Systemic Treatment of Immune-Mediated Keratoconjunctivitis Sicca with Allogeneic Stem Cells Improves the Schirmer Tear Test Score in a Canine Spontaneous Model of Disease
Abstract
:1. Introduction
2. Materials and Methods
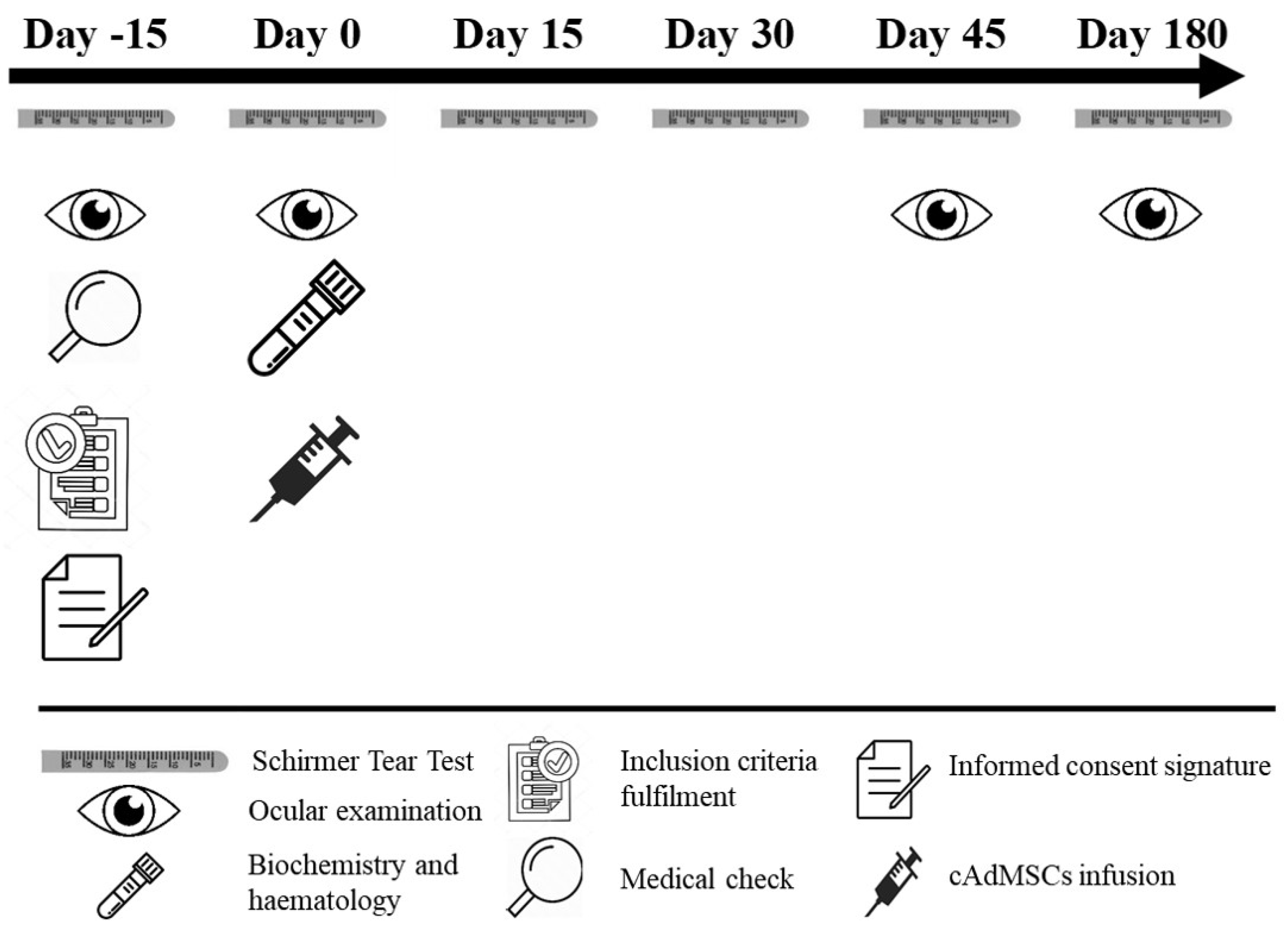
2.1. Study Design
2.2. Isolation and Culture of cAdMSCs
2.3. cATMSCs Characterization by Flow Cytometry Analysis and In Vitro Multilineage Cell Differentiation
2.4. Inclusion Criteria
2.5. Prohibited and Allowed Medications and Therapies for Experimental Group
2.6. Clinical Evaluation
2.7. cATMSCs Administration
2.8. Statistical Methods
3. Results
3.1. Characterization of Allogeneic cATMSCs by Flow Cytometry
3.2. Systemic cATMSCs Administration
3.3. Clinical Evaluation and Efficacy Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Kaswan, R.L.; Salisbury, M.A.; Ward, D.A. Spontaneous canine keratoconjunctivitis sicca. A useful model for human keratoconjunctivitis sicca: Treatment with cyclosporine eye drops. Arch. Ophthalmol. 1989, 107, 1210–1216. [Google Scholar] [CrossRef]
- Gelatt, K.N.; Peiffer, R.L., Jr.; Erickson, J.L.; Gum, G.G. Evaluation of tear formation in the dog, using a modification of the Schirmer tear test. J. Am. Vet. Med. Assoc. 1975, 166, 368–370. [Google Scholar]
- Williams, D.L. Immunopathogenesis of Keratoconjunctivitis Sicca in the Dog. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 251–268. [Google Scholar] [CrossRef]
- Kaswan, R.L.; Martin, C.L.; Dawe, D.L. Keratoconjunctivitis sicca: Immunological evaluation of 62 canine cases. Am. J. Vet. Res. 1985, 46, 376–383. [Google Scholar] [PubMed]
- Kaswan, R.L.; Martin, C.L.; Chapman, W.L. Keratoconjunctivitis sicca: Histopathologic study of nictitating membrane and lacrimal glands from 28 dogs. Am. J. Vet. Res. 1984, 45, 112–118. [Google Scholar]
- Gelatt, K. Essentials of Veterinary Ophthalmology, 3rd ed.; Gelatt, K.N., Ed.; John Wiley & Sons, Ltd: Oxford, UK, 2014. [Google Scholar]
- Roszkowska, A.M.; Oliverio, G.W.; Aragona, E.; Inferrera, L.; Severo, A.A.; Alessandrello, F.; Spinella, R.; Postorino, E.I.; Aragona, P. Ophthalmologic Manifestations of Primary Sjögren’s Syndrome. Genes 2021, 12, 365. [Google Scholar] [CrossRef] [PubMed]
