Detection and Characterization of Phosphorylation, Glycosylation, and Fatty Acid Bound to Fetuin A in Human Blood
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation of Monospecific pSer312Fet A Antibody
2.2. Enzyme-Linked Immunosorbent Assay (ELISA)
2.3. Characterization of Phospho-Ser312 Fet A Antibody
2.3.1. Dephosphorylation of pSer312Fet A
2.3.2. Phosphorylation of Fet A
2.3.3. Stability of pSer312 Fet A
2.3.4. Deglycosylation of Fet A
2.3.5. Western Blot Analysis of Commercial Fet A, rh Fet A, and pFet A from Human Plasma
2.3.6. Determination of Free Fatty Acid Composition of Fet A from Commercial Fet A and rh Fet A
2.4. Human Samples
3. Results
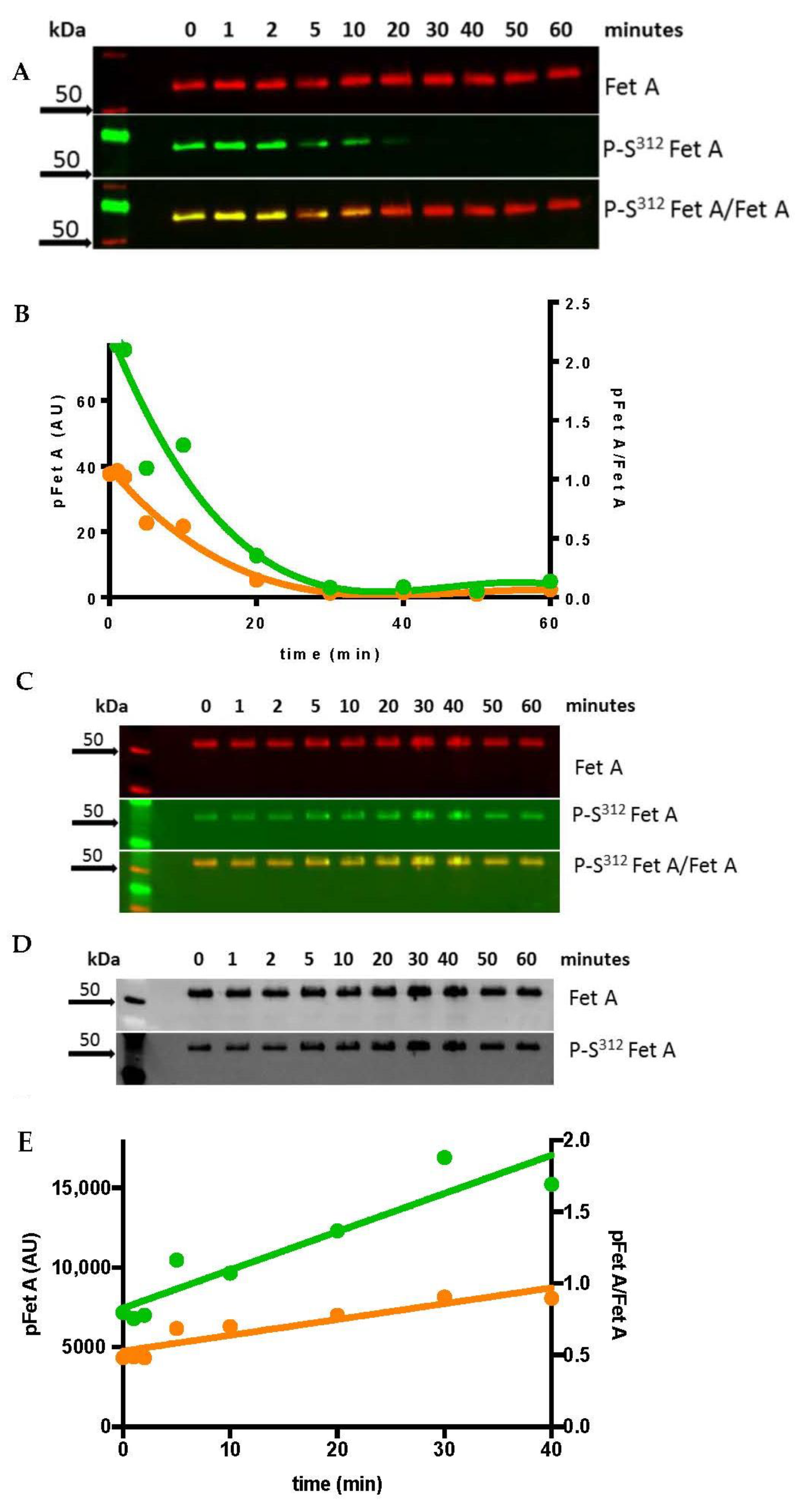
3.1. In Vitro Dephosphorylation and Phosphorylation of Fet A
3.2. Stability of pSer312 Fet A in Human Blood Samples
3.3. Precision of the Developed Assay
3.4. Effect of Deglycosylation of Fet A on pSer312
3.5. Associations of the Phosphorylation and Glycosylation Pattern of Fet A
3.6. Short-Term Metabolic Changes (OGTT) Do Not Affect Fet A or pFetA Plasma Levels
3.7. Determination of Fatty Acid Composition of Fet A
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jirak, P.; Stechemesser, L.; Moré, E.; Franzen, M.; Topf, A.; Mirna, M.; Paar, V.; Pistulli, R.; Kretzschmar, D.; Wernly, B.; et al. Clinical implications of fetuin-A. Adv. Clin. Chem. 2019, 89, 79–130. [Google Scholar] [CrossRef] [PubMed]
- Kalabay, L.; Jakab, L.; Prohászka, Z.; Füst, G.; Benkö, Z.; Telegdy, L.; Lörincz, Z.; Závodszky, P.; Arnaud, P.; Fekete, B. Human fetuin/α2HS-glycoprotein level as a novel indicator of liver cell function and short-term mortality in patients with liver cirrhosis and liver cancer. Eur. J. Gastroenterol. Hepatol. 2002, 14, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K.; Schneider, E.M.; Venkatesh, A.; Luong, J.H.T. Emerging Human Fetuin A Assays for Biomedical Diagnostics. Trends Biotechnol. 2017, 35, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Emoto, M.; Inaba, M. Fetuin-A: A multifunctional protein. Recent Patents Endocr. Metab. Immune Drug Discov. 2011, 5, 124–146. [Google Scholar] [CrossRef]
- Haglund, Å.C.; Ek, B.; Ek, P. Phosphorylation of human plasma α2-Heremans–Schmid glycoprotein (human fetuin) in vivo. Biochem. J. 2001, 357, 437–445. [Google Scholar] [CrossRef]
- Ren, G.; Kim, T.; Papizan, J.B.; Okerberg, C.K.; Kothari, V.M.; Zaid, H.; Bilan, P.J.; Araya-Ramirez, F.; Littlefield, L.A.; Bowers, R.L.; et al. Phosphorylation status of fetuin-A is critical for inhibition of insulin action and is correlated with obesity and insulin resistance. Am. J. Physiol. Metab. 2019, 317, E250–E260. [Google Scholar] [CrossRef]
