Epidemiological Impact of Myocarditis
Abstract
:1. Introduction
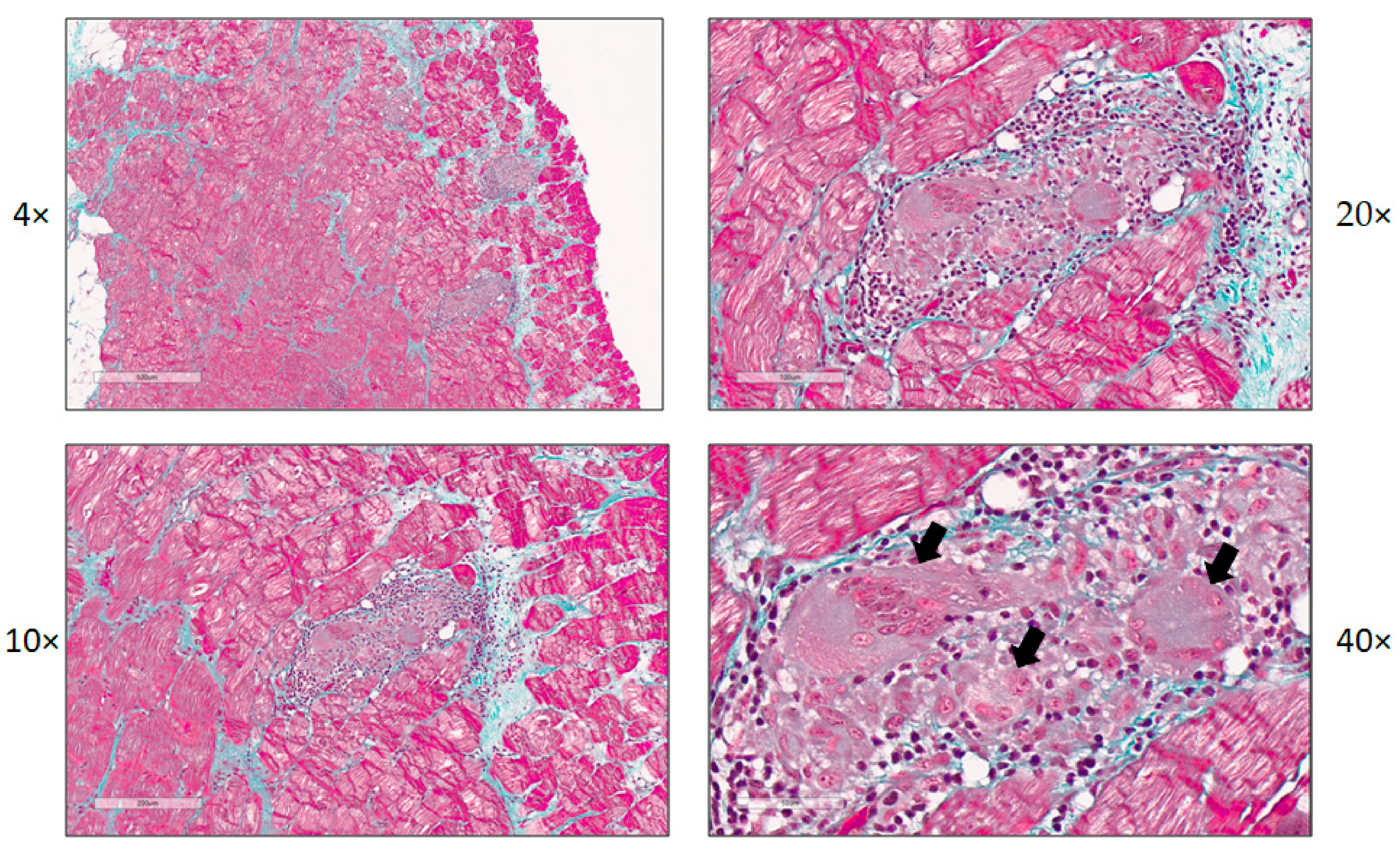
2. Etiology of Myocarditis
2.1. Infection
2.2. Toxicity or Hypersensitivity Reaction
2.3. Autoimmunity
3. Epidemiology of Myocarditis
3.1. Prevalence of Myocarditis
3.2. Sex-Specific Differences
3.3. Regional Differences in Myocarditis
3.3.1. Infectious Myocarditis
3.3.2. Toxic Myocarditis
4. Role of CMR in the Diagnosis of Myocarditis
5. Updates on SARS-CoV-2 Infection and COVID-19 Association with Myocarditis
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sagar, S.; Liu, P.P.; Cooper, L.T., Jr. Myocarditis. Lancet 2012, 379, 738–747. [Google Scholar] [CrossRef] [Green Version]
- Cooper, L.T.; Baughman, K.L.; Feldman, A.M.; Frustaci, A.; Jessup, M.; Kuhl, U.; Levine, G.N.; Narula, J.; Starling, R.C.; Towbin, J. The role of endomyocardial biopsy in the management of cardiovascular disease: A scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. Eur. Heart J. 2007, 28, 3076–3093. [Google Scholar]
- Bozkurt, B.; Colvin, M.; Cook, J.; Cooper, L.T.; Deswal, A.; Fonarow, G.C.; Francis, G.S.; Lenihan, D.; Lewis, E.F.; McNamara, D.M. Current diagnostic and treatment strategies for specific dilated cardiomyopathies: A scientific statement from the American Heart Association. Circulation 2016, 134, e579–e646. [Google Scholar] [CrossRef]
- Shanes, J.; Ghali, J.; Billingham, M.; Ferrans, V.; Fenoglio, J.; Edwards, W.; Tsai, C.; Saffitz, J.; Isner, J.; Furner, S. Interobserver variability in the pathologic interpretation of endomyocardial biopsy results. Circulation 1987, 75, 401–405. [Google Scholar] [CrossRef] [Green Version]
- Hauck, A.J.; Kearney, D.L.; Edwards, W.D. Evaluation of Postmortem Endomyocardial Biopsy Specimens from 38 Patients with Lymphocytic Myocarditis: Implications for Role of Sampling Error; Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Pollack, A.; Kontorovich, A.R.; Fuster, V.; Dec, G.W. Viral myocarditis—diagnosis, treatment options, and current controversies. Nat. Rev. Cardiol. 2015, 12, 670. [Google Scholar] [CrossRef]
- Tschöpe, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hübner, N. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2020, 1–25. [Google Scholar] [CrossRef]
- Friedrich, M.G.; Sechtem, U.; Schulz-Menger, J.; Holmvang, G.; Alakija, P.; Cooper, L.T.; White, J.A.; Abdel-Aty, H.; Gutberlet, M.; Prasad, S. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J. Am. Coll. Cardiol. 2009, 53, 1475–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baboonian, C.; McKenna, W. Eradication of viral myocarditis: Is there hope? J. Am. Coll. Cardiol. 2003, 42, 473–476. [Google Scholar] [CrossRef] [Green Version]
