Reference Ranges of Left Ventricular Hemodynamic Forces in Healthy Adults: A Speckle-Tracking Echocardiographic Study
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Conventional Echocardiography
2.3. Speckle-Tracking Analysis
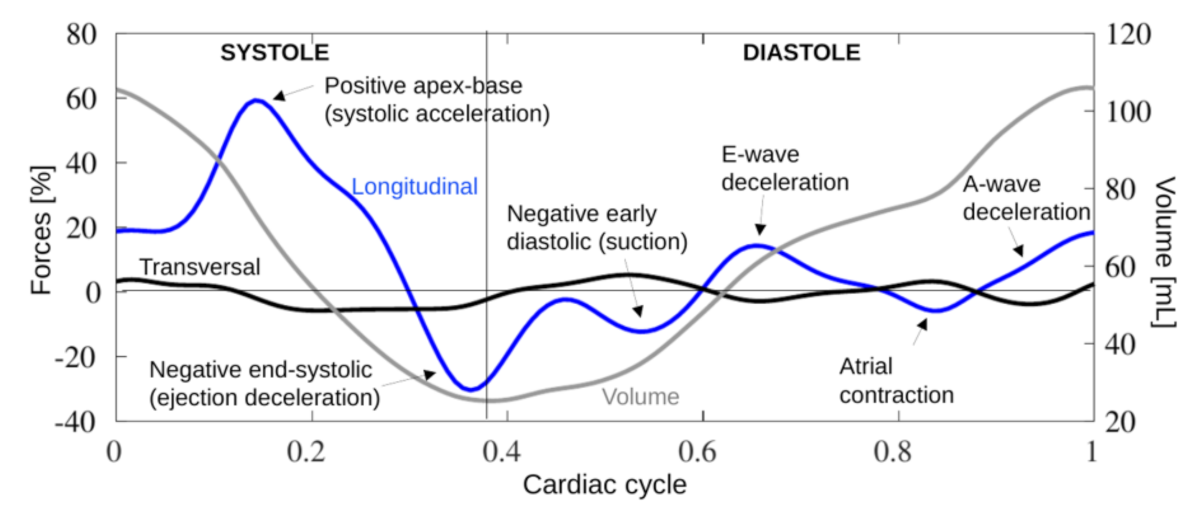
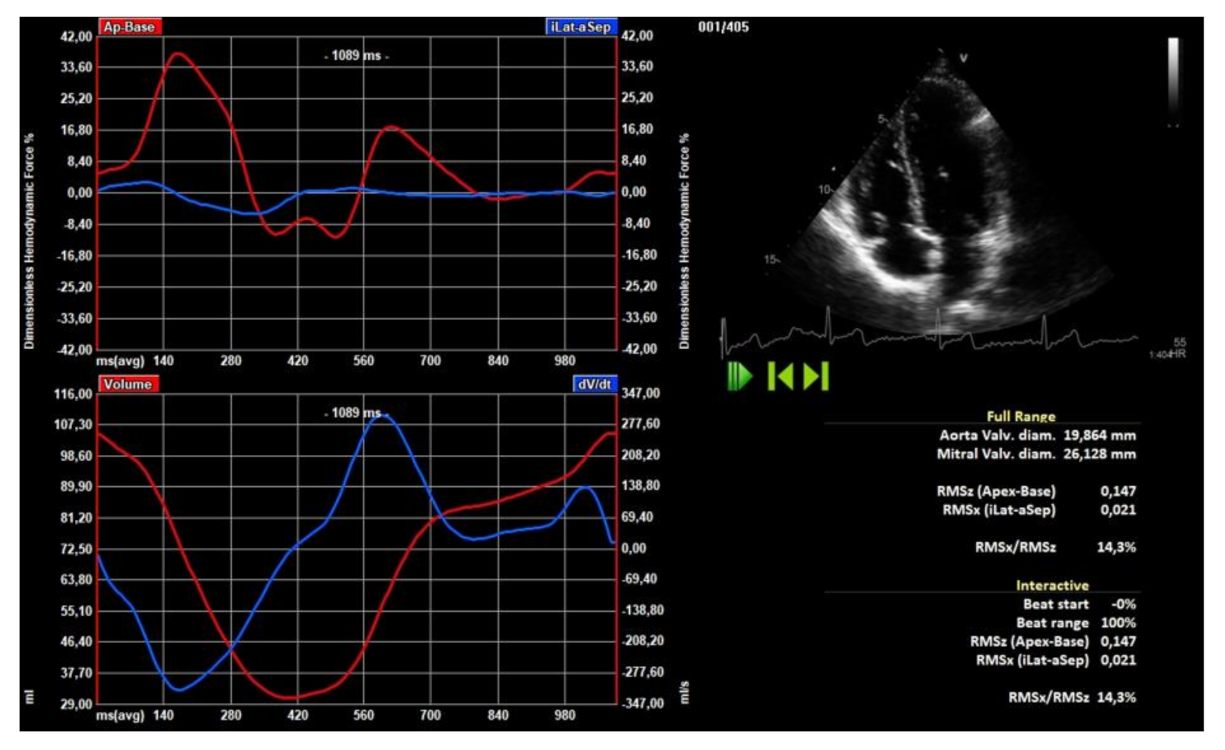
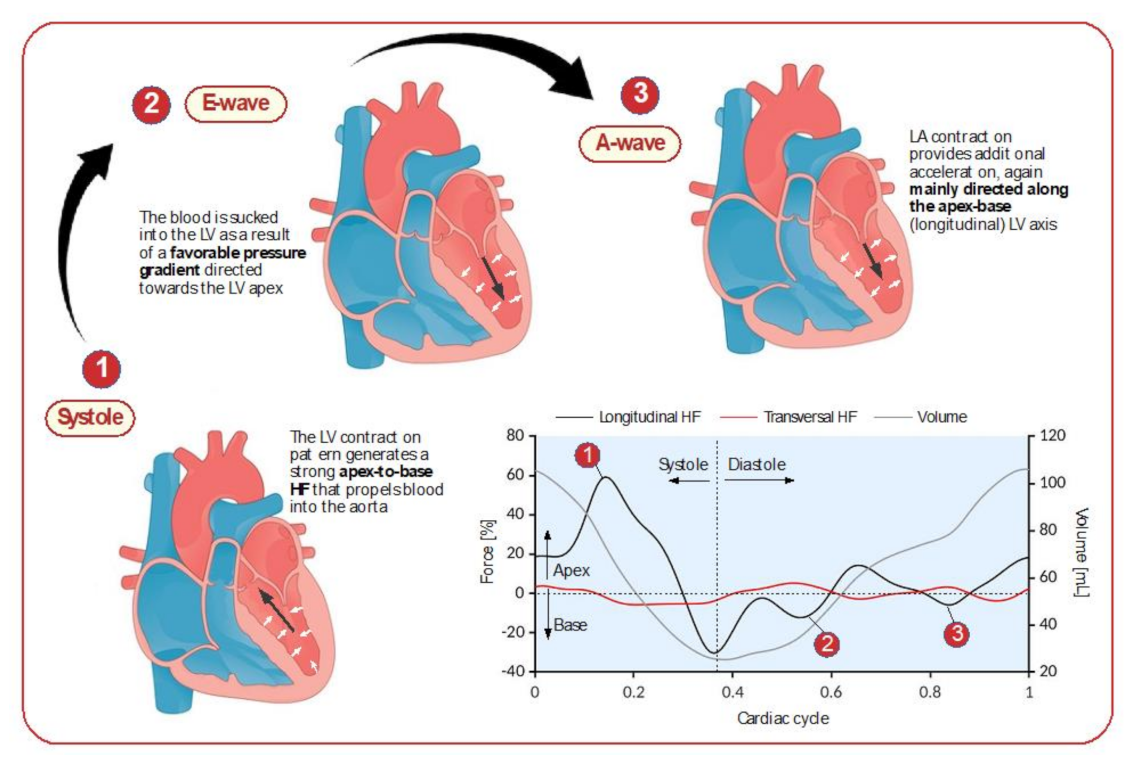
2.4. Hemodynamic Forces
2.5. Statistical Analysis
3. Results
3.1. Demographic Data
3.2. Left Heart Echo-Doppler Analysis
3.3. Right Heart Echo-Doppler Analysis
3.4. LV Deformation
3.5. LV Hemodynamic Forces
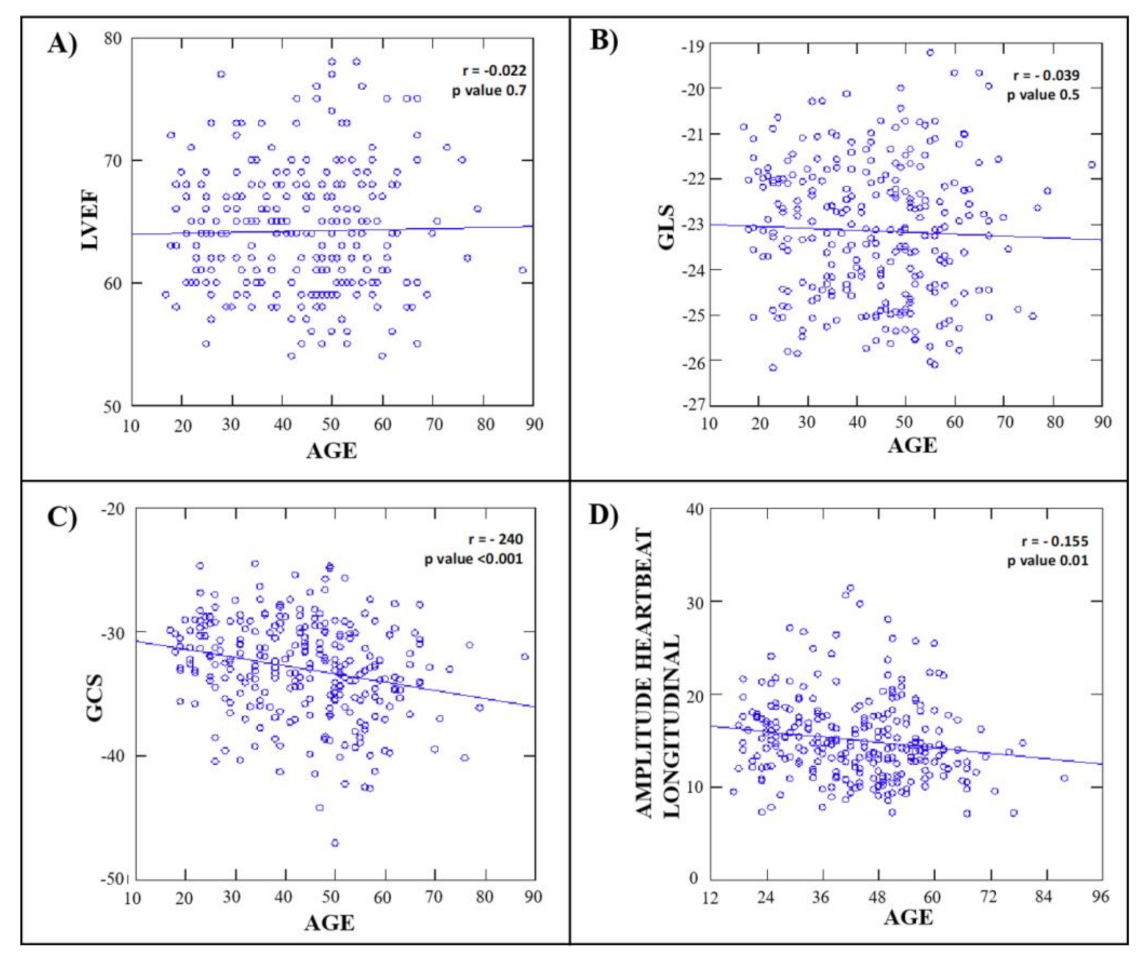
3.6. Clinical and Echocardiographic Correlates of LV Hemodynamic Force
3.7. Inter and Intra-Observer Variability
4. Discussion
4.1. Previous Studies
4.2. Uniqueness of the Present Study
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., III; Fleisher, L.A.; Jneid, H.; Mack, M.J.; McLeod, C.J.; O’Gara, P.T.; et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017, 135, e1159–e1195. [Google Scholar] [CrossRef]
- Arvidsson, P.M.; Töger, J.; Carlsson, M.; Steding-Ehrenborg, K.; Pedrizzetti, G.; Heiberg, E.; Arheden, H. Left and right ventricular hemodynamic forces in healthy volunteers and elite athletes assessed with 4D flow magnetic resonance imaging. Am. J. Physiol. Circ. Physiol. 2017, 312, H314–H328. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Badano, L.P. Definitions for a common standard for 2Dspeckle tracking echocardiography: Consensus document of the EACVI/ASE/industry task force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 28, 183–193. [Google Scholar]
- Mignot, A.; Donal, E.; Zaroui, A.; Reant, P.; Salem, A.; Hamon, C.; Monzy, S.; Roudaut, R.; Habib, G.; Lafitte, S. Global Longitudinal Strain as a Major Predictor of Cardiac Events in Patients with Depressed Left Ventricular Function: A Multicenter Study. J. Am. Soc. Echocardiogr. 2010, 23, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Ersbøll, M.; Valeur, N.; Mogensen, U.M.; Andersen, M.J.; Møller, J.E.; Velazquez, E.J.; Hassager, C.; Søgaard, P.; Køber, L. Prediction of All-Cause Mortality and Heart Failure Admissions from Global Left Ventricular Longitudinal Strain in Patients with Acute Myocardial Infarction and Preserved Left Ventricular Ejection Fraction. J. Am. Coll. Cardiol. 2013, 61, 2365–2373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedrizzetti, G.; Claus, P.; Kilner, P.J.; Nagel, E. Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use. J. Cardiovasc. Magn. Reson. 2016, 18, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maier, S.E.; Fischer, S.E.; McKinnon, G.C.; Hess, O.M.; Krayenbuehl, H.P.; Boesiger, P. Evaluation of left ventricular segmental wall motion in hypertrophic cardiomyopathy with myocardial tagging. Circulation 1992, 86, 1919–1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeon, S.B.; Reichek, N.; Tallant, B.A.; Lima, J.A.; Calhoun, L.P.; Clark, N.R.; Hoffman, E.; Ho, K.; Axel, L. Validation of in vivo myocardial strain measurement by magnetic resonance tagging with sonomicrometry. J. Am. Coll. Cardiol. 2001, 38, 555–561. [Google Scholar] [CrossRef] [Green Version]
- Courtois, M.; Kovácsjr, S.J.; Ludbrook, P.A. Transmitral pressure-flow velocity relation. Importance of regional pressure gradients in the left ventricle during diastole. Circulation 1988, 78, 661–671. [Google Scholar] [CrossRef] [Green Version]
- Firstenberg, M.S.; Vandervoort, P.M.; Greenberg, N.L.; Smedira, N.G.; McCarthy, P.M.; Garcia, M.J.; Thomas, J.D. Noninvasive estimation of transmitral pressure drop across the normal mitral valve in humans: Importance of convective and inertial forces during left ventricular filling. J. Am. Coll. Cardiol. 2000, 36, 1942–1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenberg, N.L.; Vandervoort, P.M.; Firstenberg, M.S.; Garcia, M.J.; Thomas, J.D. Estimation of diastolic intraventricular pressure gradients by Doppler M-mode echocardiography. Am. J. Physiol. Circ. Physiol. 2001, 280, H2507–H2515. [Google Scholar] [CrossRef]
- Guerra, M.; Silva, C.B.; Amorim, M.J.; Moura, C.; Bastos, P.; Leite-Moreira, A.F. Intraventricular Pressure Gradients in Heart Failure. Physiol. Res. 2013, 62, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Domenichini, F.; Pedrizzetti, G. Hemodynamic forces in a model left ventricle. Phys. Rev. Fluids 2016, 1, 083201. [Google Scholar] [CrossRef]
- Pedrizzetti, G.; Martiniello, A.R.; Bianchi, V.; D’Onofrio, A.; Caso, P.; Tonti, G. Cardiac fluid dynamics anticipates heart adaptation. J. Biomech. 2015, 48, 388–391. [Google Scholar] [CrossRef] [Green Version]