- Schäfer, C.; Heiss, A.; Schwarz, A.; Westenfeld, R.; Ketteler, M.; Floege, J.; Müller-Esterl, W.; Schinke, T.; Jahnen-Dechent, W. The serum protein α2–Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J. Clin. Investig. 2003, 112, 357–366. [Google Scholar] [CrossRef]
- De Jager, D.J.; Vervloet, M.G.; Dekker, F.W. Noncardiovascular mortality in CKD: An epidemiological perspective. Nat. Rev. Nephrol. 2014, 10, 208–214. [Google Scholar] [CrossRef]
- Ochieng, J.; Nangami, G.N.; Sakwe, A.M.; Moye, C.; Alvarez, J.; Whalen, D.S.; Thomas, P.; Lammers, P.E. Impact of Fetuin-A (AHSG) on Tumor Progression and Type 2 Diabetes. Int. J. Mol. Sci. 2018, 19, 2211. [Google Scholar] [CrossRef]
- Stefan, N.; Fritsche, A.; Weikert, C.; Boeing, H.; Joost, H.-G.; Haring, H.U.; Schulze, M.B. Plasma Fetuin-A Levels and the Risk of Type 2 Diabetes. Diabetes 2008, 57, 2762–2767. [Google Scholar] [CrossRef]
- Yin, L.; Cai, W.J.; Zhu, L.Y.; Li, J.; Su, X.H.; Wang, X.L.; Chang, X.Y.; Sun, K. Association of plasma Fetuin-A and clinical characteristics in patients with new-onset type 2 diabetes mellitus. Int. J. Clin. Exp. Med. 2015, 8, 991–999. [Google Scholar] [PubMed]
- Stefan, N.; Häring, H.-U. The role of hepatokines in metabolism. Nat. Rev. Endocrinol. 2013, 9, 144–152. [Google Scholar] [CrossRef]
- Ou, H.Y.; Yang, Y.C.; Wu, H.T.; Wu, J.S.; Lu, F.H.; Chang, C.J. Increased Fetuin-A Concentrations in Impaired Glucose Tolerance with or without Nonalcoholic Fatty Liver Disease, But Not Impaired Fasting Glucose. J. Clin. Endocrinol. Metab. 2012, 97, 4717–4723. [Google Scholar] [CrossRef] [PubMed]
- Kantartzis, K.; Machann, J.; Schick, F.; Fritsche, A.; Häring, H.U.; Stefan, N. The impact of liver fat vs visceral fat in determining categories of prediabetes. Diabetologia 2010, 53, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Hennige, A.M.; Staiger, H.; Machann, J.; Schick, F.; Krober, S.M.; Machicao, F.; Fritsche, A.; Häring, H.-U. 2-Heremans-Schmid Glycoprotein/ Fetuin-A Is Associated With Insulin Resistance and Fat Accumulation in the Liver in Humans. Diabetes Care 2006, 29, 853–857. [Google Scholar] [CrossRef]
- Stefan, N.; Haring, H.U. Circulating fetuin-A and free fatty acids interact to predict insulin resistance in humans. Nat. Med. 2013, 19, 394–395. [Google Scholar] [CrossRef]
- Mori, K.; Emoto, M.; Inaba, M. Fetuin-A and the cardiovascular system. Adv. Clin. Chem. 2012, 56, 175–195. [Google Scholar] [CrossRef]
- Ix, J.H.; Shlipak, M.G.; Brandenburg, V.M.; Ali, S.; Ketteler, M.; Whooley, M.A. Association Between Human Fetuin-A and the Metabolic Syndrome. Circulation 2006, 113, 1760–1767. [Google Scholar] [CrossRef]
