaard, P.; et al. Vectorcardiographic QRS area is associated with long-term outcome after cardiac resynchronization therapy. Heart Rhythm. 2019, 16, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Ghossein, M.A.; van Stipdonk, A.M.W.; Plesinger, F.; Kloosterman, M.; Wouters, P.C.; Salden, O.A.E.; Meine, M.; Maass, A.H.; Prinzen, F.W.; Vernooy, K. Reduction in the QRS area after cardiac resynchronization therapy is associated with survival and echocardiographic response. J. Cardiovasc. Electrophysiol. 2021, 32, 813–822. [Google Scholar] [CrossRef]
- Leyva, F.; Zegard, A.; Taylor, R.J.; Foley, P.W.X.; Umar, F.; Patel, K.; Panting, J.; van Dam, P.; Prinzen, F.W.; Marshall, H.; et al. Long-Term Outcomes of Cardiac Resynchronization Therapy Using Apical Versus Nonapical Left Ventricular Pacing. J. Am. Heart Assoc. 2018, 7, e008508. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, U.C.; Claridge, S.; Vernooy, K.; Engels, E.B.; Razavi, R.; Rinaldi, C.A.; Chen, Z.; Prinzen, F.W. Relationship between vectorcardiographic QRSarea, myocardial scar quantification, and response to cardiac resynchronization therapy. J. Electrocardiol. 2018, 51, 457–463. [Google Scholar] [CrossRef]
- Varma, N.; Ghanem, R.; Jia, P. Optimization of cardiac resynchronisation therapy: LV Lead position, qLV, or paced effects? Europace 2019, 21, 360. [Google Scholar] [CrossRef] [PubMed]
- van Everdingen, W.M.; Zweerink, A.; Cramer, M.J.; Doevendans, P.A.; Nguyen, U.C.; van Rossum, A.C.; Prinzen, F.W.; Vernooy, K.; Allaart, C.P.; Meine, M. Can We Use the Intrinsic Left Ventricular Delay (QLV) to Optimize the Pacing Configuration for Cardiac Resynchronization Therapy with a Quadripolar Left Ventricular Lead? Circ. Arrhythm. Electrophysiol. 2018, 11, e005912. [Google Scholar] [CrossRef] [PubMed]
- Saba, S.; Marek, J.; Schwartzman, D.; Jain, S.; Adelstein, E.; White, P.; Oyenuga, O.A.; Onishi, T.; Soman, P.; Gorcsan, J., III. Echocardiography-guided left ventricular lead placement for cardiac resynchronization therapy: Results of the Speckle Tracking Assisted Resynchronization Therapy for Electrode Region trial. Circ. Heart Fail. 2013, 6, 427–434. [Google Scholar] [CrossRef] [Green Version]
- Salden, O.A.E.; Broek, H.T.V.D.; van Everdingen, W.; Hoesein, F.A.A.M.; Velthuis, B.K.; Doevendans, P.A.; Cramer, M.-J.; Tuinenburg, A.E.; Leufkens, P.; Van Slochteren, F.J.; et al. Multimodality imaging for real-time image-guided left ventricular lead placement during cardiac resynchronization therapy implantations. Int. J. Cardiovasc. Imaging 2019, 35, 1327–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salden, F.C.; Luermans, J.G.; Westra, S.W.; Weijs, B.; Engels, E.B.; Heckman, L.I.; Lamerichs, L.J.; Janssen, M.H.; Clerx, K.J.; Cornelussen, R.; et al. Short-Term Hemodynamic and Electrophysiological Effects of Cardiac Resynchronization by Left Ventricular Septal Pacing. J. Am. Coll. Cardiol. 2020, 75, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Zanon, F.; Baracca, E.; Pastore, G.; Marcantoni, L.; Fraccaro, C.; Lanza, D.; Picariello, C.; Aggio, S.; Roncon, L.; Dell’Avvocata, F.; et al. Multipoint pacing by a left ventricular quadripolar lead improves the acute hemodynamic response to CRT compared with conventional biventricular pacing at any site. Heart Rhythm. 2015, 12, 975–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanon, F.; Baracca, E.; Pastore, G.; Aggio, S.; Rigatelli, G.; Dondina, C.; Marras, G.; Braggion, G.; Boaretto, G.; Cardaioli, P.; et al. Implantation of left ventricular leads using a telescopic catheter system. Pacing Clin. Electrophysiol. 2006, 29, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Brignole, M.; Auricchio, A.; Baron-Esquivias, G.; Bordachar, P.; Boriani, G.; Breithardt, O.-A.; Cleland, J.G.F.; Deharo, J.-C.; Delgado, V.; Elliott, P.M.; et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: The Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur. Heart J. 2013, 34, 2281–2329. [Google Scholar] [PubMed] [Green Version]
