The Potential Role of Liquid Biopsies in Advancing the Understanding of Neuroendocrine Neoplasms
Abstract
:1. Introduction
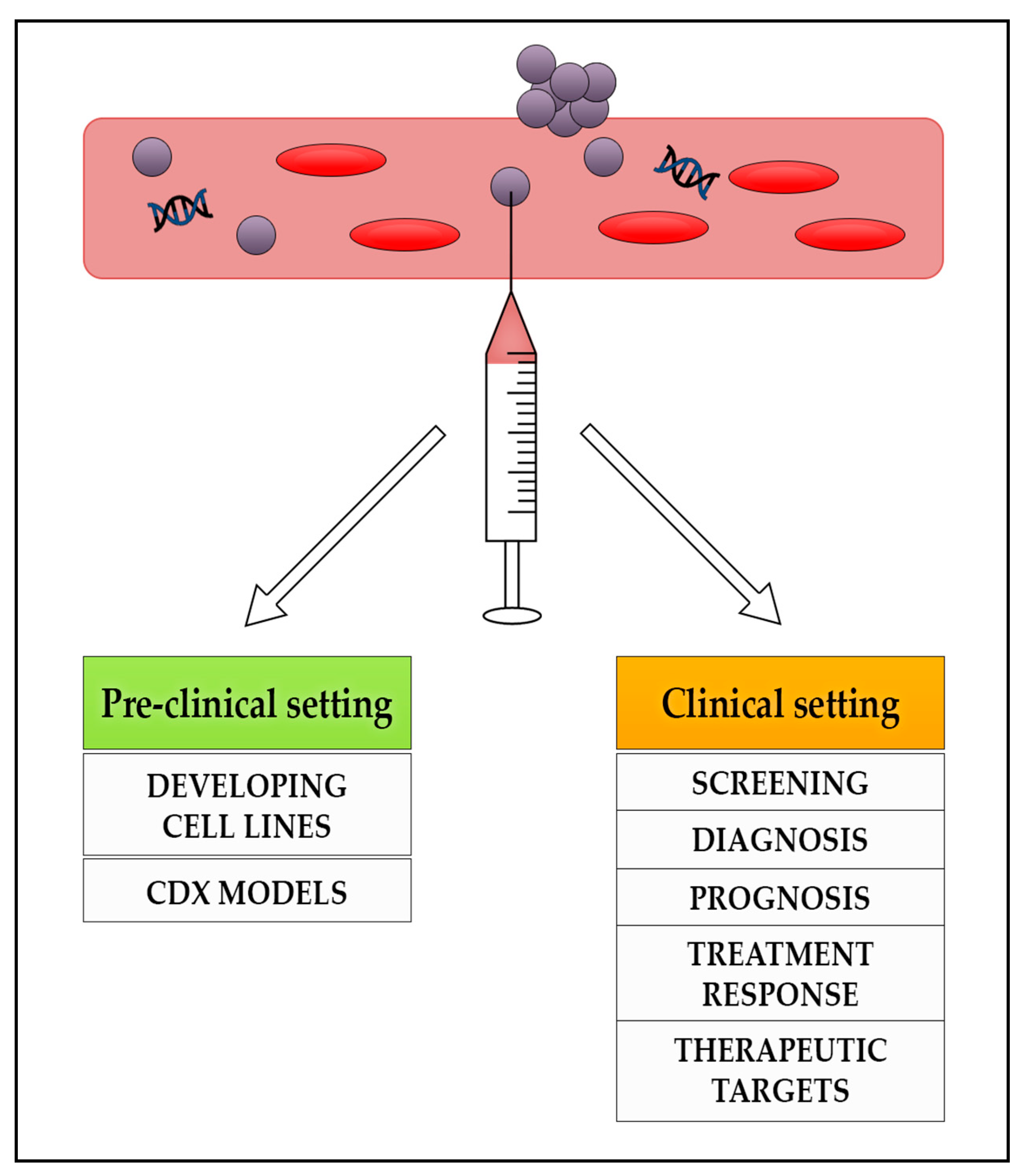
2. Liquid Biopsies
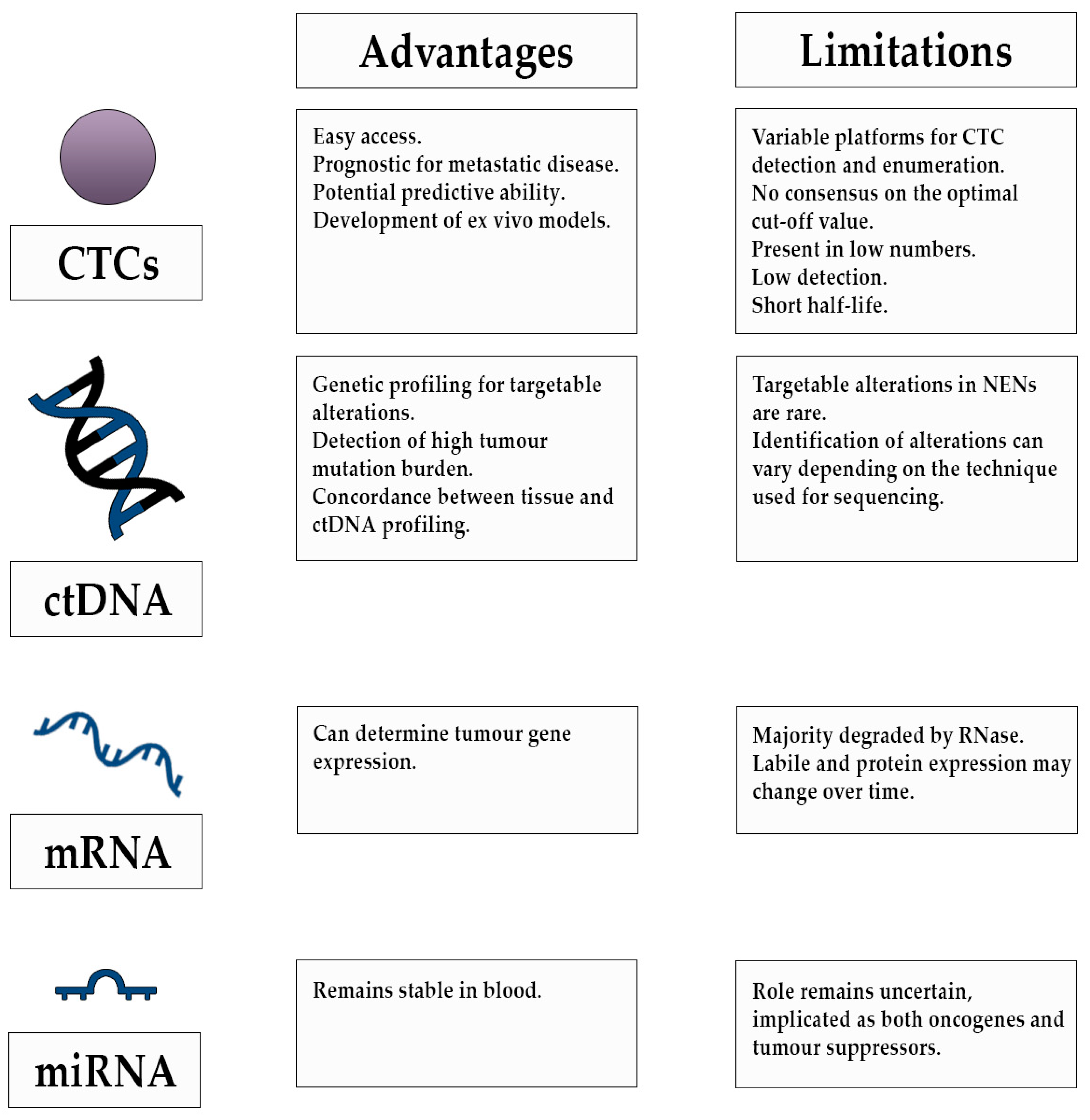
3. Circulating Tumour Cells
3.1. Potential Prognostic Ability of CTCs
3.2. Potential Predictive Ability of CTCs during Treatment
3.3. CTC Derived Models
4. Cell Free DNA (cfDNA) and Circulating Tumour DNA (ctDNA)
5. RNA
5.1. Circulating mRNA
5.2. Micro RNA
6. Future Perspectives
6.1. Diagnosis
6.2. Prognosis
6.3. Predictive
6.4. Development of Ex-Vivo Models
6.5. Targeted Treatment and Study of Resistance Mechanisms
6.6. Clinical Trials
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rizzo, F.M.; Meyer, T. Liquid Biopsies for Neuroendocrine Tumors: Circulating Tumor Cells, DNA, and MicroRNAs. Endocrinol. Metab. Clin. N. Am. 2018, 47, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Oberg, K.; Modlin, I.M.; De Herder, W.; Pavel, M.; Klimstra, D.; Frilling, A.; Metz, D.C.; Heaney, A.; Kwekkeboom, D.; Strosberg, J.; et al. Consensus on biomarkers for neuroendocrine tumour disease. Lancet Oncol. 2015, 16, e435–e446. [Google Scholar] [CrossRef] [Green Version]
- Klöppel, G. Neuroendocrine Neoplasms: Dichotomy, Origin and Classifications. Visc. Med. 2017, 33, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Ter-Minassian, M.; Chan, J.A.; Hooshmand, S.M.; Brais, L.K.; Daskalova, A.; Heafield, R.; Buchanan, L.; Qian, Z.R.; Fuchs, C.S.; Lin, X.; et al. Clinical presentation, recurrence, and survival in patients with neuroendocrine tumors: Results from a prospective institutional database. Endocr. Relat. Cancer 2013, 20, 187–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Granberg, D.; Wolin, E.; Warner, R.; Sissons, M.; Kolarova, T.; Goldstein, G.; Pavel, M.; Öberg, K.; Leyden, J. Patient-Reported Burden of a Neuroendocrine Tumor (NET) Diagnosis: Results from the First Global Survey of Patients With NETs. J. Glob. Oncol. 2016, 3, 43–53. [Google Scholar] [CrossRef]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; de Krijger, R.R.; Dietel, M.; El-Naggar, A.K.; et al. A common classification framework for neuroendocrine neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef]
- Öberg, K.E. The Management of Neuroendocrine Tumours: Current and Future Medical Therapy Options. Clin. Oncol. 2012, 24, 282–293. [Google Scholar] [CrossRef]
- Man, D.; Wu, J.; Shen, Z.; Zhu, X. Prognosis of patients with neuroendocrine tumor: A SEER database analysis. Cancer Manag. Res. 2018. [Google Scholar] [CrossRef] [Green Version]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020. [Google Scholar] [CrossRef]
- Khan, M.S.; Caplin, M.E. The use of biomarkers in neuroendocrine tumours. Frontline Gastroenterol. 2013. [Google Scholar] [CrossRef] [Green Version]
- Srirajaskanthan, R.; Kayani, I.; Quigley, A.M.; Soh, J.; Caplin, M.E.; Bomanji, J. The role of 68Ga-DOTATATE PET in patients with neuroendocrine tumors and negative or equivocal findings on 111In-DTPA-octreotide scintigraphy. J. Nucl. Med. 2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modlin, I.M.; Gustafsson, B.I.; Moss, S.F.; Pavel, M.; Tsolakis, A.V.; Kidd, M. Chromogranin A-biological function and clinical utility in neuro endocrine tumor disease. Ann. Surg. Oncol. 2010, 17, 2427–2443. [Google Scholar] [CrossRef] [PubMed]
