Elucidation of Novel Therapeutic Targets for Breast Cancer with ESR1-CCDC170 Fusion
Abstract
:1. Introduction
2. Experimental Section
2.1. Sample Acquisition and Quality Control
2.2. Case-Control Selection
2.3. Selection of Genes Affected by ESR1-CCDC170 Fusion
2.4. Pathway Analysis via ConsensusPathDB (CPDB) and Over-Representation
2.5. Druggable Pathway Analysis via CIViC and OncoKB
2.6. Statistical Analysis and Data Visualization
3. Results
3.1. Clinico-Pathological Characteristics
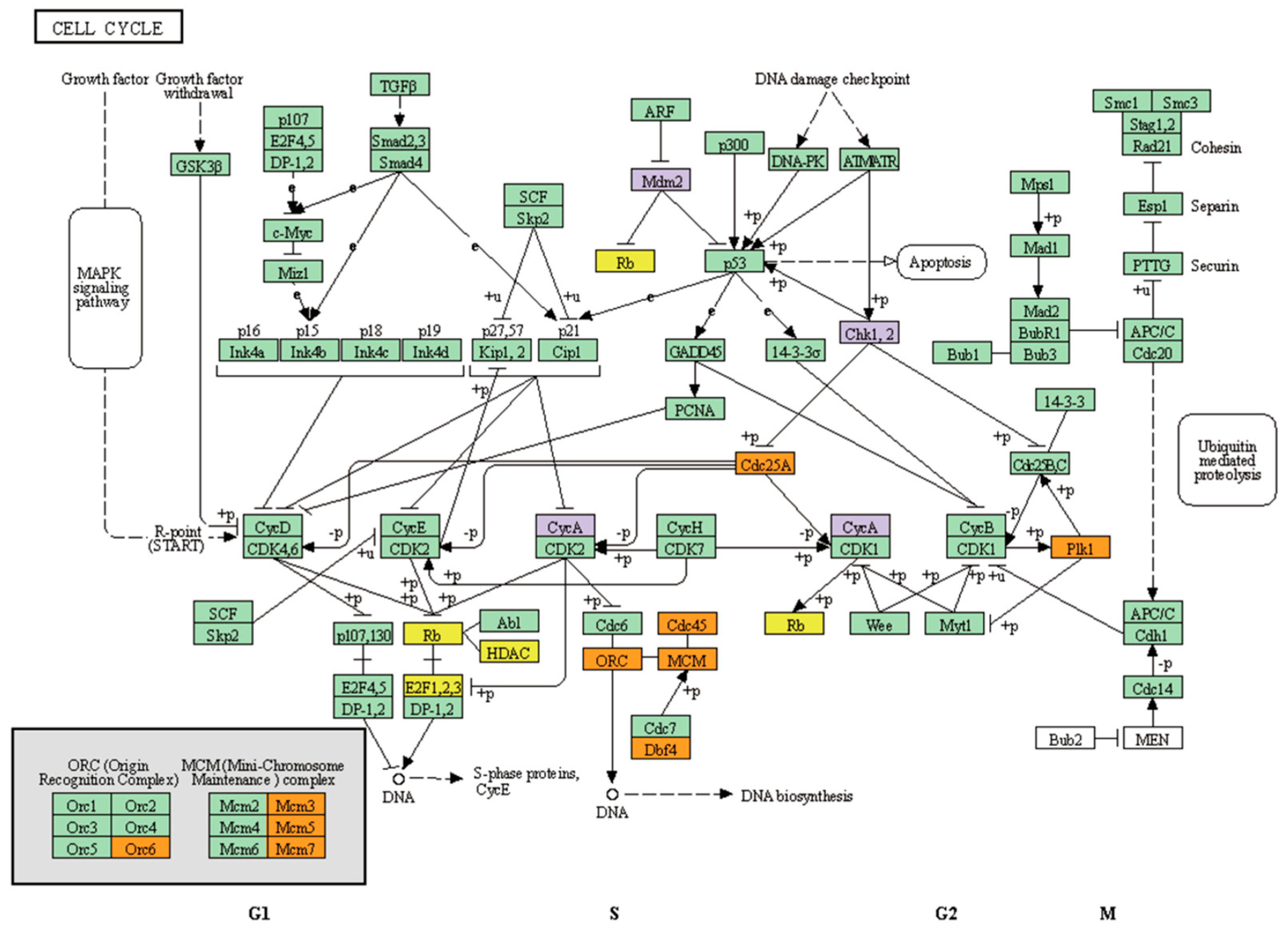
3.2. Key Pathways and Genes Altered in ESR1-CCDC170 Fusion-Positive Breast Cancer
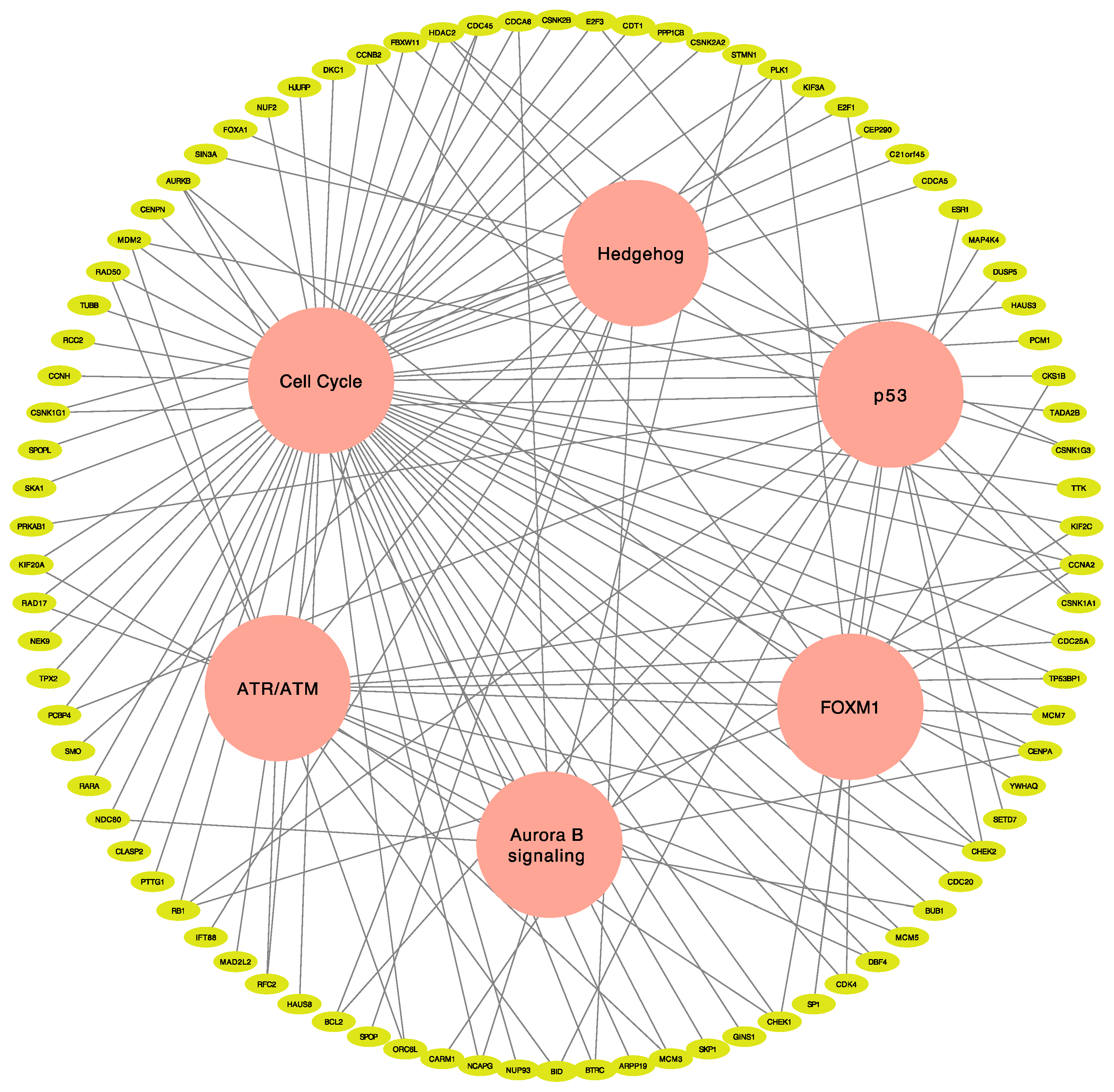
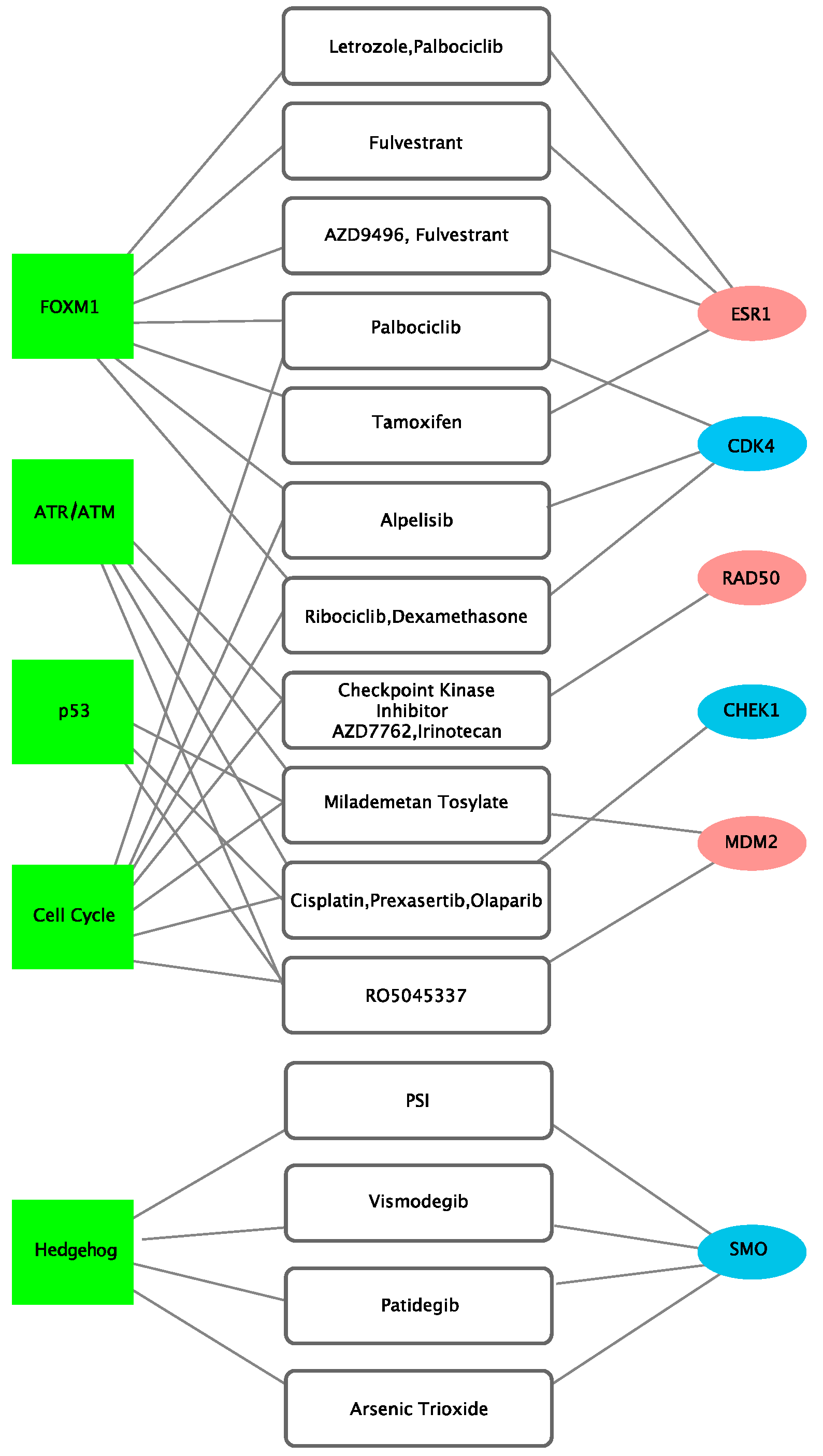
3.3. Identification of Actionable Targets and Potential Therapeutic Choice Using Network Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Waks, A.G.; Winer, E.P. Breast cancer treatment: A review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Fimereli, D.; Fumagalli, D.; Brown, D.; Gacquer, D.; Rothe, F.; Salgado, R.; Larsimont, D.; Sotiriou, C.; Detours, V. Genomic hotspots but few recurrent fusion genes in breast cancer. Genes Chromosomes Cancer 2018, 57, 331–338. [Google Scholar] [CrossRef]
- Giltnane, J.M.; Hutchinson, K.E.; Stricker, T.P.; Formisano, L.; Young, C.D.; Estrada, M.V.; Nixon, M.J.; Du, L.; Sanchez, V.; Ericsson, P.G.; et al. Genomic profiling of ER(+) breast cancers after short-term estrogen suppression reveals alterations associated with endocrine resistance. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Goksu, S.S.; Tastekin, D.; Arslan, D.; Gunduz, S.; Tatli, A.M.; Unal, D.; Salim, D.; Guler, T.; Coskun, H.S. Clinicopathologic features and molecular subtypes of breast cancer in young women (age ≤ 35). Asian Pac. J. Cancer Prev. 2014, 15, 6665–6668. [Google Scholar] [CrossRef] [Green Version]