- Izci, C.; Celik, I.; Alkan, F.; Erol, M.; Sur, E. Clinical and light microscopic studies of the conjunctival tissues of dogs with bilateral keratoconjunctivitis sicca before and after treatment with topical 2% cyclosporine. Biotech. Histochem. 2015, 90, 223–230. [Google Scholar] [CrossRef]
- Gilger, B.C.; Wilkie, D.A.; Salmon, J.H.; Peel, M.R. A topical aqueous calcineurin inhibitor for the treatment of naturally occurring keratoconjunctivitis sicca in dogs. Vet. Ophthalmol. 2013, 16, 192–197. [Google Scholar] [CrossRef]
- Sanchez, R.F.; Innocent, G.; Mould, J.; Billson, F.M. Canine keratoconjunctivitis sicca: Disease trends in a review of 229 cases. J. Small Anim. Pract. 2007, 48, 211–217. [Google Scholar] [CrossRef]
- Su, Y.; Yang, C. Keratoconjunctivitis Sicca in Sjögren’s Syndrome. N. Engl. J. Med. 2020, 383, 1663. [Google Scholar] [CrossRef] [PubMed]
- Colligris, B.; Alkozi, H.A.; Pintor, J. Recent developments on dry eye disease treatment compounds. Saudi J. Ophthalmol. 2014, 28, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Radziejewski, K.; Balicki, I. Comparative clinical evaluation of tacrolimus and cyclosporine eye drops for the treatment of canine keratoconjunctivitis sicca. Acta Vet. Hung 2016, 64, 313–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berdoulay, A.; English, R.V.; Nadelstein, B. Effect of topical 0.02% tacrolimus aqueous suspension on tear production in dogs with keratoconjunctivitis sicca. Vet. Ophthalmol. 2005, 8, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Nell, B.; Walde, I.; Billich, A.; Vit, P.; Meingassner, J.G. The effect of topical pimecrolimus on keratoconjunctivitis sicca and chronic superficial keratitis in dogs: Results from an exploratory study. Vet. Ophthalmol. 2005, 8, 39–46. [Google Scholar] [CrossRef]
- Dodi, P. Immune-mediated keratoconjunctivitis sicca in dogs: Current perspectives on management. Vet. Med. Res. Rep. 2015, 6, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera, H.D.; Weichsler, N.; Gómez, J.R.; De Jalón, J.A.G. Severe, unilateral, unresponsive keratoconjunctivitis sicca in 16 juvenile Yorkshire Terriers. Vet. Ophthalmol. 2007, 10, 285–288. [Google Scholar] [CrossRef]
- Murphy, M.B.; Moncivais, K.; Caplan, A.I. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013, 45, e54. [Google Scholar] [CrossRef] [Green Version]