- Ewang-Emukowhate, M.; Nair, D.; Caplin, M. The role of 5-hydroxyindoleacetic acid in neuroendocrine tumors: The journey so far. Int. J. Endocr. Oncol. 2019. [Google Scholar] [CrossRef] [Green Version]
- Öberg, K. Molecular genomic blood biomarkers for neuroendocrine tumours: The long and winding road from berzelius and bence jones to a neuroendocrine destination. Neuroendocrinology 2020. [Google Scholar] [CrossRef] [PubMed]
- Drucker, E.; Krapfenbauer, K. Pitfalls and limitations in translation from biomarker discovery to clinical utility in predictive and personalised medicine. EPMA J. 2013, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Modlin, I.M.; Moss, S.F.; Chung, D.C.; Jensen, R.T.; Snyderwine, E. Priorities for improving the management of gastroenteropancreatic neuroendocrine tumors. J. Natl. Cancer Inst. 2008, 100, 1282–1289. [Google Scholar] [CrossRef]
- Soda, N.; Rehm, B.H.A.; Sonar, P.; Nguyen, N.T.; Shiddiky, M.J.A. Advanced liquid biopsy technologies for circulating biomarker detection. J. Mater. Chem. B 2019, 7, 6670–6704. [Google Scholar] [CrossRef]
- Wu, J.; Hu, S.; Zhang, L.; Xin, J.; Sun, C.; Wang, L.; Ding, K.; Wang, B. Tumor circulome in the liquid biopsies for cancer diagnosis and prognosis. Theranostics 2020, 10, 4544. [Google Scholar] [CrossRef]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548. [Google Scholar] [CrossRef]
- Ashworth, T. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aust. Med. J. 1869, 14, 146. [Google Scholar]
- Miller, M.C.; Doyle, G.V.; Terstappen, L.W.M.M. Significance of Circulating Tumor Cells Detected by the CellSearch System in Patients with Metastatic Breast Colorectal and Prostate Cancer. J. Oncol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Bratulic, S.; Gatto, F.; Nielsen, J. The Translational Status of Cancer Liquid Biopsies. Regen. Eng. Transl. Med. 2019. [Google Scholar] [CrossRef] [Green Version]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.M.M.; et al. Circulating Tumor Cells, Disease Progression, and Survival in Metastatic Breast Cancer. N. Engl. J. Med. 2004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janni, W.J.; Yab, T.C.; Hayes, D.F.; Cristofanilli, M.; Bidard, F.-C.; Ignatiadis, M.; Regan, M.M.; Alix-Panabieres, C.; Barlow, W.E.; Caldas, C.; et al. Clinical utility of serial circulating tumor cell (CTC) enumeration as early treatment monitoring tool in metastatic breast cancer (MBC)—A global pooled analysis with individual patient data. In Proceedings of the 2020 San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 8–11 December 2020; p. Abstract GS4-08. [Google Scholar]
- Cohen, S.J.; Punt, C.J.A.; Iannotti, N.; Saidman, B.H.; Sabbath, K.D.; Gabrail, N.Y.; Picus, J.; Morse, M.; Mitchell, E.; Miller, M.C.; et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.R.; Valle, J.W.; McNamara, M.G. Pancreatic cancer: Are “liquid biopsies” ready for prime-time? World J. Gastroenterol. 2016, 22, 7175–7185. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Tsigani, T.; Rashid, M.; Rabouhans, J.S.; Yu, D.; Luong, T.V.; Caplin, M.; Meyer, T. Circulating tumor cells and EpCAM expression in neuroendocrine tumors. Clin. Cancer Res. 2011, 17, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.S.; Kirkwood, A.; Tsigani, T.; Garcia-Hernandez, J.; Hartley, J.A.; Caplin, M.E.; Meyer, T. Circulating tumor cells as prognostic markers in neuroendocrine tumors. J. Clin. Oncol. 