- Hartmaier, R.J.; Trabucco, S.E.; Priedigkeit, N.; Chung, J.H.; Parachoniak, C.A.; Vanden Borre, P.; Morley, S.; Rosenzweig, M.; Gay, L.M.; Goldberg, M.E.; et al. Recurrent hyperactive ESR1 fusion proteins in endocrine therapy-resistant breast cancer. Ann. Oncol. 2018, 29, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Matissek, K.J.; Onozato, M.L.; Sun, S.; Zheng, Z.; Schultz, A.; Lee, J.; Patel, K.; Jerevall, P.L.; Saladi, S.V.; Macleay, A.; et al. Expressed gene fusions as frequent drivers of poor outcomes in hormone receptor-positive breast cancer. Cancer Discov. 2018, 8, 336–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veeraraghavan, J.; Tan, Y.; Cao, X.X.; Kim, J.A.; Wang, X.; Chamness, G.C.; Maiti, S.N.; Cooper, L.J.; Edwards, D.P.; Contreras, A.; et al. Recurrent ESR1-CCDC170 rearrangements in an aggressive subset of oestrogen receptor-positive breast cancers. Nat. Commun. 2014, 5, 4577. [Google Scholar] [CrossRef] [Green Version]
- Yersal, O.; Barutca, S. Biological subtypes of breast cancer: Prognostic and therapeutic implications. World J. Clin. Oncol. 2014, 5, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Veeraraghavan, J.; Ma, J.; Hu, Y.; Wang, X.S. Recurrent and pathological gene fusions in breast cancer: Current advances in genomic discovery and clinical implications. Breast Cancer Res. Treat. 2016, 158, 219–232. [Google Scholar] [CrossRef]
- Turnbull, C.; Ahmed, S.; Morrison, J.; Pernet, D.; Renwick, A.; Maranian, M.; Seal, S.; Ghoussaini, M.; Hines, S.; Healey, C.S.; et al. Genome-wide association study identifies five new breast cancer susceptibility loci. Nat. Genet. 2010, 42, 504–507. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; He, Y.; Qin, Z.; Jiang, Y.; Jin, G.; Ma, H.; Dai, J.; Chen, J.; Hu, Z.; Guan, X.; et al. Evaluation of functional genetic variants at 6q25.1 and risk of breast cancer in a Chinese population. Breast Cancer Res. 2014, 16, 422. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Lin, L.; Veeraraghavan, J.; Hu, Y.; Wang, X.; Lee, S.; Tan, Y.; Schiff, R.; Wang, X.S. Therapeutic role of recurrent ESR1-CCDC170 gene fusions in breast cancer endocrine resistance. Breast Cancer Res. 2020, 22, 84. [Google Scholar] [CrossRef]
- Yang, S.Y.C.; Lheureux, S.; Karakasis, K.; Burnier, J.V.; Bruce, J.P.; Clouthier, D.L.; Danesh, A.; Quevedo, R.; Dowar, M.; Hanna, Y.; et al. Landscape of genomic alterations in high-grade serous ovarian cancer from exceptional long- and short-term survivors. Genome Med. 2018, 10, 81. [Google Scholar] [CrossRef] [Green Version]
- Yoshihara, K.; Wang, Q.; Torres-Garcia, W.; Zheng, S.; Vegesna, R.; Kim, H.; Verhaak, R.G. The landscape and therapeutic relevance of cancer-associated transcript fusions. Oncogene 2015, 34, 4845–4854. [Google Scholar] [CrossRef] [Green Version]
- Wilson, R.G.; Smith, A.N.; Bird, C.C. Immunohistochemical detection of abnormal cell proliferation in colonic mucosa of subjects with polyps. J. Clin. Pathol. 1990, 43, 744–747. [Google Scholar] [CrossRef] [Green Version]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandasamy, K.; Mohan, S.S.; Raju, R.; Keerthikumar, S.; Kumar, G.S.; Venugopal, A.K.; Telikicherla, D.; Navarro, J.D.; Mathivanan, S.; Pecquet, C.; et al. NetPath: A public resource of curated signal transduction pathways. Genome Biol. 2010, 11, R3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaefer, C.F.; Anthony, K.; Krupa, S.; Buchoff, J.; Day, M.; Hannay, T.; Buetow, K.H. PID: The pathway interaction database. Nucleic Acids Res. 2009, 37, D674–D679. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, A.; Sidiropoulos, K.; Garapati, P.; Gillespie, M.; Hausmann, K.; Haw, R.; Jassal, B.; Jupe, S.; Korninger, F.; McKay, S.; et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2016, 44, D481–D487. [Google Scholar] [CrossRef] [Green Version]