- Arvidsson, P.M.; Töger, J.; Pedrizzetti, G.; Heiberg, E.; Borgquist, R.; Carlsson, M.; Arheden, H. Hemodynamic forces using four-dimensional flow MRI: An independent biomarker of cardiac function in heart failure with left ventricular dyssynchrony? Am. J. Physiol. Circ. Physiol. 2018, 315, H1627–H1639. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.; Bolger, A.F.; Ebbers, T.; Carlhäll, C. Assessment of left ventricular hemodynamic forces in healthy subjects and patients with dilated cardiomyopathy using 4D flow MRI. Physiol. Rep. 2016, 4, e12685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedrizzetti, G.; Martiniello, A.R.; Bianchi, V.; D’Onofrio, A.; Caso, P.; Tonti, G. Changes in electrical activation modify the orientation of left ventricular flow momentum: Novel observations using echocardiographic particle image velocimetry. Eur. Hear. J. Cardiovasc. Imaging 2015, 17, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Lapinskas, T.; Pedrizzetti, G.; Stoiber, L.; Düngen, H.-D.; Edelmann, F.; Pieske, B.; Kelle, S. The Intraventricular Hemodynamic Forces Estimated Using Routine CMR Cine Images. JACC Cardiovasc. Imaging 2019, 12, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Ferro, M.D.; De Paris, V.; Collia, D.; Stolfo, D.; Caiffa, T.; Barbati, G.; Korcova, R.; Pinamonti, B.; Zovatto, L.; Zecchin, M.; et al. Left Ventricular Response to Cardiac Resynchronization Therapy: Insights from Hemodynamic Forces Computed by Speckle Tracking. Front. Cardiovasc. Med. 2019, 6, 59. [Google Scholar] [CrossRef]
- Pedrizzetti, G.; Arvidsson, P.M.; Töger, J.; Borgquist, R.; Domenichini, F.; Arheden, H.; Heiberg, E. On estimating intraventricular hemodynamic forces from endocardial dynamics: A comparative study with 4D flow MRI. J. Biomech. 2017, 60, 203–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedrizzetti, G.; Tanacli, R.; Lapinskas, T.; Zovatto, L.; Pieske, B.; Tonti, G.; Kelle, S. Integration between volumetric change and strain for describing the global mechanical function of the left ventricle. Med. Eng. Phys. 2019, 74, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Rudski, L.G.; Vriz, O.; Gargani, L.; Afilalo, J.; D’Andrea, A.; D’Alto, M.; Marra, A.M.; Acri, E.; Stanziola, A.A.; et al. Physiologic correlates of tricuspid annular plane systolic excursion in 1168 healthy subjects. Int. J. Cardiol. 2016, 223, 736–743. [Google Scholar] [CrossRef]