2013, 31, 365–372. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, J.C.H.; Chen, G.Y.; Jhou, D.D.W.; Chou, W.C.; Yeh, C.N.; Hwang, T.L.; Lin, H.C.; Chu, H.C.; Wang, H.M.; Yen, T.C.; et al. The Prognostic Value of Circulating Tumor Cells in Asian Neuroendocrine Tumors. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Khan, M.S.; Kirkwood, A.A.; Tsigani, T.; Lowe, H.; Goldstein, R.; Hartley, J.A.; Caplin, M.E.; Meyer, T. Early changes in circulating tumor cells are associated with response and survival following treatment of metastatic neuroendocrine neoplasms. Clin. Cancer Res. 2016. [Google Scholar] [CrossRef] [Green Version]
- Cauley, C.E.; Pitman, M.B.; Zhou, J.; Perkins, J.; Kuleman, B.; Liss, A.S.; Fernandez-Del Castillo, C.; Warshaw, A.L.; Lillemoe, K.D.; Thayer, S.P. Circulating Epithelial Cells in Patients with Pancreatic Lesions: Clinical and Pathologic Findings. J. Am. Coll. Surg. 2015. [Google Scholar] [CrossRef] [Green Version]
- Gaiser, M.R.; Daily, K.; Hoffmann, J.; Brune, M.; Enk, A.; Brownell, I. Evaluating blood levels of neuron specific enolase, chromogranin A, and circulating tumor cells as Merkel cell carcinoma biomarkers. Oncotarget 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blom, A.; Bhatia, S.; Pietromonaco, S.; Koehler, K.; Iyer, J.G.; Nagase, K.; Paulson, K.; Sabath, D.E.; Nghiem, P. Clinical utility of a circulating tumor cell assay in Merkel cell carcinoma. J. Am. Acad. Dermatol. 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, J.M.; Krebs, M.G.; Lancashire, L.; Sloane, R.; Backen, A.; Swain, R.K.; Priest, L.J.C.; Greystoke, A.; Zhou, C.; Morris, K.; et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J. Clin. Oncol. 2012. [Google Scholar] [CrossRef]
- Normanno, N.; Rossi, A.; Morabito, A.; Signoriello, S.; Bevilacqua, S.; Di Maio, M.; Costanzo, R.; De Luca, A.; Montanino, A.; Gridelli, C.; et al. Prognostic value of circulating tumor cells’ reduction in patients with extensive small-cell lung cancer. Lung Cancer 2014. [Google Scholar] [CrossRef] [PubMed]
- Hiltermann, T.J.N.; Pore, M.M.; Van den Berg, A.; Timens, W.; Boezen, H.M.; Liesker, J.J.W.; Schouwink, J.H.; Wijnands, W.J.A.; Kerner, G.S.M.A.; Kruyt, F.A.E.; et al. Circulating tumor cells in small-cell lung cancer: A predictive and prognostic factor. Ann. Oncol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, C.; Wang, X.; Ranganathan, A.; Torigian, D.; Troxel, A.; Evans, T.; Cohen, R.B.; Vaidya, B.; Rao, C.; Connelly, M.; et al. Circulating tumor cells as a predictive biomarker in patients with small cell lung cancer undergoing chemotherapy. Lung Cancer 2017. [Google Scholar] [CrossRef]
- Rizzo, F.M.; Vesely, C.; Childs, A.; Marafioti, T.; Khan, M.S.; Mandair, D.; Cives, M.; Ensell, L.; Lowe, H.; Akarca, A.U.; et al. Circulating tumour cells and their association with bone metastases in patients with neuroendocrine tumours. Br. J. Cancer 2019, 120, 294–300. [Google Scholar] [CrossRef] [Green Version]
