Osteonecrosis of the Femoral Head Safely Healed with Autologous, Expanded, Bone Marrow-Derived Mesenchymal Stromal Cells in a Multicentric Trial with Minimum 5 Years Follow-Up
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Study Authorization and Declaration
2.3. Procedures
2.3.1. Bone Marrow Harvesting
2.3.2. Cell Product Manufacturing Process in GMP Facilities
2.3.3. Cell Product
2.3.4. Surgical Procedure
2.4. Outcomes
2.4.1. Safety
2.4.2. Efficacy
2.5. Statistical Analysis
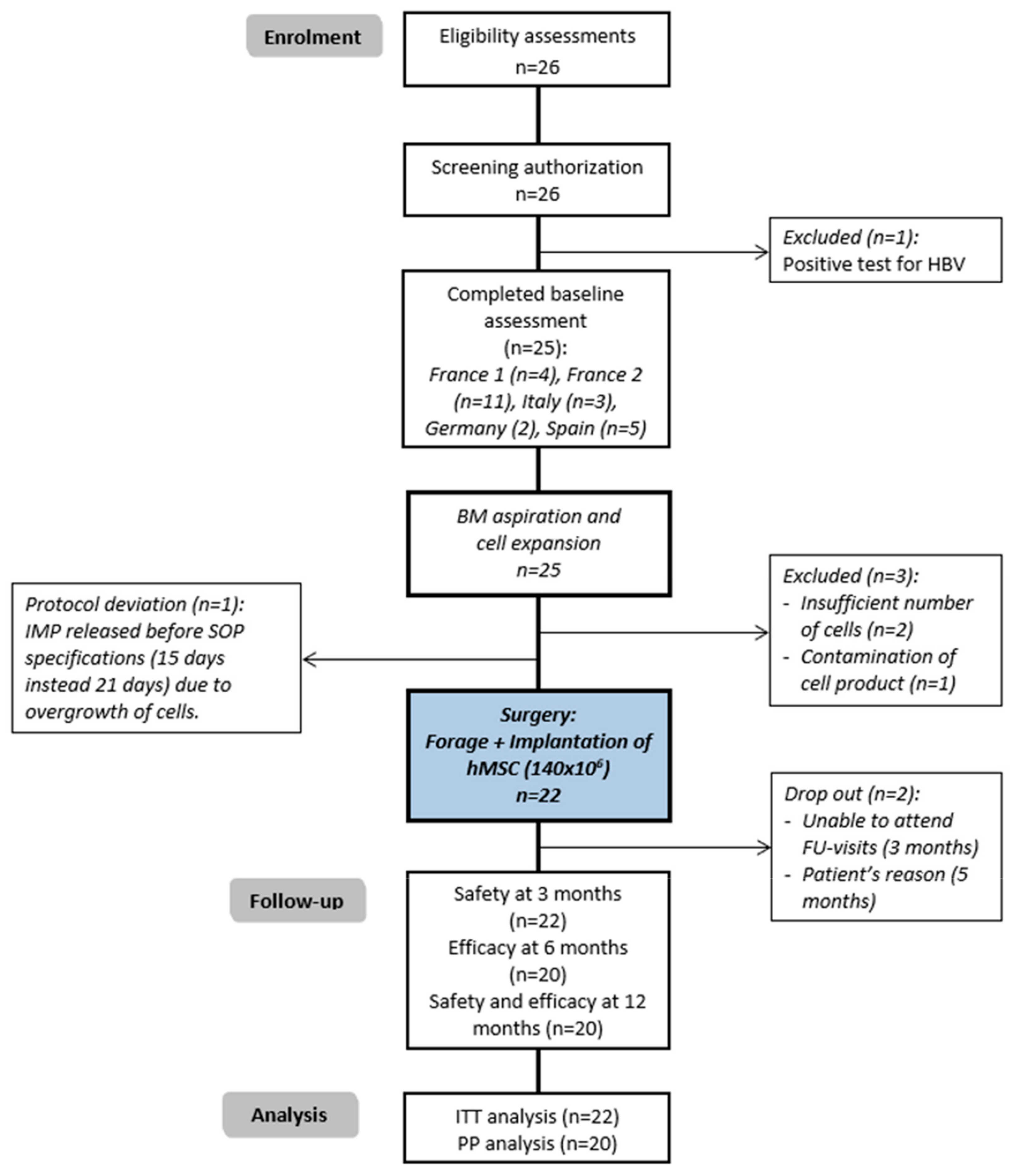
3. Results
3.1. Safety Endpoint
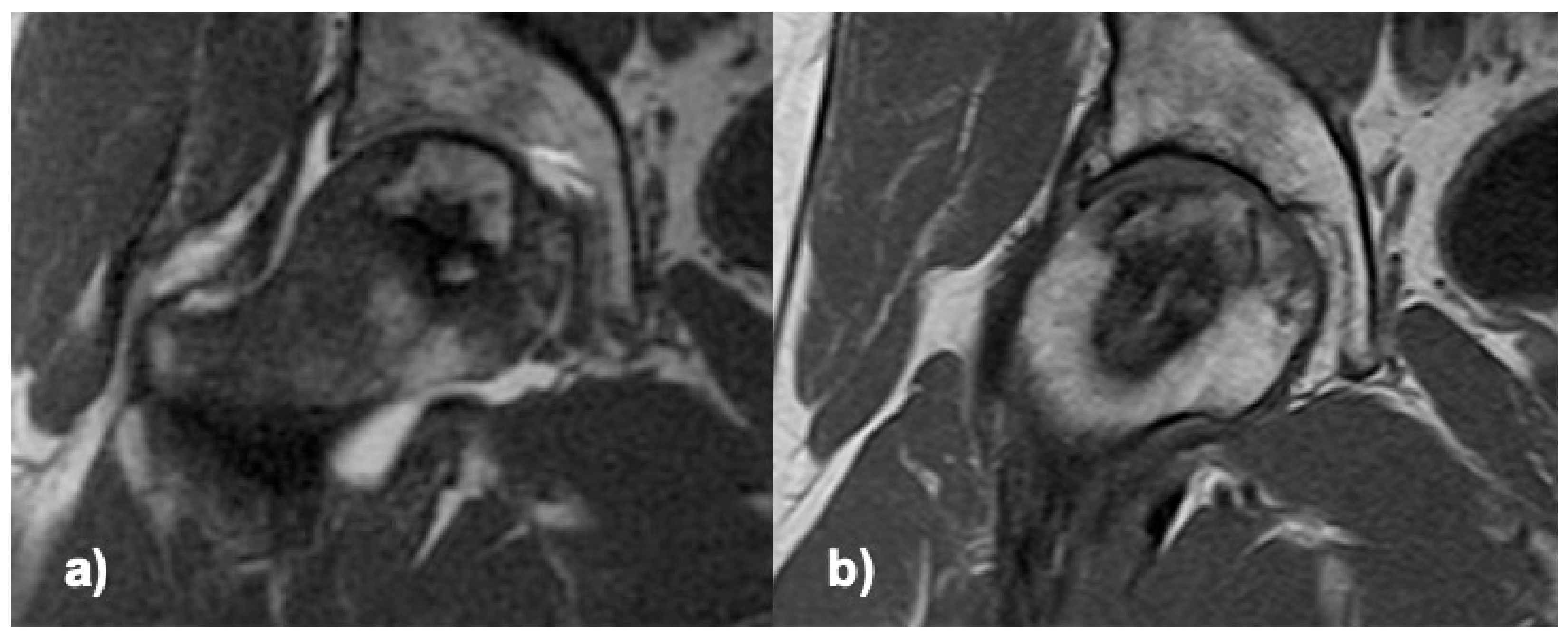
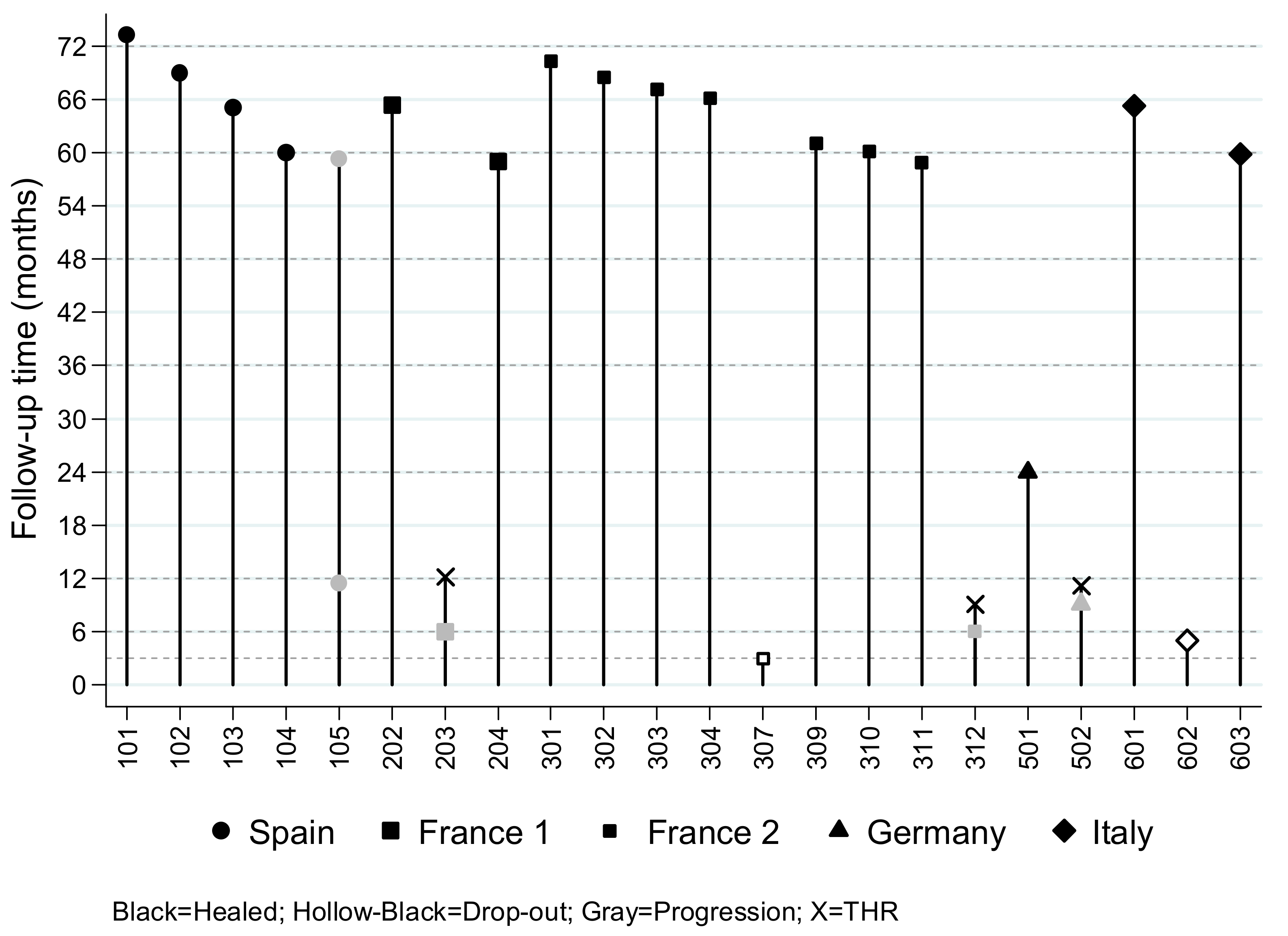
3.2. Efficacy Endpoint and Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takao, M.; Nishii, T.; Sakai, T.; Yoshikawa, H.; Sugano, N. Repair in osteonecrosis of the femoral head: MR imaging features at long-term follow-up. Clin. Rheumatol. 2010, 29, 841–848. [Google Scholar] [CrossRef]
- Hamada, H.; Takao, M.; Sakai, T.; Sugano, N. Subchondral fracture begins from the bone resorption area in osteonecrosis of the femoral head: A micro-computerised tomography study. Int. Orthop. 2018, 42, 1479–1484. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, D.; Chen, X.; Yang, J.; Shi, L.; Pang, Q. Effectiveness of various hip preservation treatments for non-traumatic osteonecrosis of the femoral head: A network meta-analysis of randomized controlled trials. J. Orthop. Sci. 2018, 23, 356–364. [Google Scholar] [CrossRef]
- Wang, C.; Meng, H.; Wang, Y.; Zhao, B.; Zhao, C.; Sun, W.; Zhu, Y.; Han, B.; Yuan, X.; Liu, R.; et al. Analysis of early stage osteonecrosis of the human femoral head and the mechanism of femoral head collapse. Int. J. Biol. Sci. 2018, 14, 156–164. [Google Scholar] [CrossRef]
- Kaneko, S.; Takegami, Y.; Seki, T.; Fukushima, W.; Sakai, T.; Ando, W.; Ishiguro, N.; Sugano, N. Surgery trends for osteonecrosis of the femoral head: A fifteen-year multi-centre study in Japan. Int. Orthop. 2020, 44, 761–769. [Google Scholar] [CrossRef]