- Kapur, S.K.; Katz, A.J. Review of the adipose derived stem cell secretome. Biochimie 2013, 95, 2222–2228. [Google Scholar] [CrossRef]
- Carrade, D.D.; Borjesson, D.L. Immunomodulation by mesenchymal stem cells in veterinary species. Comp. Med. 2013, 63, 207–217. [Google Scholar]
- Corcione, A.; Benvenuto, F.; Ferretti, E.; Giunti, D.; Cappiello, V.; Cazzanti, F.; Risso, M.; Gualandi, F.; Mancardi, G.L.; Pistoia, V.; et al. Human mesenchymal stem cells modulate B-cell functions. Blood 2006, 107, 367–372. [Google Scholar] [CrossRef] [Green Version]
- Tyndall, A.; Uccelli, A. Multipotent mesenchymal stromal cells for autoimmune diseases: Teaching new dogs old tricks. Bone Marrow. Transpl. 2009, 43, 821–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, S. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef] [Green Version]
- Villatoro, A.J.; Fernández, V.; Claros, S.; Rico-Llanos, G.A.; Becerra, J.; Andrades, J.A. Use of Adipose-Derived Mesenchymal Stem Cells in Keratoconjunctivitis Sicca in a Canine Model. Biomed. Res. Int. 2015, 2015, 527926. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, M.K.W.; Barros, M.A.; Martins, J.F.P.; Vasconcellos, J.P.C.; Morais, B.P.; Pompeia, C.; Bittencourt, M.D.; Santos Evangelho, K.D.; Kerkis, I.; Wenceslau, C.V.; et al. Allogeneic Mesenchymal Stem Cell Transplantation in Dogs with Keratoconjunctivitis Sicca. Cell Med. 2016, 8, 63–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.W.; Kang, K.S.; Koo, H.C.; Park, J.R.; Choi, E.W.; Park, Y.H. Soluble Factors–Mediated Immunomodulatory Effects of Canine Adipose Tissue–Derived Mesenchymal Stem Cells. Stem Cells Dev. 2008, 17, 681–694. [Google Scholar] [CrossRef] [Green Version]
- Abughanam, G.; Elkashty, O.A.; Liu, Y.; Bakkar, M.O.; Tran, S.D. Mesenchymal Stem Cells Extract (MSCsE)-Based Therapy Alleviates Xerostomia and Keratoconjunctivitis Sicca in Sjogren’s Syndrome-Like Disease. Int. J. Mol. Sci. 2019, 20, 4750. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, W.; Chauhan, S.K.; Dana, R. Dry Eye Disease. Arch. Ophthalmol. 2012, 130, 90. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Merino, E.M.; Usón-Casaús, J.M.; Zaragoza-Bayle, C.; Duque-Carrasco, J.; Mariñas-Pardo, L.; Hermida-Prieto, M.; Barrera-Chacón, R.; Gualtieri, M. Safety and efficacy of allogeneic adipose tissue-derived mesenchymal stem cells for treatment of dogs with inflammatory bowel disease: Endoscopic and histological outcomes. Vet. J. 2015, 206, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Merino, E.M.; Usón-Casaús, J.M.; Zaragoza-Bayle, C.; Duque-Carrasco, J.; Mariñas-Pardo, L.; Hermida-Prieto, M.; Barrera-Chacón, R.; Gualtieri, M. Safety and efficacy of allogeneic adipose tissue-derived mesenchymal stem cells for treatment of dogs with inflammatory bowel disease: Clinical and laboratory outcomes. Vet. J. 2015, 206, 385–390. [Google Scholar] [CrossRef]
- Zeira, O.; Asiag, N.; Aralla, M.; Ghezzi, E.; Pettinari, L.; Martinelli, L.; Zahirpour, D.; Dumas, M.P.; Lupi, D.; Scaccia, S.; et al. Adult autologous mesenchymal stem cells for the treatment of suspected non-infectious inflammatory diseases of the canine central nervous system: Safety, feasibility and preliminary clinical findings. J. Neuroinflam. 2015, 12, 181. [Google Scholar]
- Hamor, R.E.; Roberts, S.M.; Severin, G.A.; Chavkin, M.J. Evaluation of results for Schirmer tear tests conducted with and without application of a topical anesthetic in clinically normal dogs of 5 breeds. Am. J. Vet. Res. 2000, 61, 1422–1425. [Google Scholar] [CrossRef]
- Johnson, V.; Webb, T.; Norman, A.; Coy, J.; Kurihara, J.; Regan, D.; Dow, S. Activated Mesenchymal Stem Cells Interact with Antibiotics and Host Innate Immune Responses to Control Chronic Bacterial Infections. Sci. Rep. 2017, 7, 9575. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A.; Reilly, C.M.; Wood, J.A.; Carrade, D.D.; Deremer, S.L.; Seraphin, R.L.; Seraphin, R.L.; Clark, K.C.; Zwingenberger, A.L.; Borjesson, D.L.; et al. Safety and immunomodulatory effects of allogeneic canine adipose-derived mesenchymal stromal cells transplanted into the region of the lacrimal gland, the gland of the third eyelid and the knee joint. Cytotherapy 2013, 15, 1498–1510. [Google Scholar] [CrossRef]
