Atherogenic Index of Plasma in Obstructive Sleep Apnoea
Abstract
1. Introduction
2. Methods
2.1. Study Subjects and Design
2.2. Sleep Studies
2.3. Statistical Analyses
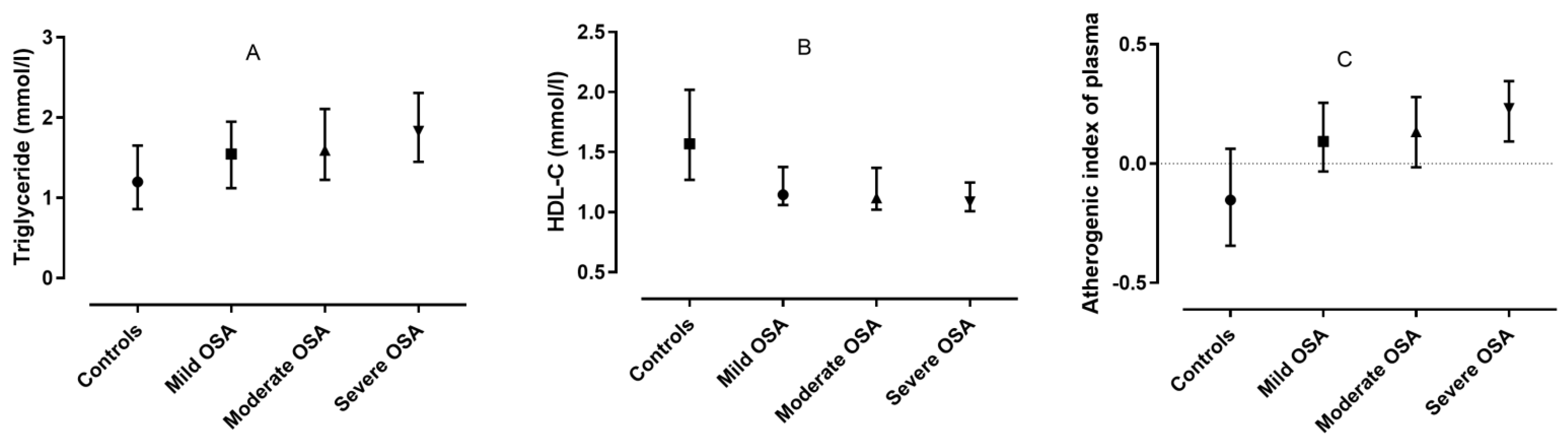
3. Results
3.1. Comparison of the OSA and Control Groups
3.2. The Relationship between Lipids and OSA
3.3. The Relationship between Lipids and OSA in Subjects Not Taking Statins
3.4. The Relationship between Lipids and OSA in Subjects Who Had PSG as a Diagnostic Test
3.5. Atherogenic Index of Plasma as a Predictor for Self-Reported Hypertension, Diabetes as Well as Cerebrovascular or Cardiovascular Disease in OSA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Marin, J.M.; Carrizo, S.J.; Vicente, E.; Agusti, A.G. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet 2005, 365, 1046–1053. [Google Scholar] [CrossRef]
- McEvoy, R.D.; Antic, N.A.; Heeley, E.; Luo, Y.; Ou, Q.; Zhang, X.; Mediano, O.; Chen, R.; Drager, L.F.; Liu, Z.; et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N. Engl. J. Med. 2016, 375, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Khatana, C.; Saini, N.K.; Chakrabarti, S.; Saini, V.; Sharma, A.; Saini, R.V.; Saini, A.K. Mechanistic Insights into the Oxidized Low-Density Lipoprotein-Induced Atherosclerosis. Oxid. Med. Cell. Longev. 2020, 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Borén, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef] [PubMed]
- Sniderman, A.D.; Couture, P.; Martin, S.S.; Degraaf, J.; Lawler, P.R.; Cromwell, W.C.; Wilkins, J.T.; Thanassoulis, G. Hypertriglyceridemia and cardiovascular risk: A cautionary note about metabolic confounding. J. Lipid Res. 2018, 59, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Barros, D.; García-Río, F. Obstructive sleep apnea and dyslipidemia: From animal models to clinical evidence. Sleep 2019, 42, zsy236. [Google Scholar] [CrossRef]
- Gileles-Hillel, A.; Kheirandish-Gozal, L.; Gozal, D. Biological plausibility linking sleep apnoea and metabolic dysfunction. Nat. Rev. Endocrinol. 2016, 12, 290–298. [Google Scholar] [CrossRef]
- Smith, S.S.; Waight, C.; Doyle, G.; Rossa, K.R.; Sullivan, K.A. Liking for high fat foods in patients with Obstructive Sleep Apnoea. Appetite 2014, 78, 185–192. [Google Scholar] [CrossRef]
- Jun, J.C.; Shin, M.-K.; Yao, Q.; Bevans-Fonti, S.; Poole, J.; Drager, L.F.; Polotsky, V.Y. Acute hypoxia induces hypertriglyceridemia by decreasing plasma triglyceride clearance in mice. Am. J. Physiol. Endocrinol. Metab. 2012, 303, e377–e388. [Google Scholar] [CrossRef]