- Aigner, N.; Schneider, W.; Eberl, V.; Knahr, K. Core decompression in early stages of femoral head osteonecrosis--an MRI-controlled study. Int. Orthop. 2002, 26, 31–35. [Google Scholar] [CrossRef]
- Bozic, K.J.; Zurakowski, D.; Thornhill, T.S. Survivorship analysis of hips treated with core decompression for nontraumatic osteonecrosis of the femoral head. J. Bone Jt. Surg. 1999, 81, 200–209. [Google Scholar] [CrossRef]
- Calori, G.M.; Mazza, E.; Colombo, A.; Mazzola, S.; Colombo, M. Core decompression and biotechnologies in the treatment of avascular necrosis of the femoral head. EFORT Open Rev. 2017, 2, 41–50. [Google Scholar] [CrossRef]
- Andriolo, L.; Merli, G.; Tobar, C.; Altamura, S.A.; Kon, E.; Filardo, G. Regenerative therapies increase survivorship of avascular necrosis of the femoral head: A systematic review and meta-analysis. Int. Orthop. 2018, 42, 1689–1704. [Google Scholar] [CrossRef]
- Brennan, M.A.; Renaud, A.; Amiaud, J.; Rojewski, M.T.; Schrezenmeier, H.; Heymann, D.; Trichet, V.; Layrolle, P. Pre-clinical studies of bone regeneration with human bone marrow stromal cells and biphasic calcium phosphate. Stem Cell Res. Ther. 2014, 5, 114. [Google Scholar] [CrossRef]
- Zhao, D.; Cui, D.; Wang, B.; Tian, F.; Guo, L.; Yang, L.; Liu, B.; Yu, X. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone 2012, 50, 325–330. [Google Scholar] [CrossRef]
- Sensebé, L.; Bourin, P.; Tarte, K. Good manufacturing practices production of mesenchymal stem/stromal cells. Hum. Gene Ther. 2011, 22, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, E.; Murgia, A.; Caselli, A.; Grisendi, G.; Piccinno, M.S.; Rasini, V.; Giordano, R.; Montemurro, T.; Bourin, P.; Sensebe, L.; et al. Transportation conditions for prompt use of ex vivo expanded and freshly harvested clinical-grade bone marrow mesenchymal stromal/stem cells for bone regeneration. Tissue Eng. Part C Methods 2014, 20, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Barrena, E.; Padilla-Eguiluz, N.G.; Avendano-Sola, C.; Payares-Herrera, C.; Velasco-Iglesias, A.; Torres, F.; Rosset, P.; Gebhard, F.; Baldini, N.; Rubio-Suarez, J.C.; et al. A Multicentric, Open-Label, Randomized, Comparative Clinical Trial of Two Different Doses of Expanded hBM-MSCs Plus Biomaterial versus Iliac Crest Autograft, for Bone Healing in Nonunions after Long Bone Fractures: Study Protocol. Stem Cells Int. 2018, 2018, 6025918. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Barrena, E.; Padilla-Eguiluz, N.; Rosset, P.; Gebhard, F.; Hernigou, P.; Baldini, N.; Rouard, H.; Sensebe, L.; Gonzalo-Daganzo, R.M.; Giordano, R.; et al. Early efficacy evaluation of mesenchymal stromal cells (MSC) combined to biomaterials to treat long bone non-unions. Injury 2020, 51 (Suppl. 1), S63–S73. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Jensen, M.P.; Chen, C.; Brugger, A.M. Interpretation of visual analog scale ratings and change scores: A reanalysis of two clinical trials of postoperative pain. J. Pain 2003, 4, 407–414. [Google Scholar] [CrossRef]
- Gómez-Barrena, E.; Rosset, P.; Gebhard, F.; Hernigou, P.; Baldini, N.; Rouard, H.; Sensebé, L.; Gonzalo-Daganzo, R.M.; Giordano, R.; Padilla-Eguiluz, N.; et al. Feasibility and safety of treating non-unions in tibia, femur and humerus with autologous, expanded, bone marrow-derived mesenchymal stromal cells associated with biphasic calcium phosphate biomaterials in a multicentric, non-comparative trial. Biomaterials 2019, 196, 100–108. [Google Scholar] [CrossRef]
- Hernigou, P.; Trousselier, M.; Roubineau, F.; Bouthors, C.; Chevallier, N.; Rouard, H.; Flouzat-Lachaniette, C.H. Stem Cell Therapy for the Treatment of Hip Osteonecrosis: A 30-Year Review of Progress. Clin. Orthop. Surg. 2016, 8, 1–8. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, L.; Jin, H.; Shan, L.; Zhou, L.; Xiao, L.; Tong, P. Autologous Stem Cells Combined Core Decompression for Treatment of Avascular Necrosis of the Femoral Head: A Systematic Meta-Analysis. Biomed. Res. Int. 2017, 2017, 6136205. [Google Scholar] [CrossRef]