- Gravanis, M.; Sternby, N. Incidence of myocarditis. A 10-year autopsy study from. Arch Pathol. Lab. Med. 1991, 115, 390–392. [Google Scholar] [PubMed]
- Chow, L.H.; Radio, S.J.; Sears, T.D.; Mcmanus, B.M. Insensitivity of right ventricular endomyocardial biopsy in the diagnosis of myocarditis. J. Am. Coll. Cardiol. 1989, 14, 915–920. [Google Scholar] [CrossRef] [Green Version]
- Dec, G. Epidemiology and Prognosis of Myocarditis and Dilated Cardiomyopathy: Predictive Value of Clinical Parameters and Biopsy Findings. In Inflammatory Cardiomyopathy (DCMi); Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Chimenti, C.; Frustaci, A. Contribution and risks of left ventricular endomyocardial biopsy in patients with cardiomyopathies: A retrospective study over a 28-year period. Circulation 2013, 128, 1531–1541. [Google Scholar] [CrossRef] [Green Version]
- Aretz, T. Myocarditis. A histopathologic definition and classification. Am. J. Cardiovasc. Pathol. 1986, 1, 3–14. [Google Scholar]
- Vaidya, V.R.; Abudan, A.A.; Vasudevan, K.; Shantha, G.; Cooper, L.T.; Kapa, S.; Noseworthy, P.A.; Cha, Y.M.; Asirvatham, S.J.; Deshmukh, A.J. The efficacy and safety of electroanatomic mapping-guided endomyocardial biopsy: A systematic review. J. Interv. Card Electrophysiol. 2018, 53, 63–71. [Google Scholar] [CrossRef]
- Grist, N. Epidemiology of viral infections of the heart. Viral Infect. Heart 1993, 88, 23–31. [Google Scholar]
- Bowles, N.E.; Ni, J.; Kearney, D.L.; Pauschinger, M.; Schultheiss, H.-P.; McCarthy, R.; Hare, J.; Bricker, J.T.; Bowles, K.R.; Towbin, J.A. Detection of viruses in myocardial tissues by polymerase chain reaction: Evidence of adenovirus as a common cause of myocarditis in children and adults. J. Am. Coll. Cardiol. 2003, 42, 466–472. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.B.; Webber, S.; Fricker, F.J.; Jaffe, R.; Demmler, G.; Kearney, D.; Zhang, Y.-H.; Bodurtha, J.; Gelb, B.; Ni, J. Acute myocarditis. Rapid diagnosis by PCR in children. Circulation 1994, 90, 330–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pankuweit, S.; Moll, R.; Baandrup, U.; Portig, I.; Hufnagel, G.; Maisch, B. Prevalence of the parvovirus B19 genome in endomyocardial biopsy specimens. Hum. Pathol. 2003, 34, 497–503. [Google Scholar] [CrossRef]
- Schenk, T.; Enders, M.; Pollak, S.; Hahn, R.; Huzly, D. High prevalence of human parvovirus B19 DNA in myocardial autopsy samples from subjects without myocarditis or dilative cardiomyopathy. J. Clin. Microbiol. 2009, 47, 106–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lotze, U.; Egerer, R.; Glück, B.; Zell, R.; Sigusch, H.; Erhardt, C.; Heim, A.; Kandolf, R.; Bock, T.; Wutzler, P. Low level myocardial parvovirus B19 persistence is a frequent finding in patients with heart disease but unrelated to ongoing myocardial injury. J. Med. Virol. 2010, 82, 1449–1457. [Google Scholar] [CrossRef] [Green Version]