- Kutmon, M.; Riutta, A.; Nunes, N.; Hanspers, K.; Willighagen, E.L.; Bohler, A.; Melius, J.; Waagmeester, A.; Sinha, S.R.; Miller, R.; et al. WikiPathways: Capturing the full diversity of pathway knowledge. Nucleic Acids Res. 2016, 44, D488–D494. [Google Scholar] [CrossRef] [PubMed]
- Jeselsohn, R.; Buchwalter, G.; de Angelis, C.; Brown, M.; Schiff, R. ESR1 mutations—A mechanism for acquired endocrine resistance in breast cancer. Nat. Rev. Clin. Oncol. 2015, 12, 573–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, J.T.; Gou, X.; Seker, S.; Ellis, M.J. ESR1 alterations and metastasis in estrogen receptor positive breast cancer. J. Cancer Metastasis Treat. 2019, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, C.X.; Reinert, T.; Chmielewska, I.; Ellis, M.J. Mechanisms of aromatase inhibitor resistance. Nat. Rev. Cancer 2015, 15, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.H.; Park, J.H.; Fan, S. Predicting and overcoming chemotherapeutic resistance in breast cancer. Adv. Exp. Med. Biol. 2017, 1026, 59–104. [Google Scholar] [CrossRef] [PubMed]
- Condorelli, R.; Spring, L.; O’Shaughnessy, J.; Lacroix, L.; Bailleux, C.; Scott, V.; Dubois, J.; Nagy, R.J.; Lanman, R.B.; Iafrate, A.J.; et al. Polyclonal RB1 mutations and acquired resistance to CDK 4/6 inhibitors in patients with metastatic breast cancer. Ann. Oncol. 2018, 29, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Wierer, M.; Verde, G.; Pisano, P.; Molina, H.; Font-Mateu, J.; Di Croce, L.; Beato, M. PLK1 signaling in breast cancer cells cooperates with estrogen receptor-dependent gene transcription. Cell Rep. 2013, 3, 2021–2032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, T.J.; Liu, J.C.; Vizeacoumar, F.; Sun, T.; Maclean, N.; Egan, S.E.; Schimmer, A.D.; Datti, A.; Zacksenhaus, E. RB1 status in triple negative breast cancer cells dictates response to radiation treatment and selective therapeutic drugs. PLoS ONE 2013, 8, e78641. [Google Scholar] [CrossRef] [Green Version]