- Papakostidis, C.; Tosounidis, T.H.; Jones, E.; Giannoudis, P.V. The role of “cell therapy” in osteonecrosis of the femoral head. A systematic review of the literature and meta-analysis of 7 studies. Acta Orthop. 2016, 87, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Hernigou, P.; Beaujean, F.; Lambotte, J.C. Decrease in the mesenchymal stem-cell pool in the proximal femur in corticosteroid-induced osteonecrosis. J. Bone Jt. Surg. 1999, 81, 349–355. [Google Scholar] [CrossRef]
- Mao, Q.; Jin, H.; Liao, F.; Xiao, L.; Chen, D.; Tong, P. The efficacy of targeted intraarterial delivery of concentrated autologous bone marrow containing mononuclear cells in the treatment of osteonecrosis of the femoral head: A five year follow-up study. Bone 2013, 57, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Wang, W.; Xu, T.; Zhang, S.; Xiao, L.; Chen, D.; Jin, H.; Tong, P. Combination treatment of biomechanical support and targeted intra-arterial infusion of peripheral blood stem cells mobilized by granulocyte-colony stimulating factor for the osteonecrosis of the femoral head: A randomized controlled clinical trial. J. Bone Miner. Res. 2015, 30, 647–656. [Google Scholar] [CrossRef]
- Min, B.W.; Song, K.S.; Cho, C.H.; Lee, S.M.; Lee, K.J. Untreated asymptomatic hips in patients with osteonecrosis of the femoral head. Clin. Orthop. Relat. Res. 2008, 466, 1087–1092. [Google Scholar] [CrossRef]
- Koo, K.H.; Kim, R.; Ko, G.H.; Song, H.R.; Jeong, S.T.; Cho, S.H. Preventing collapse in early osteonecrosis of the femoral head. A randomised clinical trial of core decompression. J. Bone Jt. Surg. 1995, 77, 870–874. [Google Scholar] [CrossRef]
- Cruz-Pardos, A.; Garcia-Rey, E.; Ortega-Chamarro, J.A.; Duran-Manrique, D.; Gomez-Barrena, E. Mid-term comparative outcomes of autologous bone-marrow concentration to treat osteonecrosis of the femoral head in standard practice. Hip Int. 2016, 26, 432–437. [Google Scholar] [CrossRef]
- Gangji, V.; De Maertelaer, V.; Hauzeur, J.P. Autologous bone marrow cell implantation in the treatment of non-traumatic osteonecrosis of the femoral head: Five year follow-up of a prospective controlled study. Bone 2011, 49, 1005–1009. [Google Scholar] [CrossRef]