- Koepsell, S.A.; Anderson, D.R.; Radio, S.J. Parvovirus B19 is a bystander in adult myocarditis. Cardiovasc. Pathol. 2012, 21, 476–481. [Google Scholar] [CrossRef]
- Bock, C.-T.; Klingel, K.; Kandolf, R. Human parvovirus B19–associated myocarditis. N. Engl. J. Med. 2010, 362, 1248–1249. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, U.; Lassner, D.; Dorner, A.; Rohde, M.; Escher, F.; Seeberg, B.; Hertel, E.; Tschope, C.; Skurk, C.; Gross, U. A distinct subgroup of cardiomyopathy patients characterized by transcriptionally active cardiotropic erythrovirus and altered cardiac gene expression. Basic Res. Cardiol. 2013, 108, 372. [Google Scholar] [CrossRef]
- Bock, C.-T.; Düchting, A.; Utta, F.; Brunner, E.; Sy, B.T.; Klingel, K.; Lang, F.; Gawaz, M.; Felix, S.B.; Kandolf, R. Molecular phenotypes of human parvovirus B19 in patients with myocarditis. World J. Cardiol. 2014, 6, 183. [Google Scholar] [CrossRef] [PubMed]
- Van Linthout, S.; Elsanhoury, A.; Klein, O.; Sosnowski, M.; Miteva, K.; Lassner, D.; Abou-El-Enein, M.; Pieske, B.; Kühl, U.; Tschöpe, C. Telbivudine in chronic lymphocytic myocarditis and human parvovirus B19 transcriptional activity. ESC Heart Fail. 2018, 5, 818–829. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Z.; Huang, Y.; Li, J.; Hong, W.; Qin, Z.; Tong, Y.; Li, J.; Lv, M.; Li, M.; et al. Characterization of the Myocarditis during the worst outbreak of dengue infection in China. Medicine 2016, 95, e4051. [Google Scholar] [CrossRef]
- Nugent, A.W.; Daubeney, P.E.; Chondros, P.; Carlin, J.B.; Cheung, M.; Wilkinson, L.C.; Davis, A.M.; Kahler, S.G.; Chow, C.; Wilkinson, J.L. The epidemiology of childhood cardiomyopathy in Australia. N. Engl. J. Med. 2003, 348, 1639–1646. [Google Scholar] [CrossRef]
- Kanungo, R.; Vijayalakshmi, N.; Nalini, P.; Bhattacharya, S. Diphtheria due to non-toxigenic Corynebacterium diphtheriae: A report of two cases. Indian J. Med. Microbiol. 2002, 20, 50. [Google Scholar] [PubMed]
- Putterman, C.; Caraco, Y.; Shalit, M. Acute nonrheumatic perimyocarditis complicating streptococcal tonsillitis. Cardiology 1991, 78, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Aung, M.; Raith, E.; Williams, E.; Burrell, A.J. Severe meningococcal serogroup W sepsis presenting as myocarditis: A case report and review of literature. J. Intensive Care Soc. 2019, 20, 182–186. [Google Scholar] [CrossRef] [Green Version]
- Baysal, K.; Sancak, R.; Ozturk, F.; Uysal, S.; Gurses, N. Cardiac involvement due to Salmonella typhi infections in children. Ann. Trop. Paediatr. 1998, 18, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.S. Complications of bacteriologically confirmed typhoid fever in children. J. Trop. Pediatrics 2002, 48, 102–108. [Google Scholar] [CrossRef] [Green Version]