- You, S.; Kublin, C.L.; Avidan, O.; Miyasaki, D.; Zoukhri, D. Isolation and propagation of mesenchymal stem cells from the lacrimal gland. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2087–2094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massingale, M.L.; Li, X.; Vallabhajosyula, M.; Chen, D.; Wei, Y.; Asbell, P.A. Analysis of Inflammatory Cytokines in the Tears of Dry Eye Patients. Cornea 2009, 28, 1023–1027. [Google Scholar] [CrossRef]
- Lu, L.; Li, Y.; Du, M.J.; Zhang, C.; Zhang, X.Y.; Tong, H.Z.; Liu, L.; Han, W.; Li, W.; Yan, L.; et al. Characterization of a Self-renewing and Multi-potent Cell Population Isolated from Human Minor Salivary Glands. Sci. Rep. 2015, 5, 10106. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Zhang, X. Lacrimal gland development: From signaling interactions to regenerative medicine. Dev. Dyn. 2017, 246, 970–980. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Shin, J.M.; Yeum, C.E.; Chae, G.T.; Chun, M.H.; Oh, S.J. Intravitreal delivery of mesenchymal stem cells loaded onto hydrogel affects the regulatory expression of endogenous NGF and BDNF in ischemic rat retina. Tissue Eng. Regen. Med. 2012, 9, 249–258. [Google Scholar] [CrossRef]
- Li, B.; Xing, Y.; Gan, Y.; He, J.; Hua, H. Labial gland-derived mesenchymal stem cells and their exosomes ameliorate murine Sjögren’s syndrome by modulating the balance of Treg and Th17 cells. Stem Cell Res. 2021, 12, 478. [Google Scholar] [CrossRef]
- Hendrix, D.V.; Adkins, E.A.; Ward, D.A.; Stuffle, J.; Skorobohach, B. An Investigation Comparing the Efficacy of Topical Ocular Application of Tacrolimus and Cyclosporine in Dogs. Vet. Med. Int. 2011, 2011, 487592. [Google Scholar] [CrossRef] [Green Version]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef] [Green Version]
- Gazdic, M.; Volarevic, V.; Arsenijevic, N.; Stojkovic, M. Mesenchymal Stem Cells: A Friend or Foe in Immune-Mediated Diseases. Stem Cell Rev. Rep. 2015, 11, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Harman, R.; Carlson, K.; Gaynor, J.; Gustafson, S.; Dhupa, S.; Clement, K.; Hoelzler, M.; McCarthy, T.; Schwartz, P.; Adams, C.; et al. A Prospective, Randomized, Masked, and Placebo-Controlled Efficacy Study of Intraarticular Allogeneic Adipose Stem Cells for the Treatment of Osteoarthritis in Dogs. Front. Vet. Sci. 2016, 3, 81. [Google Scholar] [CrossRef] [PubMed]