- Sen, R.K.; Tripathy, S.K.; Aggarwal, S.; Marwaha, N.; Sharma, R.R.; Khandelwal, N. Early results of core decompression and autologous bone marrow mononuclear cells instillation in femoral head osteonecrosis: A randomized control study. J. Arthroplast. 2012, 27, 679–686. [Google Scholar] [CrossRef]
- Tabatabaee, R.M.; Saberi, S.; Parvizi, J.; Mortazavi, S.M.; Farzan, M. Combining Concentrated Autologous Bone Marrow Stem Cells Injection with Core Decompression Improves Outcome for Patients with Early-Stage Osteonecrosis of the Femoral Head: A Comparative Study. J. Arthroplast. 2015, 30, 11–15. [Google Scholar] [CrossRef]
- Aoyama, T.; Goto, K.; Kakinoki, R.; Ikeguchi, R.; Ueda, M.; Kasai, Y.; Maekawa, T.; Tada, H.; Teramukai, S.; Nakamura, T.; et al. An exploratory clinical trial for idiopathic osteonecrosis of femoral head by cultured autologous multipotent mesenchymal stromal cells augmented with vascularized bone grafts. Tissue Eng. Part B Rev. 2014, 20, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Jiang, P.; Lei, X.; Ni, C.; Zhang, Y.; Zhang, B.; Zheng, Q.; Li, D. Efficacy and safety of stem cell therapy for the early-stage osteonecrosis of femoral head: A systematic review and meta-analysis of randomized controlled trials. Stem Cell Res. Ther. 2020, 11, 445. [Google Scholar] [CrossRef] [PubMed]
- Hernigou, P.; Poignard, A.; Beaujean, F.; Rouard, H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J. Bone Jt. Surg. 2005, 87, 1430–1437. [Google Scholar] [CrossRef]
| Variables | Mean n | SD % | Min | Max |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 43.1 | 10.9 | 21.0 | 62.0 |
| Height (cm) | 173.7 | 8.7 | 152 | 190 |
| Weight (Kg) | 77.4 | 20.0 | 50 | 118 |
| BMI | 25.7 | 5.6 | 17.3 | 36.8 |
| Male sex | 19 | 86% | ||
| ASA: | ||||
| I | 5 | 22.7% | ||
| II | 14 | 63.6% | ||
| III | 3 | 12.6% | ||
| Alcohol history (yes) | 7 | 32% | ||
| No. drinks per day 1 | 2.2 | 1.9 | 1 | 6 |
| Duration (years) 2 | 12.8 | 6.9 | 2 | 20 |