- De Pooter, J.; El Haddad, M.; De Buyzere, M.; Aranda, H.A.; Cornelussen, R.; Stegemann, B.; Rinaldi, C.A.; Sterlinski, M.; Sokal, A.; Francis, D.P.; et al. Biventricular Paced QRS Area Predicts Acute Hemodynamic CRT Response Better Than QRS Duration or QRS Amplitudes. J. Cardiovasc. Electrophysiol. 2017, 28, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Hsing, J.M.; Selzman, K.A.; Leclercq, C.; Pires, L.A.; McLaughlin, M.G.; McRae, S.E.; Peterson, B.J.; Zimetbaum, P.J. Paced left ventricular QRS width and ECG parameters predict outcomes after cardiac resynchronization therapy: PROSPECT-ECG substudy. Circ. Arrhythm. Electrophysiol. 2011, 4, 851–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gold, M.R.; Thebault, C.; Linde, C.; Abraham, W.T.; Gerritse, B.; Ghio, S.; Sutton, M.S.J.; Daubert, J.-C. Effect of QRS duration and morphology on cardiac resynchronization therapy outcomes in mild heart failure: Results from the Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction (REVERSE) study. Circulation 2012, 126, 822–829. [Google Scholar] [CrossRef] [Green Version]
- De Pooter, J.; El Haddad, M.; Timmers, L.; Van Heuverswyn, F.; Jordaens, L.; Duytschaever, M.; Stroobandt, R. Different Methods to Measure QRS Duration in CRT Patients: Impact on the Predictive Value of QRS Duration Parameters. Ann. Noninvasive Electrocardiol. 2016, 21, 305–315. [Google Scholar] [CrossRef] [PubMed]
- De Pooter, J.; El Haddad, M.; Stroobandt, R.; De Buyzere, M.; Timmermans, F. Accuracy of computer-calculated and manual QRS duration assessments: Clinical implications to select candidates for cardiac resynchronization therapy. Int. J. Cardiol. 2017, 236, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.O.; Hellkamp, A.S.; van Bommel, R.J.; Schalij, M.J.; Borleffs, C.J.; Bax, J.J. QRS fusion complex analysis using wave interference to predict reverse remodeling during cardiac resynchronization therapy. Heart Rhythm. 2014, 11, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Butter, C.; Auricchio, A.; Stellbrink, C.; Fleck, E.; Ding, J.; Yu, Y.; Huvelle, E.; Spinelli, J. Effect of resynchronization therapy stimulation site on the systolic function of heart failure patients. Circulation 2001, 104, 3026–3029. [Google Scholar] [CrossRef] [Green Version]
- Macias, A.; Gavira, J.J.; Castano, S.; Alegria, E.; Garcia-Bolao, I. Left ventricular pacing site in cardiac resynchronization therapy: Clinical follow-up and predictors of failed lateral implant. Eur. J. Heart Fail. 2008, 10, 421–427. [Google Scholar] [CrossRef] [Green Version]
- Kronborg, M.B.; Albertsen, A.E.; Nielsen, J.C.; Mortensen, P.T. Long-term clinical outcome and left ventricular lead position in cardiac resynchronization therapy. Europace 2009, 11, 1177–1182. [Google Scholar] [CrossRef]
- Wilton, S.B.; Shibata, M.A.; Sondergaard, R.; Cowan, K.; Semeniuk, L.; Exner, D.V. Relationship between left ventricular lead position using a simple radiographic classification scheme and long-term outcome with resynchronization therapy. J. Interv. Card. Electrophysiol. 2008, 23, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.-X.; Powell, B.D.; Asirvatham, S.J.; Friedman, P.A.; Rea, R.F.; Webster, T.L.; Brooke, K.L.; Hodge, D.O.; Wiste, H.J.; Yang, Y.-Z.; et al. Left ventricular lead position for cardiac resynchronization: A comprehensive cinegraphic, echocardiographic, clinical, and survival analysis. Europace 2012, 14, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Wouters, P.C.; Vernooy, K.; Cramer, M.J.; Prinzen, F.W.; Meine, M. Optimizing lead placement for pacing in dyssynchronous heart failure: The patient in the lead. Heart Rhythm. 2021, 18, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Spartalis, M.; Tzatzaki, E.; Spartalis, E.; Damaskos, C.; Athanasiou, A.; Livanis, E.; Voudris, V. The Role of Echocardiography in the Optimization of Cardiac Resynchronization Therapy: Current Evidence and Future Perspectives. Open Cardiovasc. Med. J. 2017, 11, 133–145. [Google Scholar] [CrossRef] [Green Version]