- Weikert, C.; Stefan, N.; Schulze, M.B.; Pischon, T.; Berger, K.; Joost, H.-G.; Häring, H.-U.; Boeing, H.; Fritsche, A. Plasma Fetuin-A Levels and the Risk of Myocardial Infarction and Ischemic Stroke. Circulation 2008, 118, 2555–2562. [Google Scholar] [CrossRef]
- Roshanzamir, F.; Miraghajani, M.; Rouhani, M.H.; Mansourian, M.; Ghiasvand, R.; Safavi, S.M. The association between circulating fetuin-A levels and type 2 diabetes mellitus risk: Systematic review and meta-analysis of observational studies. J. Endocrinol. Investig. 2018, 41, 33–47. [Google Scholar] [CrossRef]
- Mathews, S.T.; Singh, G.P.; Ranalletta, M.; Cintron, V.J.; Qiang, X.; Goustin, A.S.; Jen, K.-L.C.; Charron, M.J.; Jahnen-Dechent, W.; Grunberger, G. Improved Insulin Sensitivity and Resistance to Weight Gain in Mice Null for the Ahsg Gene. Diabetes 2002, 51, 2450–2458. [Google Scholar] [CrossRef] [PubMed]
- Auberger, P.; Falquerho, L.; Contreres, J.O.; Pages, G.; Le Cam, G.; Rossi, B.; Le Cam, A. Characterization of a natural inhibitor of the insulin receptor tyrosine kinase: cDNA cloning, purification, and anti-mitogenic activity. Cell 1989, 58, 631–640. [Google Scholar] [CrossRef]
- Goustin, A.S.; Abou-Samra, A.B. The “thrifty” gene encoding Ahsg/Fetuin-A meets the insulin receptor: Insights into the mechanism of insulin resistance. Cell. Signal. 2011, 23, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Mathews, S.T.; Chellam, N.; Srinivas, P.R.; Cintron, V.J.; Leon, M.A.; Goustin, A.S.; Grunberger, G. Alpha2-HSG, a specific inhibitor of insulin receptor autophosphorylation, interacts with the insulin receptor. Mol. Cell. Endocrinol. 2000, 164, 87–98. [Google Scholar] [CrossRef]
- Srinivas, P.R.; Deutsch, D.D.; Mathews, S.T.; Goustin, A.S.; Leon, M.A.; Grunberger, G. Recombinant human alpha 2-HS glycoprotein inhibits insulin-stimulated mitogenic pathway without affecting metabolic signalling in Chinese hamster ovary cells overexpressing the human insulin receptor. Cell Signal. 1996, 8, 567–573. [Google Scholar] [CrossRef]
- Goustin, A.S.; Derar, N.; Abou-Samra, A.B. Ahsg-fetuin blocks the metabolic arm of insulin action through its interaction with the 95-kD β-subunit of the insulin receptor. Cell Signal. 2013, 25, 981–988. [Google Scholar] [CrossRef]
- Akhoundi, C.; Amiot, M.; Auberger, P.; Le Cam, A.; Rossi, B. Insulin and interleukin-1 differentially regulate pp63, an acute phase phosphoprotein in hepatoma cell line. J. Biol. Chem. 1994, 269, 15925–15930. [Google Scholar] [CrossRef]
- Heinrichsdorff, J.; Olefsky, J.M. Fetuin-A: The missing link in lipid-induced inflammation. Nat. Med. 2012, 18, 1182–1183. [Google Scholar] [CrossRef]