- McGovern, S.L.; Qi, Y.; Pusztai, L.; Symmans, W.F.; Buchholz, T.A. Centromere protein-A, an essential centromere protein, is a prognostic marker for relapse in estrogen receptor-positive breast cancer. Breast Cancer Res. 2012, 14, R72. [Google Scholar] [CrossRef] [Green Version]
- Nevanlinna, H.; Bartek, J. The CHEK2 gene and inherited breast cancer susceptibility. Oncogene 2006, 25, 5912–5919. [Google Scholar] [CrossRef] [Green Version]
- Shan, W.; Jiang, Y.; Yu, H.; Huang, Q.; Liu, L.; Guo, X.; Li, L.; Mi, Q.; Zhang, K.; Yang, Z. HDAC2 overexpression correlates with aggressive clinicopathological features and DNA-damage response pathway of breast cancer. Am. J. Cancer Res. 2017, 7, 1213–1226. [Google Scholar] [PubMed]


| Total (n = 1095) | Control (n = 48) | Fusion (n = 11) | p-Values | |
|---|---|---|---|---|
| age | 46–72 | 44–68 | 49–71 | 0.001 |
| sex | ||||
| - female | 1082 (98.8%) | 48 (100.0%) | 11 (100.0%) | |
| Vital status | 1 | |||
| - alive | 991 (90.5%) | 42 (87.5%) | 10 (90.9%) | |
| - dead | 104 (9.5%) | 6 (13.6%) | 1 (9.1%) | |
| stage | NS | |||
| - stage I | 90 (8.3%) | 2 (4.2%) | 1 (9.1%) | |
| - stage Ia | 85 (7.8%) | 3 (6.2%) | 1 (9.1%) | |
| - stage Ib | 6 (0.6%) | 0 (0.0%) | 0 (0.0%) | |
| - stage II | 6 (0.6%) | 0 (0.0%) | 0 (0.0%) | |
| - stage IIa | 359 (33.0%) | 24 (50.0%) | 3 (27.3%) | |
| - stage IIb | 257 (23.6%) | 9 (18.8%) | 3 (27.3%) | |
| - stage III | 2 (0.2%) | 0 (0.0%) | 0 (0.0%) | |
| - stage IIIa | 156 (14.4%) | 4 (8.3%) | 2 (18.2%) | |
| - stage IIIb | 27 (2.5%) | 0 (0.0%) | 0 (0.0%) | |
| - stage IIIc | 65 (6.0%) | 3 (6.2%) | 1 (9.1%) | |
| - stage Iv | 20 (1.8%) | 2 (4.2%) | 0 (0.0%) | |