- Li, J.; Grigoryev, D.; Ye, S.Q.; Thorne, L.; Schwartz, A.R.; Smith, P.L.; O’Donnell, C.P.; Polotsky, V.Y. Chronic intermittent hypoxia upregulates genes of lipid biosynthesis in obese mice. J. Appl. Physiol. 2005, 99, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Barceló, A.; Piérola, J.; De La Peña, M.; Esquinas, C.; Fuster, A.; Sánchez-De-La-Torre, M.; Carrera, M.; Alonso-Fernández, A.; Ladaria, A.; Bosch, M.; et al. Free fatty acids and the metabolic syndrome in patients with obstructive sleep apnoea. Eur. Respir. J. 2010, 37, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Bikov, A.; Lazar, Z.; Horvath, P.; Tarnoki, D.L.; Tarnoki, A.D.; Fesus, L.; Horvath, M.; Meszaros, M.; Losonczy, G.; Kunos, L. Association Between Serum Lipid Profile and Obstructive Respiratory Events During REM and Non-REM Sleep. Lung 2019, 197, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Shi, G.; Xue, S.; Lu, W. The atherogenic index of plasma is a strong and independent predictor for coronary artery disease in the Chinese Han population. Medicine 2017, 96, e8058. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-T.; Gao, Y.; Zheng, Y.-Y.; Ma, Y.-T.; Xie, X. Atherogenic index of plasma (AIP): A novel predictive indicator for the coronary artery disease in postmenopausal women. Lipids Health Dis. 2018, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dobiásová, M.; Frohlich, J. The plasma parameter log (TG/HDL-C) as an atherogenic index: Correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)). Clin. Biochem. 2001, 34, 583–588. [Google Scholar] [CrossRef]
- Edwards, M.K.; Blaha, M.J.; Loprinzi, P.D. Atherogenic Index of Plasma and Triglyceride/High-Density Lipoprotein Cholesterol Ratio Predict Mortality Risk Better Than Individual Cholesterol Risk Factors, Among an Older Adult Population. Mayo Clin. Proc. 2017, 92, 680–681. [Google Scholar] [CrossRef]
- Shimizu, Y.; Yoshimine, H.; Nagayoshi, M.; Kadota, K.; Takahashi, K.; Izumino, K.; Inoue, K.; Maeda, T. Serum triglyceride levels in relation to high-density lipoprotein cholesterol (TG-HDL) ratios as an efficient tool to estimate the risk of sleep apnea syndrome in non-overweight Japanese men. Environ. Health Prev. Med. 2016, 21, 321–326. [Google Scholar] [CrossRef][Green Version]
- Wu, W.-T.; Tsai, S.-S.; Shih, T.-S.; Lin, M.-H.; Chou, T.-C.; Ting, H.; Wu, T.-N.; Liou, S.-H. The Association between Obstructive Sleep Apnea and Metabolic Markers and Lipid Profiles. PLoS ONE 2015, 10, e0130279. [Google Scholar] [CrossRef][Green Version]
- Hermans, M.P.; Mahadeb, Y.P.; Katchunga, P.; Cirhuza, J.C.; Ahn, S.A.; Rousseau, M.F. Novel sexual dimorphisms of sleep apnea syndrome in diabetes. Diabetes Metab. Syndr. Clin. Res. Rev. 2014, 8, 36–44. [Google Scholar] [CrossRef]