- Vallur, R.; Kalbacher, H.; Feil, R. Catalytic Activity of cGMP-Dependent Protein Kinase Type I in Intact Cells Is Independent of N-Terminal Autophosphorylation. PLoS ONE 2014, 9, e98946. [Google Scholar] [CrossRef][Green Version]
- Waraich, R.S.; Zaidi, N.; Moeschel, K.; Beck, A.; Weigert, C.; Voelter, W.; Kalbacher, H.; Lehmann, R. Development and precise characterization of phospho-site-specific antibody of Ser357 of IRS-1: Elimination of cross reactivity with adjacent Ser358. Biochem. Biophys. Res. Commun. 2008, 376, 26–31. [Google Scholar] [CrossRef]
- Samara, P.; Kalbacher, H.; Ioannou, K.; Radu, D.L.; Livaniou, E.; Promponas, V.J.; Voelter, W.; Tsitsilonis, O. Development of an ELISA for the quantification of the C-terminal decapeptide prothymosin α(100–109) in sera of mice infected with bacteria. J. Immunol. Methods 2013, 395, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Mathews, S.T.; Graff, E.; Judd, R.L.; Kothari, V. Comparison of Chemiluminescence vs. Infrared Techniques for Detection of Fetuin-A in Saliva. Methods. Mol. Biol. 2015, 1314, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Peter, A.; Cegan, A.; Staiger, H.; Machann, J.; Schick, F.; Claussen, C.D.; Fritsche, A.; Häring, H.-U.; Schleicher, E. Low hepatic stearoyl-CoA desaturase 1 activity is associated with fatty liver and insulin resistance in obese humans. Diabetologia 2008, 51, 648–656. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kantartzis, K.; Thamer, C.; Peter, A.; Machann, J.; Schick, F.; Schraml, C.; Konigsrainer, A.; Krober, S.; Niess, A.; Fritsche, A.; et al. High cardiorespiratory fitness is an independent predictor of the reduction in liver fat during a lifestyle intervention in non-alcoholic fatty liver disease. Gut 2009, 58, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Peter, A.; Kantartzis, K.; Machann, J.; Schick, F.; Staiger, H.; Machicao, F.; Schleicher, E.; Fritsche, A.; Häring, H.-U.; Stefan, N. Relationships of Circulating Sex Hormone-Binding Globulin With Metabolic Traits in Humans. Diabetes 2010, 59, 3167–3173. [Google Scholar] [CrossRef]
- Peter, A.; Kovarova, M.; Staiger, H.; Machann, J.; Schick, F.; Königsrainer, A.; Königsrainer, I.; Schleicher, E.; Fritsche, A.; Häring, H.-U.; et al. The hepatokines fetuin-A and fetuin-B are upregulated in the state of hepatic steatosis and may differently impact on glucose homeostasis in humans. Am. J. Physiol. Metab. 2018, 314, E266–E273. [Google Scholar] [CrossRef]
- Tagliabracci, V.S.; Wiley, S.E.; Guo, X.; Kinch, L.N.; Durrant, E.; Wen, J.; Xiao, J.; Cui, J.; Nguyen, K.B.; Engel, J.L.; et al. A Single Kinase Generates the Majority of the Secreted Phosphoproteome. Cell 2015, 161, 1619–1632. [Google Scholar] [CrossRef]