- Ferrara, F.; Gargani, L.; Ruohonen, S.; Vriz, O.; Scalese, M.; Russo, V.; Marra, A.M.; Stanziola, A.A.; Cittadini, A.; D’Andrea, A.; et al. Reference values and correlates of right atrial volume in healthy adults by two-dimensional echocardiography. Echocardiography 2018, 35, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., III; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography: Endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [CrossRef] [PubMed]
- Mor-Avi, V.; Lang, R.M.; Badano, L.P.; Belohlavek, M.; Cardim, N.M.; Derumeaux, G.; Galderisi, M.; Marwick, T.; Nagueh, S.F.; Sengupta, P.P.; et al. Current and Evolving Echocardiographic Techniques for the Quantitative Evaluation of Cardiac Mechanics: ASE/EAE Consensus Statement on Methodology and Indications endorsed by the Japanese Society of Echocardiography. J. Am. Soc. Echocardiogr. 2011, 24, 277–313. [Google Scholar] [CrossRef] [PubMed]
- Rushmer, R.F.; Harding, D.; Baker, D.; Watson, N. Initial Ventricular Impulse. Circulation 1964, 29, 268–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasipoularides, A.; Murgo, J.P.; Miller, J.W.; Craig, W.E. Nonobstructive left ventricular ejection pressure gradients in man. Circ. Res. 1987, 61, 220–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brutsaert, D.L. Nonuniformity: A physiologic modulator of contraction and relaxation of the normal heart. J. Am. Coll. Cardiol. 1987, 9, 341–348. [Google Scholar] [CrossRef]
- Butler, C.K.; Wong, A.Y.K.; Armour, J.A. Systolic pressure gradients between the wall of the left ventricle, the left ventricular chamber, and the aorta during positive inotropic states: Implications for left ventricular efficiency. Can. J. Physiol. Pharmacol. 1988, 66, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Courtois, M.; Kovács, S.J.; Ludbrook, P.A. Physiological early diastolic intraventricular pressure gradient is lost during acute myocardial ischemia. Circulation 1990, 81, 1688–1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerra, M.; Amorim, M.J.; Brás-Silva, C.; Leite-Moreira, A.F. Intraventricular pressure gradients throughout the cardiac cycle: Effects of ischaemia and modulation by afterload. Exp. Physiol. 2013, 98, 149–160. [Google Scholar] [CrossRef]
- Smiseth, O.A.; Steine, K.; Sandbæk, G.; Stugaard, M.; Gjølberg, T.Ø. Mechanics of intraventricular filling: Study of LV early diastolic pressure gradients and flow velocities. Am. J. Physiol. Circ. Physiol. 1998, 275, H1062–H1069. [Google Scholar] [CrossRef]