- Cao, B.; Fan, Z.; Zhang, Y.; Li, T. Independent association of severity of obstructive sleep apnea with lipid metabolism of atherogenic index of plasma (AIP) and apoB/apoAI ratio. Sleep Breath. 2020, 24, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Lachowska, M.; Wysocki, J.; Balcerzak, J.; Prus, M.; Niemczyk, K. Sleep Quality and Atherogenic Risk in Sleep Apnea Patients. Electron. J. Gen. Med. 2016, 13, 28–36. [Google Scholar] [CrossRef]
- Silva, L.O.E.; Guimarães, T.D.M.; Luz, G.P.; Coelho, G.; Badke, L.; Almeida, I.R.; Millani-Carneiro, A.; Tufik, S.; Bittencourt, L.; Togeiro, S.M. Metabolic Profile in Patients with Mild Obstructive Sleep Apnea. Metab. Syndr. Relat. Disord. 2018, 16, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Meszaros, M.; Tarnoki, A.D.; Tarnoki, D.L.; Kovacs, D.T.; Forgo, B.; Lee, J.; Sung, J.; Vestbo, J.; Müller, V.; Kunos, L.; et al. Obstructive sleep apnea and hypertriglyceridaemia share common genetic background: Results of a twin study. J. Sleep Res. 2020, 29, e12979. [Google Scholar] [CrossRef] [PubMed]
- Bikov, A.; Frent, S.; Pleava, R.; Kunos, L.; Bokhari, S.; Meszaros, M.; Mihaicuta, S. The Burden of Associated Comorbidities in Patients with Obstructive Sleep Apnea—Regional Differences in Two Central–Eastern European Sleep Centers. J. Clin. Med. 2020, 9, 3583. [Google Scholar] [CrossRef]
- Kushida, C.A.; Littner, M.R.; Morgenthaler, T.; Alessi, C.A.; Bailey, D.; Coleman, J.; Friedman, L.; Hirshkowitz, M.; Kapen, S.; Kramer, M.; et al. Practice Parameters for the Indications for Polysomnography and Related Procedures: An Update for 2005. Sleep 2005, 28, 499–523. [Google Scholar] [CrossRef]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for Scoring Respiratory Events in Sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef]
- Delong, E.R.; Delong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837. [Google Scholar] [CrossRef]
- Gottlieb, D.J.; Punjabi, N.M. Diagnosis and Management of Obstructive Sleep Apnea: A Review. JAMA 2020, 323, 1389–1400. [Google Scholar] [CrossRef]
- Bikov, A.; Kolossvary, M.; Jermendy, A.L.; Drobni, Z.D.; Tarnoki, A.D.; Tarnoki, D.L.; Forgó, B.; Kovacs, D.T.; Losonzcy, G.; Kunos, L.; et al. Comprehensive coronary plaque assessment in patients with obstructive sleep apnea. J. Sleep Res. 2019, 28, e12828. [Google Scholar] [CrossRef]