| History of smoking (yes) | 11 | 50% | ||
| No. packs per day | 1.1 | 0.7 | 0.2 | 2 |
| Duration (years) 3 | 19.8 | 8.8 | 5 | 30 |
| Months since diagnosis of ONFH 4 | 2.3 | 2.2 | 0.1 | 7.6 |
| Cause of ONFH: | ||||
| Alcohol consumption | 1 | 4.5% | ||
| Corticosteroids | 5 | 22.7% | ||
| Idiopathic | 11 | 50.0% | ||
| Other 5 | 5 | 22.7% | ||
| Description of the affected femur | ||||
| Laterality (Right) | 13 | 59% | ||
| ARCO classification: | ||||
| IIA | 15 | 68.2% | ||
| IIB | 6 | 27.3% | ||
| IIC | 1 | 4.5% | ||
| Loss of bone in AP X-ray (none) 6 | 18 | 82% | ||
| Preoperative clinical data | ||||
| Total Harris Hip Score | 63.0 | 19.0 | 40 | 91 |
| Spontaneous pain in VAS scale (mm) 7 | 30.3 | 20.5 | 1 | 76 |
| Weight-bearing pain in VAS scale (mm) 8 | 57.9 | 21.1 | 10 | 100 |
| Mean | SD | Min | Max | |
|---|---|---|---|---|
| BM aspiration | ||||
| Aspirated bone marrow volume | 51.20 | 8.80 | 31.50 | 65.00 |
| Cell count WBC/mL BM aspirate (×107) | 2.07 | 11.40 | 11.20 | 61.00 |
| CFU-F of BM/×106 WBC | 45 | 53 | 0 | 190 |
| Doubling time and population doubling in p0 and p1 of expansion | ||||
| Doubling time in P0 (h) | 23.80 | 3.30 | 12.40 | 28.20 |
| Doubling time in P1 (h) | 54.80 | 31.50 | 22.10 | 186.00 |
| Number if population doublings in P0 | 14.60 | 3.00 | 12.40 | 27.10 |
| Number if population doublings in P1 | 3.20 | 1.20 | 0.90 | 6.50 |
| Cumulative population | 17.70 | 3.40 | 13.80 | 30.00 |
| Yield | ||||
| MSC/ul BM aspirate in P0 (×103) | 8.46 | 10.80 | 0.24 | 51.00 |
| MSC/ul BM aspirate in P1 (×104)z | 6.90 | 9.31 | 0.21 | 35.20 |
| Overall harvest (×108) | 2.87 | 1.87 | 0.42 | 7.38 |
| Identity: Surface markets after P1 | ||||
| %CD34 positive cells | 0.29 | 0.26 | −0.09 | 1.30 |
| %CD45 positive cells | 0.49 | 1.14 | 0.00 | 5.40 |
| %CD73 positive cells | 97.81 | 4.77 | 78.00 | 100.00 |
| %CD90 positive cells | 99.30 | 0.49 | 93.20 | 100.00 |
| %CD105 positive cells | 97.38 | 4.20 | 82.40 | 100.00 |
| %MHC cII positive cells | 3.62 | 7.01 | 0.00 | 27.60 |
| Viability | ||||