- Childs, A.; Vesely, C.; Ensell, L.; Lowe, H.; Luong, T.V.; Caplin, M.E.; Toumpanakis, C.; Thirlwell, C.; Hartley, J.A.; Meyer, T. Expression of somatostatin receptors 2 and 5 in circulating tumour cells from patients with neuroendocrine tumours. Br. J. Cancer 2016, 115, 1540–1547. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.H.; Wick, J.A.; Sittampalam, G.S.; Nirmalanandhan, V.S.; Ganti, A.K.; Neupane, P.C.; Williamson, S.K.; Godwin, A.K.; Schmitt, S.; Smart, N.J.; et al. A multicenter pilot study examining the role of circulating tumor cells as a blood-based tumor marker in patients with extensive small-cell lung cancer. Front. Oncol. 2014. [Google Scholar] [CrossRef] [Green Version]
- Carter, L.; Rothwell, D.G.; Mesquita, B.; Smowton, C.; Leong, H.S.; Fernandez-Gutierrez, F.; Li, Y.; Burt, D.J.; Antonello, J.; Morrow, C.J.; et al. Molecular analysis of circulating tumor cells identifies distinct copy-number profiles in patients with chemosensitive and chemorefractory small-cell lung cancer. Nat. Med. 2017. [Google Scholar] [CrossRef]
- Tayoun, T.; Faugeroux, V.; Oulhen, M.; Aberlenc, A.; Pawlikowska, P.; Farace, F. CTC-Derived Models: A Window into the Seeding Capacity of Circulating Tumor Cells (CTCs). Cells 2019, 8, 1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lallo, A.; Schenk, M.W.; Frese, K.K.; Blackhall, F.; Dive, C. Circulating tumor cells and CDX models as a tool for preclinical drug development. Transl. Lung Cancer Res. 2017, 6, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baccelli, I.; Schneeweiss, A.; Riethdorf, S.; Stenzinger, A.; Schillert, A.; Vogel, V.; Klein, C.; Saini, M.; Bäuerle, T.; Wallwiener, M.; et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat. Biotechnol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Faugeroux, V.; Pailler, E.; Oulhen, M.; Deas, O.; Brulle-Soumare, L.; Hervieu, C.; Marty, V.; Alexandrova, K.; Andree, K.C.; Stoecklein, N.H.; et al. Genetic characterization of a unique neuroendocrine transdifferentiation prostate circulating tumor cell-derived eXplant model. Nat. Commun. 2020. [Google Scholar] [CrossRef] [Green Version]
- Passiglia, F.; Rizzo, S.; Di Maio, M.; Galvano, A.; Badalamenti, G.; Listì, A.; Gulotta, L.; Castiglia, M.; Bazan, V.; Russo, A.; et al. The diagnostic accuracy of circulating tumor DNA for the detection of EGFR-T790M mutation in NSCLC: A systematic review and meta-analysis. Sci. Rep. 2018. [Google Scholar] [CrossRef] [Green Version]
- Tarazona, N.; Gimeno-Valiente, F.; Gambardella, V.; Zuñiga, S.; Rentero-Garrido, P.; Huerta, M.; Roselló, S.; Martinez-Ciarpaglini, C.; Carbonell-Asins, J.A.; Carrasco, F.; et al. Targeted next-generation sequencing of circulating-tumor DNA for tracking minimal residual disease in localized colon cancer. Ann. Oncol. 2019. [Google Scholar] [CrossRef] [Green Version]
- Di Domenico, A.; Wiedmer, T.; Marinoni, I.; Perren, A. Genetic and epigenetic drivers of neuroendocrine tumours (NET). Endocr. Relat. Cancer 2017, 24, R315–R334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, Y.; Shi, C.; Edil, B.H.; De Wilde, R.F.; Klimstra, D.S.; Maitra, A.; Schulick, R.D.; Tang, L.H.; Wolfgang, C.L.; Choti, M.A.; et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science (80-.) 2011. [Google Scholar] [CrossRef] [Green Version]
- Zakka, K.; Nagy, R.; Drusbosky, L.; Akce, M.; Wu, C.; Alese, O.B.; El-Rayes, B.F.; Kasi, P.M.; Mody, K.; Starr, J.; et al. Blood-based next-generation sequencing analysis of neuroendocrine neoplasms. Oncotarget 2020. [Google Scholar] [CrossRef] [PubMed]