- Eriksson, J.; Zajac, J.; Alehagen, U.; Bolger, A.F.; Ebbers, T.; Carlhäll, C.-J. Left ventricular hemodynamic forces as a marker of mechanical dyssynchrony in heart failure patients with left bundle branch block. Sci. Rep. 2017, 7, 2971. [Google Scholar] [CrossRef] [PubMed]
- Leiner, T.; Bogaert, J.; Friedrich, M.G.; Mohiaddin, R.; Muthurangu, V.; Myerson, S.; Powell, A.J.; Raman, S.V.; Pennell, D.J. SCMR Position Paper (2020) on clinical indications for cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2020, 22, 1–37. [Google Scholar] [CrossRef]
- Ferro, M.D.; Stolfo, D.; De Paris, V.; Lesizza, P.; Korcova, R.; Collia, D.; Tonti, G.; Sinagra, G.; Pedrizzetti, G. Cardiac fluid dynamics meets deformation imaging. Cardiovasc. Ultrasound 2018, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- Faganello, G.; Collia, D.; Furlotti, S.; Pagura, L.; Zaccari, M.; Pedrizzetti, G.; Di Lenarda, A. A new integrated approach to cardiac mechanics: Reference values for normal left ventricle. Int. J. Cardiovasc. Imaging 2020, 36, 2173–2185. [Google Scholar] [CrossRef] [PubMed]
- Muraru, D.; Cucchini, U.; Mihăilă, S.; Miglioranza, M.H.; Aruta, P.; Cavalli, G.; Cecchetto, A.; Padayattil-Josè, S.; Peluso, D.; Iliceto, S.; et al. Left Ventricular Myocardial Strain by Three-Dimensional Speckle-Tracking Echocardiography in Healthy Subjects: Reference Values and Analysis of Their Physiologic and Technical Determinants. J. Am. Soc. Echocardiogr. 2014, 27, 858–871.e1. [Google Scholar] [CrossRef] [PubMed]
- Alcidi, G.M.; Esposito, R.; Evola, V.; Santoro, C.; Lembo, M.; Sorrentino, R.; Iudice, F.L.; Borgia, F.; Novo, G.; Trimarco, B.; et al. Normal reference values of multilayer longitudinal strain according to age decades in a healthy population: A single-centre experience. Eur. Hear. J. Cardiovasc. Imaging 2017, 19, 1390–1396. [Google Scholar] [CrossRef]
- Marwick, T.H.; Leano, R.L.; Brown, J.; Sun, J.-P.; Hoffmann, R.; Lysyansky, P.; Becker, M.; Thomas, J.D. Myocardial Strain Measurement With 2-Dimensional Speckle-Tracking Echocardiography: Definition of normal range. JACC Cardiovasc. Imaging 2009, 2, 80–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugimoto, T.; Dulgheru, R.; Bernard, C.; Ilardi, F.; Contu, L.; Addetia, K.; Caballero, L.; Akhaladze, N.; Athanassopoulos, G.D.; Barone, D.; et al. Echocardiographic reference ranges for normal left ventricular 2D strain: Results from the EACVI NORRE study. Eur. Hear. J. Cardiovasc. Imaging 2017, 18, 833–840. [Google Scholar] [CrossRef]