| % Viable cells in aspirate | 95.37 | 3.20 | 89.40 | 99.99 |
| % Viable cells after P0 | 94.92 | 4.70 | 82.70 | 100.00 |
| % Viable cells after P1 | 96.29 | 2.82 | 88.30 | 99.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Barrena, E.; Padilla-Eguiluz, N.G.; Rosset, P.; Hernigou, P.; Baldini, N.; Ciapetti, G.; Gonzalo-Daganzo, R.M.; Avendaño-Solá, C.; Rouard, H.; Giordano, R.; et al. Osteonecrosis of the Femoral Head Safely Healed with Autologous, Expanded, Bone Marrow-Derived Mesenchymal Stromal Cells in a Multicentric Trial with Minimum 5 Years Follow-Up. J. Clin. Med. 2021, 10, 508. https://doi.org/10.3390/jcm10030508
Gómez-Barrena E, Padilla-Eguiluz NG, Rosset P, Hernigou P, Baldini N, Ciapetti G, Gonzalo-Daganzo RM, Avendaño-Solá C, Rouard H, Giordano R, et al. Osteonecrosis of the Femoral Head Safely Healed with Autologous, Expanded, Bone Marrow-Derived Mesenchymal Stromal Cells in a Multicentric Trial with Minimum 5 Years Follow-Up. Journal of Clinical Medicine. 2021; 10(3):508. https://doi.org/10.3390/jcm10030508
Chicago/Turabian StyleGómez-Barrena, Enrique, Norma G. Padilla-Eguiluz, Philippe Rosset, Philippe Hernigou, Nicola Baldini, Gabriela Ciapetti, Rosa M. Gonzalo-Daganzo, Cristina Avendaño-Solá, Hélène Rouard, Rosaria Giordano, and et al. 2021. "Osteonecrosis of the Femoral Head Safely Healed with Autologous, Expanded, Bone Marrow-Derived Mesenchymal Stromal Cells in a Multicentric Trial with Minimum 5 Years Follow-Up" Journal of Clinical Medicine 10, no. 3: 508. https://doi.org/10.3390/jcm10030508
APA StyleGómez-Barrena, E., Padilla-Eguiluz, N. G., Rosset, P., Hernigou, P., Baldini, N., Ciapetti, G., Gonzalo-Daganzo, R. M., Avendaño-Solá, C., Rouard, H., Giordano, R., Dominici, M., Schrezenmeier, H., Layrolle, P., & on behalf of the REBORNE Consortium. (2021). Osteonecrosis of the Femoral Head Safely Healed with Autologous, Expanded, Bone Marrow-Derived Mesenchymal Stromal Cells in a Multicentric Trial with Minimum 5 Years Follow-Up. Journal of Clinical Medicine, 10(3), 508. https://doi.org/10.3390/jcm10030508