- Wolf, M.; Riedlinger, I.; Lehmann, R.; Haring, H.U.; Schleicher, E.; Peter, A. Comparison of the automated KRYPTOR chromogranin A assay with the DAKO ELISA. Clin. Lab. 2014, 60, 2103–2106. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Franc, V.; Heck, A.J.R. Similar Albeit Not the Same: In-Depth Analysis of Proteoforms of Human Serum, Bovine Serum, and Recombinant Human Fetuin. J. Proteome Res. 2018, 17, 2861–2869. [Google Scholar] [CrossRef]
- Peter, A.; Kovářová, M.; Nadalin, S.; Cermak, T.; Königsrainer, A.; Machicao, F.; Stefan, N.; Häring, H.-U.; Schleicher, E. PNPLA3 variant I148M is associated with altered hepatic lipid composition in humans. Diabetologia 2014, 57, 2103–2107. [Google Scholar] [CrossRef]
- Cayatte, A.J.; Kumbla, L.; Subbiah, M.T. Marked acceleration of exogenous fatty acid incorporation into cellular triglycerides by fetuin. J. Biol. Chem. 1990, 265, 5883–5888. [Google Scholar] [CrossRef]
- Denecke, B.; Gräber, S.; Schäfer, C.; Heiss, A.; Wöltje, M.; Jahnen-Dechent, W. Tissue distribution and activity testing suggest a similar but not identical function of fetuin-B and fetuin-A. Biochem. J. 2003, 376, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.; Machann, J.; Guthoff, M.; Nawroth, P.P.; Nadalin, S.; Saleem, M.A.; Heyne, N.; Königsrainer, A.; Fend, F.; Schick, F.; et al. The protective effect of human renal sinus fat on glomerular cells is reversed by the hepatokine fetuin-A. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Bhattacharya, S. Plasma fetuin-A triggers inflammatory changes in macrophages and adipocytes by acting as an adaptor protein between NEFA and TLR-4. Diabetologia 2016, 59, 859–860. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alexander, M.; Loomis, A.K.; Van Der Lei, J.; Duarte-Salles, T.; Prieto-Alhambra, D.; Ansell, D.; Pasqua, A.; Lapi, F.; Rijnbeek, P.; Mosseveld, M.; et al. Non-alcoholic fatty liver disease and risk of incident acute myocardial infarction and stroke: Findings from matched cohort study of 18 million European adults. BMJ 2019, 367, l5367. [Google Scholar] [CrossRef] [PubMed]






| Species | Fet A Sequence Alignment |
|---|---|
| human | -slgspSgevshprk- |
| pig | -svesasgeafhvgk- |
| rat | -svesasgevlhspk- |
| BMI 20 kg/m2 | BMI 35 kg/m2 | |||||
|---|---|---|---|---|---|---|
| Fet A (AU) | pFet A (AU) | pFet A/FetA | Fet A (AU) | pFet A (AU) | pFet A/FetA | |
| Mean | 14,222 | 10,647 | 0.75 | 16,702 | 11,681 | 0.697 |
| SD | 2031 | 1989 | 0.082 | 2189 | 2746 | 0.119 |
| SEM | 454 | 445 | 0.018 | 489 | 614 | 0.026 |
| CV | 14% | 19% | 11% | 13% | 24% | 17% |
| N | 20 | 20 | 20 | 20 | 20 | 20 |