- Klinker, M.W. Mesenchymal stem cells in the treatment of inflammatory and autoimmune diseases in experimental animal models. World J. Stem Cells 2015, 7, 556. [Google Scholar] [CrossRef] [PubMed]
- Ohman, L.; Edqvist, L.E.; Johansson, E.D. Absorption of topically applied hydrocortisone from the eye of the rhesus monkey. Acta Ophthalmol. 1982, 60, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Glaze, M.B.; Crawford, M.A.; Nachreiner, R.F.; Casey, H.W.; Nafe, L.A.; Kearney, M.T. Ophthalmic corticosteroid therapy: Systemic effects in the dog. J. Am. Vet. Med. Assoc. 1988, 192, 73–75. [Google Scholar] [PubMed]
- Holmberg, B.J.; Maggs, D.J. The use of corticosteroids to treat ocular inflammation. Vet. Clin. N. Am. Small Anim. Pract. 2004, 34, 693–705. [Google Scholar] [CrossRef]
- Williams, D. Canine Keratoconjunctivitis Sicca: Current Concepts in Diagnosis and Treatment. J. Clin. Ophthalmol. Optom. 2018, 2, 1. [Google Scholar]

| Dog Characteristics | cAdMSCs (n = 14) | Control (n = 14) | Mann-Whitney U Test p Value | Chi-Square p Value |
|---|---|---|---|---|
| Age (Years; mean ± SD) | 7.00 ± 1.88 | 6.71 ± 1.90 | 0.701 | |
| Weight (Kg; mean ± SD) | 10.69 ± 7.31 | 10.32 ± 6.18 | 0.635 | |
| KCS history (Years; mean ± SD) | 2.36 ± 1.28 | 2.23 ± 1.17 | 0.734 | |
| Sex (n (%)) | 1.000 | |||
| Male | 5 (35.70) | 9 (64.30) | ||
| Female | 9 (64.30) | 5 (35.70) | ||
| Breed (n (%)) | 0.766 | |||
| British bulldog | 1 (7.10) | - | ||
| Cocker spaniel | - | 1 (7.14) | ||
| Crossbreed | 3 (21.50) | 2 (14.29) | ||
| French bulldog | 1 (7.10) | 3 (21.43) | ||
| Golden | - | 1 (7.14) | ||
| Ponter | - | 1 (7.14) | ||
| Pug | 1 (7.10) | 1 (7.14) | ||
| Schnauzer | 1 (7.10) | - | ||
| Shih-Tzu | 3 (21.50) | 2 (14.29) | ||
| Weim | 1 (7.10) | - | ||
| West Highland White Terrier | 1 (7.10) | 1 (7.14) | ||
| Yorkshire Terrier | 2 (14.40) | 2 (14.29) |
| Day 0 (Visit 1) n = 21 | Day 45 (Visit 4) n = 21 | Day 180 (Visit 5) n = 10 | |
|---|---|---|---|
| Eye characteristics (mean ± SD) (range 0–10) | |||
| Corneal vascularization | 2.05 ± 2.82 | 2.14 ± 2.97 | 2.10 ± 2.56 |
| Overlying epithelium | 1.48 ± 2.87 | 1.48 ± 2.87 | 1.30 ± 2.21 |
| Palpebral closing limitation | 1.00 ± 2.43 | 1.00 ± 2.43 | 0.50 ± 0.97 |
| Subepithelial oedema | 2.52 ± 3.67 | 2.67 ± 3.86 | 2.70 ± 2.91 |