- Töger, J.; Arvidsson, P.M.; Bock, J.; Kanski, M.; Pedrizzetti, G.; Carlsson, M.; Arheden, H.; Heiberg, E. Hemodynamic forces in the left and right ventricles of the human heart using 4D flow magnetic resonance imaging: Phantom validation, reproducibility, sensitivity to respiratory gating and free analysis software. PLoS ONE 2018, 13, e0195597. [Google Scholar] [CrossRef] [PubMed]
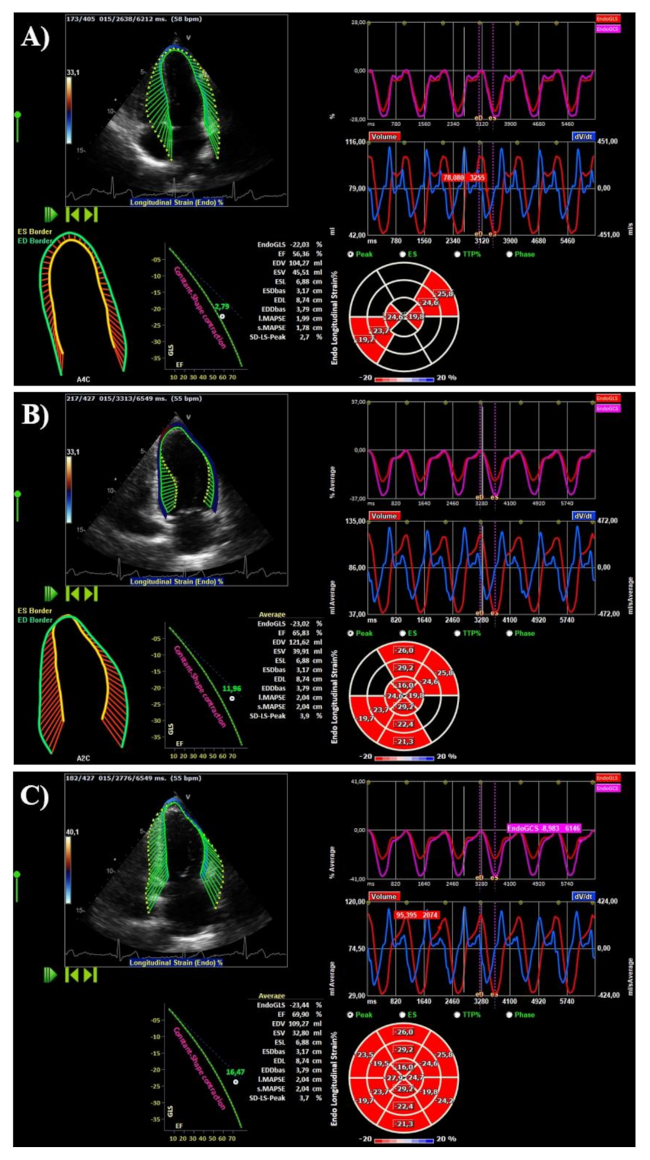
| Variable | Overall | Women | Men | p Value |
|---|---|---|---|---|
| (n = 269) | (n = 146) | (n = 123) | ||
| (Mean ± SD) | (Mean ± SD) | (Mean ± SD) | ||
| Age (years) | 43.4 ± 14.0 | 43.7 ± 13.9 | 43.1 ± 14.1 | 0.7 |
| Height (cm) | 168 ± 9 | 162 ± 7 | 175 ± 7 | <0.001 |
| Weight (kg) | 70 ± 12 | 63 ± 9 | 78 ± 10 | <0.001 |
| BMI (kg/m2) | 24.6 ± 3.1 | 24.0 ± 3.1 | 25.4 ± 2.9 | <0.001 |
| BSA (m2) | 1.78 ± 0.19 | 1.67 ± 0.14 | 1.91 ± 0.16 | <0.001 |
| Systolic BP (mmHg) | 122 ± 12 | 120 ± 13 | 124 ± 11 | <0.001 |
| Diastolic BP (mmHg) | 77 ± 8 | 75 ± 9 | 78 ± 8 | 0.007 |
| Mean BP (mmHg) | 92 ± 9 | 90 ± 9 | 93 ± 8 | 0.002 |
| Pulse pressure (mmHg) | 45 ± 10 | 44 ± 10 | 45 ± 9 | 0.167 |
| HR (b/m) | 73 ± 12 | 74 ± 12 | 71 ± 12 | 0.018 |
| Variable | Overall | Women | Men | p Value |
|---|---|---|---|---|
| (n = 269) | (n = 146) | (n = 123) | ||
| (Mean ± SD) | (Mean ± SD) | (Mean ± SD) | ||
| Septal wall thickness in diastole (mm) | 8.9 ± 1.4 | 8.3 ± 1.3 | 9.6 ± 1.2 | <0.001 |
| Inferolateral wall thickness (mm) diastole | 8.9 ± 1.4 | 8.4 ± 1.5 | 9.6 ± 1.3 | <0.001 |
| LV end-diastolic diameter (mm) | 45.0 ± 4.0 | 43.4 ± 3.6 | 46.9 ± 3.7 | <0.001 |
| Proximal ascending aorta (mm) | 28.9 ± 3.1 | 27.8 ± 2.9 | 30.3 ± 2.8 | <0.001 |
| LV mass/BSA (g/m2) | 85.6 ± 20.2 | 77.5 ± 17.4 | 95.4 ± 19.1 | <0.001 |
| LA Volume (mL) | 32.5 ± 7.1 | 31.0 ± 6.7 | 34.4 ± 7.0 | <0.001 |
| LV EDV (mL) | 99 ± 21 | 88 ± 15 | 113 ± 21 | <0.001 |
| LV ESV (mL) | 37 ± 10 | 32 ± 8 | 42 ± 9 | <0.001 |
| LV EF (biplane) (%) | 64.2 ± 5 | 63.7 ± 4.4 | 64.9 ± 5.6 | 0.049 |
| Mitral Peak E/e’ ratio | 5.6 ± 1.5 | 5.7 ± 1.5 | 5.5 ± 1.4 | 0.319 |
| SV (mL) | 64 ± 13 | 60.0 ± 11.6 | 68.5 ± 12.6 | <0.001 |
| CO (L/min) | 4.6 ± 1.1 | 4.4 ± 1.0 | 4.8 ± 1.1 | 0.005 |
| Variable | Overall | Women | Men | p Value |
|---|---|---|---|---|
| (n = 269) | (n = 146) | (n = 123) | ||
| (Mean ± SD) | (Mean ± SD) | (Mean ± SD) | ||
| RV basal diameter (mm) | 34.6 ± 2.9 | 33.5 ± 2.8 | 35.8 ± 2.4 | <0.001 |