- Johns, M.W. Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness Scale. Chest 1993, 103, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, C.; Jia, P.; Zhang, J.; Feng, L.; Wei, S.; Luo, Y.; Su, L.; Zhao, C.; Dong, H.; et al. The Association between the Phenotype of Excessive Daytime Sleepiness and Blood Pressure in Patients with Obstructive Sleep Apnea-Hypopnea Syndrome. Int. J. Med. Sci. 2014, 11, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Kuniyoshi, F.H.S.; Covassin, N.; Singh, P.; Gami, A.S.; Chahal, C.A.A.; Somers, V.K. Excessive Daytime Sleepiness Independently Predicts Increased Cardiovascular Risk After Myocardial Infarction. J. Am. Heart Assoc. 2018, 7, e007221. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.C.; Drager, L.F.; Najjar, S.S.; Gottlieb, S.S.; Brown, C.D.; Smith, P.L.; Schwartz, A.R.; Polotsky, V.Y. Effects of sleep apnea on nocturnal free fatty acids in subjects with heart failure. Sleep 2011, 34, 1207–1213. [Google Scholar] [CrossRef]
- Barter, P.J.; Brandrup-Wognsen, G.; Palmer, M.K.; Nicholls, S.J. Effect of statins on HDL-C: A complex process unrelated to changes in LDL-C: Analysis of the VOYAGER Database. J. Lipid Res. 2010, 51, 1546–1553. [Google Scholar] [CrossRef]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef]
- Gündüz, C.; Basoglu, O.K.; Hedner, J.; Zou, D.; Bonsignore, M.R.; Hein, H.; Staats, R.; Pataka, A.; Barbe, F.; Sliwinski, P.; et al. Obstructive sleep apnoea independently predicts lipid levels: Data from the European Sleep Apnea Database. Respirology 2018, 23, 1180–1189. [Google Scholar] [CrossRef]
- Drager, L.F.; Togeiro, S.M.; Polotsky, V.Y.; Lorenzi-Filho, G. Obstructive sleep apnea: A cardiometabolic risk in obesity and the metabolic syndrome. J. Am. Coll. Cardiol. 2013, 62, 569–576. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Langsted, A.; Mora, S.; Kolovou, G.; Baum, H.; Bruckert, E.; Watts, G.F.; Sypniewska, G.; Wiklund, I.; Borén, J.; et al. Fasting is not routinely required for determination of a lipid profile: Clinical and laboratory implications including flagging at desirable concentration cut-points—A joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur. Heart J. 2016, 37, 1944–1958. [Google Scholar]

| OSA (n = 461) | Control (n = 99) | p-Value | |
|---|---|---|---|
| Age (years) | 54/46–62/ | 46/34.5–59.5/ | <0.001 |
| Gender (% male) | 65 | 31 | <0.001 |
| BMI (kg/m2) | 31.8/28.3–35.8/ | 24.8/21.7–28.1/ | <0.001 |
| Smokers (ever%) | 42 | 14 | <0.001 |
| Cigarette pack years | 0/0–15/ | 0/0–0/ | <0.001 |
| Hypertension (%) | 68 | 38 | <0.001 |
| Cardiovascular or cerebrovascular disease (%) | 29 | 5 | <0.001 |
| Cardiac arrhythmia (%) | 26 | 12 | 0.002 |
| Diabetes mellitus (%) | 20 | 13 | 0.09 |
| COPD (%) | 12 | 6 | 0.08 |
| Statin users (%) | 24 | 6 | <0.001 |
| TGs (mmol/L) | 1.7/1.3–2.1/ | 1.2/0.9–1.6/ | <0.001 |
| TC (mmol/L) | 5.1/4.3–5.9/ | 5.2/4.7–6.1/ | 0.061 |
| LDL-C (mmol/L) | 2.9/2.4–3.8/ | 3.1/2.4–3.8/ | 0.868 |
| HDL-C (mmol/L) | 1.1/1.0–1.3/ | 1.6/1.3–2.0/ | <0.001 |