- Wang, V.E.; Young, L.; Ali, S.; Miller, V.A.; Urisman, A.; Wolfe, J.; Bivona, T.G.; Damato, B.; Fogh, S.; Bergsland, E.K. A Case of Metastatic Atypical Neuroendocrine Tumor with ALK Translocation and Diffuse Brain Metastases. Oncologist 2017. [Google Scholar] [CrossRef] [Green Version]
- Boons, G.; Vandamme, T.; Peeters, M.; Beyens, M.; Driessen, A.; Janssens, K.; Zwaenepoel, K.; Roeyen, G.; Van Camp, G.; De Beeck, K.O. Cell-free DNA from metastatic pancreatic neuroendocrine tumor patients contains tumor-specific mutations and copy number variations. Front. Oncol. 2018, 8, 467. [Google Scholar] [CrossRef] [Green Version]
- Beltran, H.; Romanel, A.; Conteduca, V.; Casiraghi, N.; Sigouros, M.; Franceschini, G.M.; Orlando, F.; Fedrizzi, T.; Ku, S.Y.; Dann, E.; et al. Circulating tumor DNA profile recognizes transformation to castration-resistant neuroendocrine prostate cancer. J. Clin. Investig. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharabi, A.; Kim, S.S.; Kato, S.; Sanders, P.D.; Patel, S.P.; Sanghvi, P.; Weihe, E.; Kurzrock, R. Exceptional Response to Nivolumab and Stereotactic Body Radiation Therapy (SBRT) in Neuroendocrine Cervical Carcinoma with High Tumor Mutational Burden: Management Considerations from the Center For Personalized Cancer Therapy at UC San Diego Moores Cance. Oncologist 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klempner, S.J.; Gershenhorn, B.; Tran, P.; Lee, T.K.; Erlander, M.G.; Gowen, K.; Schrock, A.B.; Morosini, D.; Ross, J.S.; Stephens, P.J.; et al. BRAFV600E mutations in high-grade colorectal neuroendocrine tumors may predict responsiveness to BRAF-MEK combination therapy. Cancer Discov. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerard, L.; Garcia, J.; Gauthier, A.; Lopez, J.; Durand, A.; Hervieu, V.; Lemelin, A.; Chardon, L.; Landel, V.; Gibert, B.; et al. ctDNA in neuroendocrine carcinoma of gastroenteropancreatic origin or of unknown primary: The CIRCAN-NEC pilot study. Neuroendocrinology 2020. [Google Scholar] [CrossRef]
- Modlin, I.M.; Kidd, M.; Malczewska, A.; Drozdov, I.; Bodei, L.; Matar, S.; Chung, K.M. The NETest: The Clinical Utility of Multigene Blood Analysis in the Diagnosis and Management of Neuroendocrine Tumors. Endocrinol. Metab. Clin. N. Am. 2018, 47, 485–504. [Google Scholar] [CrossRef]
- Fernandez-Mercado, M.; Manterola, L.; Larrea, E.; Goicoechea, I.; Arestin, M.; Armesto, M.; Otaegui, D.; Lawrie, C.H. The circulating transcriptome as a source of non-invasive cancer biomarkers: Concepts and controversies of non-coding and coding RNA in body fluids. J. Cell. Mol. Med. 2015. [Google Scholar] [CrossRef]
- Kopreski, M.S.; Benko, F.A.; Kwak, L.W.; Gocke, C.D. Detection of tumor messenger RNA in the serum of patients with malignant melanoma. Clin. Cancer Res. 1999, 5, 1961–1965. [Google Scholar]
- García, V.; García, J.M.; Peña, C.; Silva, J.; Domínguez, G.; Lorenzo, Y.; Diaz, R.; Espinosa, P.; de Sola, J.G.; Cantos, B.; et al. Free circulating mRNA in plasma from breast cancer patients and clinical outcome. Cancer Lett. 2008. [Google Scholar] [CrossRef]
- Kidd, M.; Drozdov, I.A.; Matar, S.; Gurunlian, N.; Ferranti, N.J.; Malczewska, A.; Bennett, P.; Bodei, L.; Modlin, I.M. Utility of a ready-to-use PCR system for neuroendocrine tumor diagnosis. PLoS ONE 2018. [Google Scholar] [CrossRef]