| RV longitudinal diameter (mm) | 62.7 ± 6.1 | 60.6 ± 6.2 | 65.2 ± 6.1 | <0.001 |
| RA Volume (mL) | 28.8 ± 7.9 | 26.0 ± 5.8 | 32.1 ± 8.8 | <0.001 |
| TAPSE (mm) | 22.7 ± 2.4 | 22.7 ± 2.4 | 22.7 ± 2.4 | 0.916 |
| S′(cm/s) | 13.5 ± 2.2 | 13.4 ± 2.0 | 13.7 ± 2.3 | 0.251 |
| PASP (mmHg) | 21.8 ± 4.8 | 21.5 ± 4.4 | 22.3 ± 5.3 | 0.170 |
| RVOT AcT (ms) | 136.9 ± 18.3 | 139.8 ± 18.2 | 134.5 ± 18.2 | 0.049 |
| Variable | Overall | Women | Men | p Value |
|---|---|---|---|---|
| (n = 269) | (n = 146) | (n = 123) | ||
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| (95% CI) | (95% CI) | (95% CI) | ||
| GLS, % | −23.1 ± 1.5 (−20.1 to −26.1) | −23.1 ± 1.5 (−20.1 to −26.1) | −23.1 ± 1.5 (−20.1 to −26.1) | 1.0 |
| GCS, % | −33.0 ± 3.9 (−25.3 to −40.1) | −33.1 ± 3.9 (−25.4 to −40.8) | −32.8 ± 3.8 (−25.3 to −40.3) | 0.4 |
| Variable | Overall | Women | Men | p Value |
|---|---|---|---|---|
| (n = 269) | (n = 146) | (n = 123) | ||
| (Mean ± SD) | (Mean ± SD) | (Mean ± SD) | ||
| (95% CI) | (95% CI) | (95% CI) | ||
| Longitudinal force, FL | ||||
| Whole cycle, % | 15.0 ± 4.4 (6.3 to 23.7) | 14.6 ± 4.1 (6.5 to 22.7) | 15.6 ± 4.7 (6.3 to 24.9) | 0.06 |
| Systolic impulse, % | 19.8 ± 6.1 (7.8 to 31.8) | 18.9 ± 5.6 (7.9 to 29.9) | 20.8 ± 6.5 (8.0 to 33.6) | 0.009 |
| Systolic, % | 20.8 ± 6.2 (8.6 to 33.0) | 19.8 ± 5.6 (8.8 to 30.8) | 22.0 ± 6.7 (8.8 to 35.2) | 0.004 |
| Diastolic, % | 8.1 ± 2.7 (2.8 to 13.4) | 8.1 ± 2.8 (2.6 to 13.6) | 8.1 ± 2.6 (3.0 to 13.2) | 0.9 |
| Transversal force, FT | ||||
| Whole cycle, % | 2.4 ± 0.9 (0.6 to 4.2) | 2.2 ± 0.8 (0.6 to 3.8) | 2.6 ± 1.0 (0.6 to 4.6) | 0.004 |
| Systolic, % | 2.7 ± 1.0 (0.7 to 4.7) | 2.5 ± 0.9 (0.7 to 4.3) | 2.9 ± 1.1 (0.7 to 5.1) | <0.001 |
| Diastolic, % | 2.1 ± 1.1 (0.0 to 3.3) | 2.0 ± 1.0 (0.0 to 4.0) | 2.2 ± 1.1 (0.0 to 4.4) | 0.1 |
| Alignment angle, ° | 14.0 ± 3.6 (6.9 to 21.1) | 13.5 ± 3.3 (7.0 to 20.0) | 14.7 ± 3.3 (8.2 to 21.2) | 0.004 |
| Variables Related with Amplitude Heart-Beat Longitudinal | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| r | 95% CI | p Value | Std Coefficient (β) | p Value | |
| Gender | −0.114 | −0.230 to 0.006 | 0.6 | ||
| Age (years) | −0.155 | −0.270 to −0.036 | 0.01 | −0.232 | <0.001 |
| BSA (m2) | 0.139 | 0.019 to 0.254 | 0.02 | 0.149 | 0.003 |
| Pulse pressure (mmHg) | 0.206 | 0.089 to 0.319 | 0.001 | 0.186 | <0.001 |
| Heart rate (bpm) | 0.448 | 0.346 to 0.539 | <0.001 | 0.396 | <0.001 |