| Abrasion/Ulcers | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Blepharospasm | 1.24 ± 1.95 | 1.00 ± 2.39 | 0.00 ± 0.00 |
| Pigmentary keratitis | 2.38 ± 3.53 | 2.24 ± 3.48 | 2.40 ± 2.80 |
| Keratohelcosis | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Ocular surface damage (mean ± SD) (range 0–3) | |||
| Nasal conjunctiva surface damage | 1.14 ± 1.11 | 0.67 ± 1.02 | 0.30 ± 0.48 |
| Temporal conjunctiva surface damage | 1.00 ± 1.14 | 0.48 ± 0.93 | 0.30 ± 0.48 |
| Cornea surface damage | 1.38 ± 1.40 | 1.14 ± 1.31 | 1.10 ± 1.29 |
| Total ocular surface damage | 3.52 ± 2.79 | 2.29 ± 2.85 | 1.70 ± 1.83 |
| Conjunctivitis type (n (%)) | n = 20 | n = 17 | n = 10 |
| Mucoid | 13 (65.00) | 7 (41.20) | 2 (20.00) |
| Muco-purulent | 4 (20.00) | 2 (11.80) | 0 (0.00) |
| Transparent serous | 1 (5.00) | 8 (47.00) | 6 (60.00) |
| Serous | 0 (0.00) | 0 (0.00) | 2 (20.00) |
| Purulent | 2 (10.00) | 0 (0.00) | 0 (0.00) |
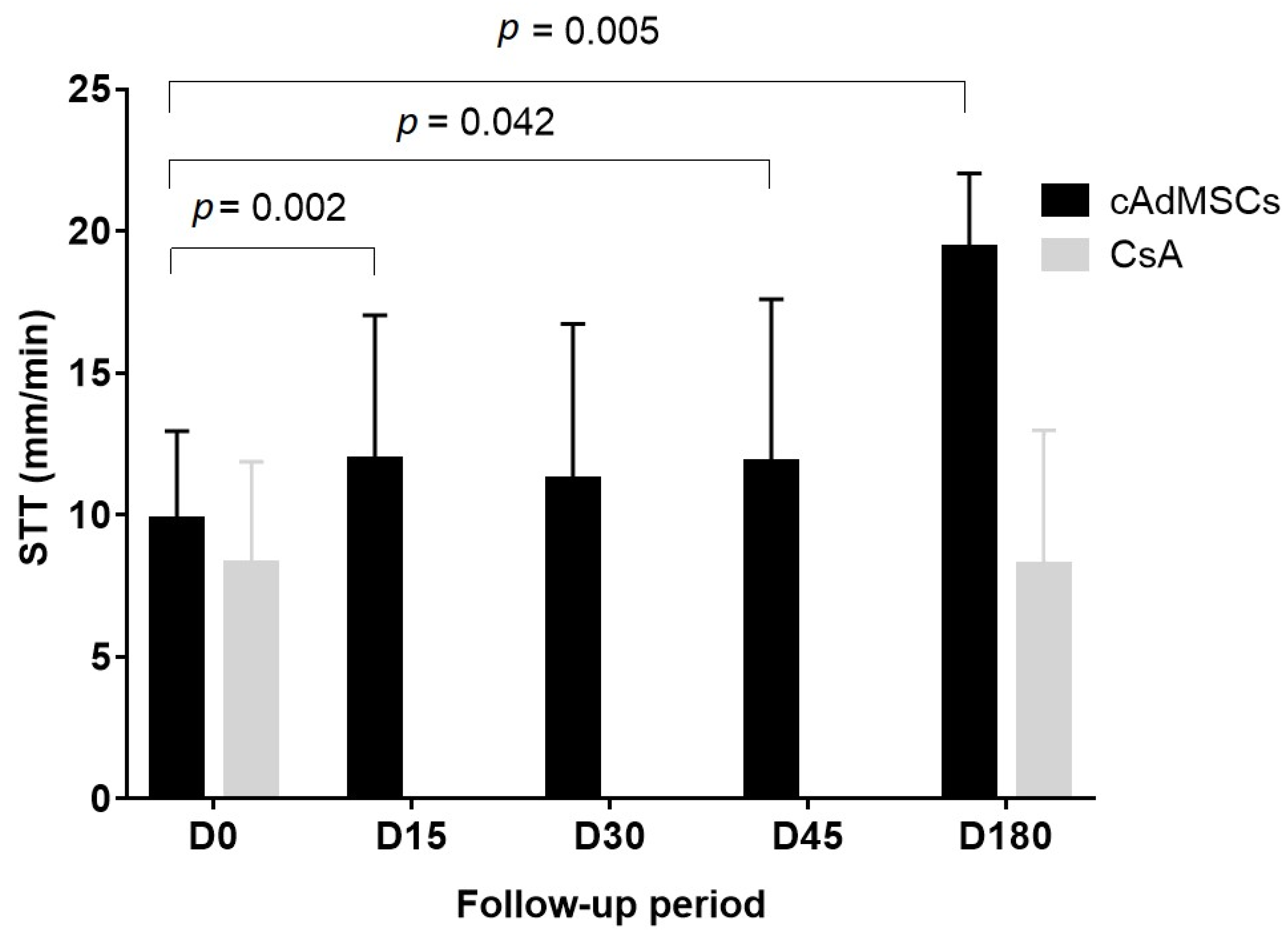
| Day 0 n = 21 | Day 15 n = 21 | Day 30 n = 21 | Day 45 n = 21 | Day 180 n = 10 | |
|---|---|---|---|---|---|
| STT (Mean ±SD) | 9.71 ± 3.34 | 11.95 ± 5.08 | 11.24 ± 5.50 | 11.86 ± 5.74 | 19.40 ± 2.63 |
| Wilcoxon signed-rank test (vs. Day 0) | p = 0.002 | p = 0.294 | p = 0.042 | p = 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hermida-Prieto, M.; García-Castro, J.; Mariñas-Pardo, L. Systemic Treatment of Immune-Mediated Keratoconjunctivitis Sicca with Allogeneic Stem Cells Improves the Schirmer Tear Test Score in a Canine Spontaneous Model of Disease. J. Clin. Med. 2021, 10, 5981. https://doi.org/10.3390/jcm10245981
Hermida-Prieto M, García-Castro J, Mariñas-Pardo L. Systemic Treatment of Immune-Mediated Keratoconjunctivitis Sicca with Allogeneic Stem Cells Improves the Schirmer Tear Test Score in a Canine Spontaneous Model of Disease. Journal of Clinical Medicine. 2021; 10(24):5981. https://doi.org/10.3390/jcm10245981
Chicago/Turabian StyleHermida-Prieto, Manuel, Javier García-Castro, and Luis Mariñas-Pardo. 2021. "Systemic Treatment of Immune-Mediated Keratoconjunctivitis Sicca with Allogeneic Stem Cells Improves the Schirmer Tear Test Score in a Canine Spontaneous Model of Disease" Journal of Clinical Medicine 10, no. 24: 5981. https://doi.org/10.3390/jcm10245981