- Genç, C.G.; Jilesen, A.P.J.; Nieveen van Dijkum, E.J.M.; Klümpen, H.J.; van Eijck, C.H.J.; Drozdov, I.; Malczewska, A.; Kidd, M.; Modlin, I. Measurement of circulating transcript levels (NETest) to detect disease recurrence and improve follow-up after curative surgical resection of well-differentiated pancreatic neuroendocrine tumors. J. Surg. Oncol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ćwikła, J.B.; Bodei, L.; Kolasinska-Ćwikła, A.; Sankowski, A.; Modlin, I.M.; Kidd, M. Circulating transcript analysis (NETest) in GEP-NETs treated with somatostatin analogs defines therapy. J. Clin. Endocrinol. Metab. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodei, L.; Kidd, M.S.; Singh, A.; van der Zwan, W.A.; Severi, S.; Drozdov, I.A.; Malczewska, A.; Baum, R.P.; Kwekkeboom, D.J.; Paganelli, G.; et al. PRRT neuroendocrine tumor response monitored using circulating transcript analysis: The NETest. Eur. J. Nucl. Med. Mol. Imaging 2020. [Google Scholar] [CrossRef] [PubMed]
- Malczewska, A.; Kidd, M.; Matar, S.; Kos-Kudla, B.; Modlin, I.M. A Comprehensive Assessment of the Role of miRNAs as Biomarkers in Gastroenteropancreatic Neuroendocrine Tumors. Neuroendocrinology 2018. [Google Scholar] [CrossRef] [PubMed]
- Li, S.C.; Essaghir, A.; Martijn, C.; Lloyd, R.V.; Demoulin, J.B.; Öberg, K.; Giandomenico, V. Global microRNA profiling of well-differentiated small intestinal neuroendocrine tumors. Mod. Pathol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Garzon, R.; Fabbri, M.; Cimmino, A.; Calin, G.A.; Croce, C.M. MicroRNA expression and function in cancer. Trends Mol. Med. 2006, 12, 580–587. [Google Scholar] [CrossRef]
- Lloyd, K.A.; Moore, A.R.; Parsons, B.N.; O’Hara, A.; Boyce, M.; Dockray, G.J.; Varro, A.; Pritchard, D.M. Gastrin-induced miR-222 promotes gastric tumor development by suppressing p27kip1. Oncotarget 2016. [Google Scholar] [CrossRef] [Green Version]
- Vicentini, C.; Fassan, M.; D’Angelo, E.; Corbo, V.; Silvestris, N.; Nuovo, G.J.; Scarpa, A. Clinical application of microRNA testing in neuroendocrine tumors of the gastrointestinal tract. Molecules 2014, 19, 2458–2468. [Google Scholar] [CrossRef] [Green Version]
- Miller, H.C.; Frampton, A.E.; Malczewska, A.; Ottaviani, S.; Stronach, E.A.; Flora, R.; Kaemmerer, D.; Schwach, G.; Pfragner, R.; Faiz, O.; et al. MicroRNAs associated with small bowel neuroendocrine tumours and their metastases. Endocr. Relat. Cancer 2016. [Google Scholar] [CrossRef] [Green Version]
- Helo, P.; Cronin, A.M.; Danila, D.C.; Wenske, S.; Gonzalez-Espinoza, R.; Anand, A.; Koscuiszka, M.; Väänänen, R.M.; Pettersson, K.; Chun, F.K.H.; et al. Circulating prostate tumor cells detected by Reverse transcription-PCR in men with localized or castration-refractory prostate cancer: Concordance with CellSearch assay and association with bone metastases and with survival. Clin. Chem. 2009. [Google Scholar] [CrossRef]
- Van De Stolpe, A.; Pantel, K.; Sleijfer, S.; Terstappen, L.W.; Den Toonder, J.M.J. Circulating tumor cell isolation and diagnostics: Toward routine clinical use. Cancer Res. 2011, 71, 5955–5960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Q.; Ling, S.; Zheng, S.; Xu, X. Liquid biopsy in hepatocellular carcinoma: Circulating tumor cells and circulating tumor DNA. Mol. Cancer 2019, 18, 114. [Google Scholar] [CrossRef] [PubMed]