| AIP | 0.18/0.03–0.31/ | −0.15/−0.34–0.06/ | <0.001 |
| ESS | 8/5–11/ | 6/4–9/ | <0.001 |
| TST (min) * | 423/375–463/ | 400/358–425/ | 0.003 |
| SPT (min) * | 470/431–503/ | 423/397–444/ | <0.001 |
| Sleep% * | 91/82–97/ | 95/88–99/ | 0.008 |
| REM% * | 15.1/11.6–20.2/ | 16.1/12.2–20.8/ | 0.946 |
| AHI (1/h) | 28.6/14.9–47.7/ | 2.3/1.2–3.5/ | <0.001 |
| ODI (1/h) | 26.0/13.5–47.9/ | 1.1/0.4–2.2/ | <0.001 |
| TST90% (%) | 7.3/1.3–25.4/ | 0/0–0.1/ | <0.001 |
| MinSatO2 (%) | 82/75–87/ | 91/88–93/ | <0.001 |
| B | p | |
|---|---|---|
| TGs (mmol/L) | 0.33 | 0.07 |
| TC (mmol/L) | −0.27 | 0.02 |
| LDL-C (mmol/L) | −0.07 | 0.24 |
| HDL-C (mmol/L) | −1.02 | <0.001 |
| AIP | 2.02 | <0.001 |
| β | p-Value | |
|---|---|---|
| TGs (mmol/L) | 0.30 | 0.12 |
| TC (mmol/L) | −0.23 | 0.054 |
| LDL-C (mmol/L) | −0.07 | 0.21 |
| HDL-C (mmol/L) | −0.84 | 0.02 |
| AIP | 1.81 | <0.001 |
| B | p-value | |
|---|---|---|
| TGs (mmol/L) | 0.12 | 0.49 |
| TC (mmol/L) | −0.34 | 0.02 |
| LDL-C (mmol/L) | −0.05 | 0.45 |
| HDL-C (mmol/L) | −0.98 | 0.002 |
| AIP | 1.79 | 0.004 |
| Cerebrovascular and Cardiovascular Disease | Diabetes Mellitus | Arterial Hypertension | |||||
|---|---|---|---|---|---|---|---|
| AUC | 95% CI | AUC | 95% CI | AUC | 95% CI | ||
| AIP | 0.604 | 0.558 to 0.649 | 0.627 | 0.581 to 0.671 | 0.553 | 0.506 to 0.599 | |
| HDL-C | 0.586 | 0.539 to 0.631 | 0.579 | 0.532 to 0.625 | 0.561 | 0.515 to 0.607 | |
| LDL-C | 0.551 | 0.505 to 0.598 | 0.560 | 0.513 to 0.606 | 0.507 | 0.460 to 0.553 | |
| TC | 0.563 | 0.517 to 0.609 | 0.522 | 0.475 to 0.568 | 0.510 | 0.463 to 0.556 | |
| TGs | 0.601 | 0.555 to 0.646 | 0.626 | 0.580 to 0.670 | 0.536 | 0.489 to 0.582 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bikov, A.; Meszaros, M.; Kunos, L.; Negru, A.G.; Frent, S.M.; Mihaicuta, S. Atherogenic Index of Plasma in Obstructive Sleep Apnoea. J. Clin. Med. 2021, 10, 417. https://doi.org/10.3390/jcm10030417
Bikov A, Meszaros M, Kunos L, Negru AG, Frent SM, Mihaicuta S. Atherogenic Index of Plasma in Obstructive Sleep Apnoea. Journal of Clinical Medicine. 2021; 10(3):417. https://doi.org/10.3390/jcm10030417
Chicago/Turabian StyleBikov, Andras, Martina Meszaros, Laszlo Kunos, Alina Gabriela Negru, Stefan Marian Frent, and Stefan Mihaicuta. 2021. "Atherogenic Index of Plasma in Obstructive Sleep Apnoea" Journal of Clinical Medicine 10, no. 3: 417. https://doi.org/10.3390/jcm10030417
APA StyleBikov, A., Meszaros, M., Kunos, L., Negru, A. G., Frent, S. M., & Mihaicuta, S. (2021). Atherogenic Index of Plasma in Obstructive Sleep Apnoea. Journal of Clinical Medicine, 10(3), 417. https://doi.org/10.3390/jcm10030417