| pFet A (1) | pFet A (2) | pFet A Sum | pFet A Deglyco | |||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| Gender | 0.21 | 0.22 | 0.42 | 0.01 | 0.18 | 0.23 | 0.32 | 0.06 |
| Age | −0.14 | 0.43 | +0.10 | 0.58 | +0.02 | 0.92 | +0.08 | 0.63 |
| BMI | +0.17 | 0.33 | −0.03 | 0.86 | +0.14 | 0.42 | +0.10 | 0.56 |
| Fasting glucose | +0.08 | 0.22 | +0.06 | 0.73 | +0.01 | 0.95 | +0.14 | 0.42 |
| 2 h glucose OGTT | −0.05 | 0.77 | +0.15 | 0.39 | +0.11 | 0.54 | +0.29 | 0.09 |
| Fasting insulin | +0.03 | 0.88 | +0.08 | 0.66 | −0.08 | 0.64 | +0.30 | 0.08 |
| 2 h insulin OGTT | +0.12 | 0.49 | +0.12 | 0.49 | −0.00 | 0.97 | +0.30 | 0.08 |
| HOMA-IR | +0.04 | 0.82 | +0.08 | 0.63 | −0.08 | 0.67 | +0.31 | 0.07 |
| Insulin sensitivity OGTT | −0.11 | 0.51 | −0.10 | 0.56 | +0.01 | 0.94 | −0.27 | 0.11 |
| Fatty Acid Species | FFA Plasma Sample | Commercial Fet A | rh Fet A |
|---|---|---|---|
| C16:0 | 29.61 | 44.09 | 42.32 |
| C18:0 | 15.80 | 49.37 | 48.62 |
| C18:1N9 (MUFA) | 36.86 | 2.52 | 4.88 |
| C18:2N6 | 14.87 | 1.69 | 1.41 |
| C20:0 | 0.23 | 1.18 | 1.74 |
| C20:4N6 | 1.72 | 0.83 | 0.26 |
| C22:6N3 | 0.91 | 0.33 | 0.77 |
| SFA (sum) | 45.64 | 94.64 | 92.68 |
| PUFA (sum) | 17.55 | 2.85 | 2.44 |
| MUFA/SFA | 1.23 | 37.5 | 19.3 |
| uSFA/SFA | 0.84 | 17.6 | 12.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovářová, M.; Kalbacher, H.; Peter, A.; Häring, H.-U.; Didangelos, T.; Stefan, N.; Birkenfeld, A.; Schleicher, E.; Kantartzis, K. Detection and Characterization of Phosphorylation, Glycosylation, and Fatty Acid Bound to Fetuin A in Human Blood. J. Clin. Med. 2021, 10, 411. https://doi.org/10.3390/jcm10030411
Kovářová M, Kalbacher H, Peter A, Häring H-U, Didangelos T, Stefan N, Birkenfeld A, Schleicher E, Kantartzis K. Detection and Characterization of Phosphorylation, Glycosylation, and Fatty Acid Bound to Fetuin A in Human Blood. Journal of Clinical Medicine. 2021; 10(3):411. https://doi.org/10.3390/jcm10030411
Chicago/Turabian StyleKovářová, Markéta, Hubert Kalbacher, Andreas Peter, Hans-Ulrich Häring, Triantafyllos Didangelos, Norbert Stefan, Andreas Birkenfeld, Erwin Schleicher, and Konstantinos Kantartzis. 2021. "Detection and Characterization of Phosphorylation, Glycosylation, and Fatty Acid Bound to Fetuin A in Human Blood" Journal of Clinical Medicine 10, no. 3: 411. https://doi.org/10.3390/jcm10030411
APA StyleKovářová, M., Kalbacher, H., Peter, A., Häring, H.-U., Didangelos, T., Stefan, N., Birkenfeld, A., Schleicher, E., & Kantartzis, K. (2021). Detection and Characterization of Phosphorylation, Glycosylation, and Fatty Acid Bound to Fetuin A in Human Blood. Journal of Clinical Medicine, 10(3), 411. https://doi.org/10.3390/jcm10030411