- Potdar, P.; Lotey, N. Role of circulating tumor cells in future diagnosis and therapy of cancer. J. Cancer Metastasis Treat. 2015. [Google Scholar] [CrossRef] [Green Version]
- Paoletti, C.; Muñiz, M.C.; Thomas, D.G.; Griffith, K.A.; Kidwell, K.M.; Tokudome, N.; Brown, M.E.; Aung, K.; Craig Miller, M.; Blossom, D.L.; et al. Development of circulating tumor cell-endocrine therapy index in patients with hormone receptor-positive Breast Cancer. Clin. Cancer Res. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craig, Z.; Swain, J.; Batman, E.; Wadsley, J.; Reed, N.; Faluyi, O.; Cave, J.; Sharma, R.; Chau, I.; Wall, L.; et al. NET-02 trial protocol: A multicentre, randomised, parallel group, open-label, phase II, single-stage selection trial of liposomal irinotecan (nal-IRI) and 5-fluorouracil (5-FU)/folinic acid or docetaxel as second-line therapy in patients with progressive poorly differentiated extrapulmonary neuroendocrine carcinoma (NEC). BMJ Open 2020. [Google Scholar] [CrossRef] [Green Version]
| Author | Tumour Type | N | Biomarkers | CTC Cut-Off Value | CTC Detection | Outcomes |
|---|---|---|---|---|---|---|
| Hseih et al., 2019 [29] | Unresectable locally advanced and metastatic NENs | 35 | CTCs (EpCAM independent) | Three cut-offs: ≥1, ≥5, ≥20 cells/mL blood) | 43% had detectable CTCs | CTC counts associated with cancer stages (I-III vs. IV, p = 0.015), liver metastasis (p = 0.026), and NET grading (p = 0.03). Baseline CTC counts prognostic factors for PFS survival (p = 0.015) and OS (p = 0.023). |
| Rizzo et al., 2019 [38] | Metastatic bronchial, midgut or pancreatic NENs | 254 | CTCs with CXCR4 expression EpCAM +ve | ≥1 CTC 7.5/mL blood | 43% had detectable CTCs | Bone metastases significantly associated with CTCs (p < 0.0001) CXCR4-positive CTCs in patients with bone metastases was 56% compared to 35% in those without (p = 0.18) |
| Khan et al., 2016 [30] | Metastatic NENs commencing therapy | 138 | CTCs with EpCAM expression | ≥1 CTC 7.5/mL blood | 68% had detectable CTCs. | Changes in CTCs had strong association with OS (HR, 4.13; p = 0.0002). Better prognosis in patients with 0 CTCs before and after therapy; followed by those with ≥50% reduction in CTCs (HR 3.31) Poor outcomes in patients with a <50% reduction or increase in CTCs (HR, 5.07). |
| Childs et al., 2016 [39] | Metastatic midgut, pancreatic or CUP NETS | 31 | CTCs with SSTR expression EpCAM +ve | ≥1 CTC 7.5/mL blood | 68% had detectable CTCs | 33% had expression of SSTR2/SSTR5 87% (n = 27) of all patients had SSTR-positive tumours according to somatostatin receptor scintigraphy or 68Ga PET CT |
| Khan et al., 2013 [28] | Metastatic NENs | 175 | CTCs with EpCAM expression | ≥1 CTC per 7.5 mL | 49% patients had ≥ one CTC, 42% had ≥ two CTCs, and ≥ 30% had five CTCs | ≥one CTC associated with worse PFS and OS (hazard ratios [HRs], 6.6 and 8.0, p < 0.001). CTCs associated with poor prognosis. Grade 1, HRs were 5.0 for PFS (p < 0.017) and 7.2 for OS (p < 0.023); Grade 2, HRs were 3.5 for PFS (p < 0.018) and 5.2 for OS (p < 0.036). |
| Khan et al., 2011 [27] | Metastatic NENS | 74 | CTCs with EpCAM expression | NR | 43% of midgut and 21% of pancreatic NETs had detectable CTCs 68% > 5 CTCs | Absence of CTCs strongly associated with stable disease (p < 0.001) Moderate correlation between CTC levels and burden of liver metastases (B = 8.91, p < 0.001) |