| LV mass (gr) | 0.076 | −0.044 to 0.195 | 0.21 | ||
| LA vol (mL) | 0.011 | −0.109 to 0.131 | 0.86 | ||
| LV EF (%) | 0.120 | 0.000 to 0.237 | 0.05 | ||
| LV SV (mL) | −0.095 | −0.213 to 0.025 | 0.121 | ||
| E/e’ | −0.119 | −0.044 to 0.195 | 0.052 | ||
| GLS | −0.153 | −0.268 to −0.034 | 0.01 | −0.056 | 0.266 |
| GCS | −0.254 | −0.390 to −0.170 | <0.001 | −0.328 | <0.001 |
| Variables | ICC | 95% Confidence Interval | p Value | |
|---|---|---|---|---|
| Lower Bound | Upper Bound | |||
| Intra-observer variability | ||||
| GLS | 0.98 | 0.96 | 0.99 | <0.01 |
| GCS | 0.98 | 0.95 | 0.99 | <0.01 |
| Amplitude Heart Beat Longitudinal | 0.98 | 0.95 | 0.99 | <0.01 |
| Inter-observer variability | ||||
| GLS | 0.98 | 0.94 | 0.99 | <0.01 |
| GCS | 0.98 | 0.94 | 0.99 | <0.01 |
| Amplitude Heart Beat Longitudinal | 0.97 | 0.93 | 0.98 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrara, F.; Capuano, F.; Cocchia, R.; Ranieri, B.; Contaldi, C.; Lacava, G.; Capone, V.; Chianese, S.; Rega, S.; Annunziata, R.; et al. Reference Ranges of Left Ventricular Hemodynamic Forces in Healthy Adults: A Speckle-Tracking Echocardiographic Study. J. Clin. Med. 2021, 10, 5937. https://doi.org/10.3390/jcm10245937
Ferrara F, Capuano F, Cocchia R, Ranieri B, Contaldi C, Lacava G, Capone V, Chianese S, Rega S, Annunziata R, et al. Reference Ranges of Left Ventricular Hemodynamic Forces in Healthy Adults: A Speckle-Tracking Echocardiographic Study. Journal of Clinical Medicine. 2021; 10(24):5937. https://doi.org/10.3390/jcm10245937
Chicago/Turabian StyleFerrara, Francesco, Francesco Capuano, Rosangela Cocchia, Brigida Ranieri, Carla Contaldi, Graziella Lacava, Valentina Capone, Salvatore Chianese, Salvatore Rega, Roberto Annunziata, and et al. 2021. "Reference Ranges of Left Ventricular Hemodynamic Forces in Healthy Adults: A Speckle-Tracking Echocardiographic Study" Journal of Clinical Medicine 10, no. 24: 5937. https://doi.org/10.3390/jcm10245937
APA StyleFerrara, F., Capuano, F., Cocchia, R., Ranieri, B., Contaldi, C., Lacava, G., Capone, V., Chianese, S., Rega, S., Annunziata, R., Sepe, C., Salzano, A., Citro, R., D’Andrea, A., Mauro, C., Cademartiri, F., Pedrizzetti, G., & Bossone, E. (2021). Reference Ranges of Left Ventricular Hemodynamic Forces in Healthy Adults: A Speckle-Tracking Echocardiographic Study. Journal of Clinical Medicine, 10(24), 5937. https://doi.org/10.3390/jcm10245937