- Duckett, S.G.; Ginks, M.; Shetty, A.; Bostock, J.; Gill, J.S.; Hamid, S.; Kapetanakis, S.; Cunliffe, E.; Razavi, R.; Carr-White, G.; et al. Invasive acute hemodynamic response to guide left ventricular lead implantation predicts chronic remodeling in patients undergoing cardiac resynchronization therapy. J. Am. Coll. Cardiol. 2011, 58, 1128–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stellbrink, C.; Breithardt, O.-A.; Franke, A.; Sack, S.; Bakker, P.; Auricchio, A.; Pochet, T.; Salo, R.; Kramer, A.; Spinelli, J. Impact of cardiac resynchronization therapy using hemodynamically optimized pacing on left ventricular remodeling in patients with congestive heart failure and ventricular conduction disturbances. J. Am. Coll. Cardiol. 2001, 38, 1957–1965. [Google Scholar] [CrossRef] [Green Version]
- Mullens, W.; Verga, T.; Grimm, R.A.; Starling, R.C.; Wilkoff, B.L.; Tang, W.H.W. Persistent hemodynamic benefits of cardiac resynchronization therapy with disease progression in advanced heart failure. J. Am. Coll. Cardiol. 2009, 53, 600–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogaard, M.D.; Houthuizen, P.; Bracke, F.A.; Doevendans, P.A.; Prinzen, F.W.; Meine, M.; van Gelder, B.M. Baseline left ventricular dP/dtmax rather than the acute improvement in dP/dtmax predicts clinical outcome in patients with cardiac resynchronization therapy. Eur. J. Heart Fail. 2011, 13, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Shimano, M.; Yoshida, Y.; Inden, Y.; Muramatsu, T.; Tsuji, Y.; Tsuboi, N.; Hirayama, H.; Shibata, R.; Murohara, T. Maximum derivative of left ventricular pressure predicts cardiac mortality after cardiac resynchronization therapy. Clin. Cardiol. 2010, 33, E18–E23. [Google Scholar] [CrossRef] [PubMed]
- Prinzen, F.W.; Auricchio, A. The “missing” link between acute hemodynamic effect and clinical response. J. Cardiovasc. Transl. Res. 2012, 5, 188–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| All Patients (n = 52) | |
|---|---|
| Age (y) | 70 ± 9 |
| Male (n, %) | 41 (79) |
| NYHA | |
| II (n, %) | 28 (54) |
| III (n, %) | 24 (46) |
| Ischemic CMP (n, %) | 26 (50) |
| Baseline LVEF (%) | 29 ± 9 |
| Sinus rhythm (n, %) | 37 (71) |
| QRS duration (ms) | 169 ± 18 |
| LBBB (n, %) | 35 (67) |
| IVCD (n, %) | 6 (12) |
| RBBB (n, %) | 4 (8) |
| Upgrade from RV pacing (n, %) | 7 (13) |
| QRS area (µVs) | 108 ± 44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghossein, M.A.; Zanon, F.; Salden, F.; van Stipdonk, A.; Marcantoni, L.; Engels, E.; Luermans, J.; Westra, S.; Prinzen, F.; Vernooy, K. Left Ventricular Lead Placement Guided by Reduction in QRS Area. J. Clin. Med. 2021, 10, 5935. https://doi.org/10.3390/jcm10245935
Ghossein MA, Zanon F, Salden F, van Stipdonk A, Marcantoni L, Engels E, Luermans J, Westra S, Prinzen F, Vernooy K. Left Ventricular Lead Placement Guided by Reduction in QRS Area. Journal of Clinical Medicine. 2021; 10(24):5935. https://doi.org/10.3390/jcm10245935
Chicago/Turabian StyleGhossein, Mohammed Ali, Francesco Zanon, Floor Salden, Antonius van Stipdonk, Lina Marcantoni, Elien Engels, Justin Luermans, Sjoerd Westra, Frits Prinzen, and Kevin Vernooy. 2021. "Left Ventricular Lead Placement Guided by Reduction in QRS Area" Journal of Clinical Medicine 10, no. 24: 5935. https://doi.org/10.3390/jcm10245935