| Author | Tumour Type | N | Biomarkers | Outcome | Clinical Relevance |
|---|---|---|---|---|---|
| Zakka et al., 2020 [50] | Pancreatic NET, gastrointestinal NEC, large cell lung NEC, nasopharyngeal NEC | 320 | ctDNA analysis | Genomic alterations found in 87.5% of samples Total of 1012 alterations identified Mutations in TP53 52%, KRAS, 22%, EGFR 12%, PIK3CA,11%, BRAF 10%, MYC 10%, CCNE1 10%, CDK6 8%, RB1 7%, NF1 7%, MET 7%, FGFR1 7%, APC 7%, ERBB2 6% and 5%. | Evaluation of ctDNA was feasible in NENS and may help determine driver mutations for targeted therapy |
| Wang et al., 2017 [51] | Metastatic atypical carcinoid tumour of the lung | 1 | ctDNA analysis | ctDNA analysis revealed ALK translocation Treated with ALK inhibitor alectinib with partial response. Approximately 60% shrinkage of dominant brain metastases | ctDNA is a feasible alternative platform for identifying driver mutations when tissue sampling is limited. It may help determine targeted therapy |
| Boons et al., 2018 [52] | Pancreatic NET undergoing surgery | 10 | cfDNA analysis | Tumor-specific variants were detected in 2 PNET patients, at variant allele fractions of 19% and 21%. In the metastatic patients, there was correlation between copy number variations of tumour tissue profiles and cfDNA. | Copy number variation analysis in cfDNA has potential as a liquid biopsy |
| Beltran et al., 2020 [53] | Castration-resistant neuroendocrine prostate cancer (CRPC-NE) | 17 | cfDNA and ctDNA analysis | High concordance between cfDNA and biopsy tissue genomic alterations Mutations found in RB1 (69%) and TP53 (63%) in CRPC-NE patients. Prior exposure to cytotoxic chemotherapy was associated with higher cfDNA | Evulation of cfDNA is feasible in CRPC-NE and may help determine genomic changes associated with the disease |
| Sharabi et al., 2017 [54] | High-grade, large-cell neuroendocrine carcinoma of the cervix | 1 | ctDNA analysis | Multiple alterations in ctDNA suspicious for high tumour mutational burden. Nivolumab commenced on the basis of ctDNA results as tumour tissue awaited Tissue biopsy confirmed mismatch repair gene defect, concordant with ctDNA. | Evaluation of ctDNA is feasible and may help determine driver mutations for targeted therapy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, D.; Lamarca, A.; Valle, J.W.; McNamara, M.G. The Potential Role of Liquid Biopsies in Advancing the Understanding of Neuroendocrine Neoplasms. J. Clin. Med. 2021, 10, 403. https://doi.org/10.3390/jcm10030403
Shah D, Lamarca A, Valle JW, McNamara MG. The Potential Role of Liquid Biopsies in Advancing the Understanding of Neuroendocrine Neoplasms. Journal of Clinical Medicine. 2021; 10(3):403. https://doi.org/10.3390/jcm10030403
Chicago/Turabian StyleShah, Dinakshi, Angela Lamarca, Juan W Valle, and Mairéad G McNamara. 2021. "The Potential Role of Liquid Biopsies in Advancing the Understanding of Neuroendocrine Neoplasms" Journal of Clinical Medicine 10, no. 3: 403. https://doi.org/10.3390/jcm10030403
APA StyleShah, D., Lamarca, A., Valle, J. W., & McNamara, M. G. (2021). The Potential Role of Liquid Biopsies in Advancing the Understanding of Neuroendocrine Neoplasms. Journal of Clinical Medicine, 10(3), 403. https://doi.org/10.3390/jcm10030403