| - stage x | 14 (1.3%) | 1 (2.1%) | 0 (0.0%) | |
| ER status | 0 | |||
| - positive | 808 (77.2%) | 10 (20.8%) | 10 (90.9%) | |
| - negative | 237 (22.5%) | 38 (79.2%) | 0 (0.0%) | |
| - indeterminate | 2 (0.2%) | 0 (0.0%) | 1 (9.1%) | |
| PR status | 0 | |||
| - positive | 700 (66.9%) | 2 (4.3%) | 7 (63.6%) | |
| - negative | 342 (32.7%) | 45 (95.7%) | 4 (36.4%) | |
| - indeterminate | 4 (0.4%) | 0 (0.0%) | 0 (0.0%) | |
| HER2 IHC | 0 | |||
| 0 | 61 (9.8%) | 7 (21.9%) | 0 (0.0%) | |
| - 1+ | 270 (43.4%) | 12 (37.5%) | 4 (44.4%) | |
| - 2+ | 199 (32.1%) | 10 (31.2%) | 1 (11.1%) | |
| - 3+ | 90 (14.5%) | 3 (9.4%) | 4 (44.4%) | |
| subtype | 0 | |||
| - Basal | 190 (18.0%) | 42 (89.4%) | 0 (0.0%) | |
| - Her2 | 82 (7.8%) | 5 (10.6%) | 1 (9.1%) | |
| - LumA | 566 (53.6%) | 0 (0.0%) | 5 (45.5%) | |
| - LumB | 217 (20.6%) | 0 (0.0%) | 5 (45.5%) | |
| PIK3CA mutation | 301 (31.2%) | 3 (6.8%) | 1 (10.0%) | NS |
| CDH1 mutation | 96 (10.0%) | 0 (0.0%) | 1 (10.0%) | NS |
| TP53 mutation | 264 (27.4%) | 29 (65.9%) | 3 (30.0%) | NS |
| BRCA1 mutation | 11 (1.1%) | 0 (0.0%) | 0 (0.0%) | NS |
| BRCA2 mutation | 12 (1.2%) | 1 (2.7%) | 1 (10.0%) | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.H.; Yun, J.W.; Kim, H.Y.; Heo, C.Y.; Lee, S. Elucidation of Novel Therapeutic Targets for Breast Cancer with ESR1-CCDC170 Fusion. J. Clin. Med. 2021, 10, 582. https://doi.org/10.3390/jcm10040582
Jeong JH, Yun JW, Kim HY, Heo CY, Lee S. Elucidation of Novel Therapeutic Targets for Breast Cancer with ESR1-CCDC170 Fusion. Journal of Clinical Medicine. 2021; 10(4):582. https://doi.org/10.3390/jcm10040582
Chicago/Turabian StyleJeong, Jae Heon, Jae Won Yun, Ha Young Kim, Chan Yeong Heo, and Sejoon Lee. 2021. "Elucidation of Novel Therapeutic Targets for Breast Cancer with ESR1-CCDC170 Fusion" Journal of Clinical Medicine 10, no. 4: 582. https://doi.org/10.3390/jcm10040582