- van der Linde, M.R.; Crijns, H.J.; Lie, K.I. Transient complete AV block in Lyme disease: Electrophysiologic observations. Chest 1989, 96, 219–221. [Google Scholar] [CrossRef]
- Van der Linde, M. Lyme carditis: clinical characteristics of 105 cases. Scand. J. Infect. Dis. Suppl. 1991, 77, 81–84. [Google Scholar]
- Klein, J.; Stanek, G.; Bittner, R.; Horvat, R.; Holzinger, C.; Glogar, D. Lyme borreliosis as a cause of myocarditis and heart muscle disease. Eur. Heart J. 1991, 12, (Suppl. D). 73–75. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.; Oberoi, M.; Oliver, T. A Rare Case of Myocarditis, Intracardiac Thrombus And Embolic Stroke Caused By Mycoplasma Pneumoniae. J. Card. Fail. 2020, 26, S62. [Google Scholar] [CrossRef]
- Odeh, M.; Oliven, A. Chlamydial infections of the heart. Eur. J. Clin. Microbiol. Infect. Dis. 1992, 11, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Havaldar, P. Diphtheria in the eighties: Experience in a south Indian district hospital. J. Indian Med. Assoc. 1992, 90, 155. [Google Scholar]
- Gwaltney, J.; Mandell, G.; Douglas, R., Jr. Principles and Practices of Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 1989; pp. 651–656. [Google Scholar]
- Ledford, D.K. Immunologic aspects of vasculitis and cardiovascular disease. JAMA 1997, 278, 1962–1971. [Google Scholar] [CrossRef]
- Kodliwadmath, A. Phenytoin-induced Stevens–Johnson syndrome with myocarditis: A rare case report. Int. Med. Case Rep. J. 2017, 10, 229. [Google Scholar] [CrossRef] [Green Version]
- Noël, M.-C.; Powell, V.; Burton, L.; Panda, R.; Remington, G. Clozapine-related myocarditis and rechallenge: A case series and clinical review. J. Clin. Psychopharmacol. 2019, 39, 380–385. [Google Scholar] [CrossRef]
- Paratz, E.D.; Cunningham, N.J.; MacIsaac, A.I. The cardiac complications of methamphetamines. HeartLung Circ. 2016, 25, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, S.S.; Fradley, M.G.; Cohen, J.V.; Nohria, A.; Reynolds, K.L.; Heinzerling, L.M.; Sullivan, R.J.; Damrongwatanasuk, R.; Chen, C.L.; Gupta, D. Myocarditis in patients treated with immune checkpoint inhibitors. J. Am. Coll. Cardiol. 2018, 71, 1755–1764. [Google Scholar] [CrossRef]
- Salem, J.-E.; Manouchehri, A.; Moey, M.; Lebrun-Vignes, B.; Bastarache, L.; Pariente, A.; Gobert, A.; Spano, J.-P.; Balko, J.M.; Bonaca, M.P. Cardiovascular toxicities associated with immune checkpoint inhibitors: An observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018, 19, 1579–1589. [Google Scholar] [CrossRef]
- Halsell, J.S.; Riddle, J.R.; Atwood, J.E.; Gardner, P.; Shope, R.; Poland, G.A.; Gray, G.C.; Ostroff, S.; Eckart, R.E.; Hospenthal, D.R. Myopericarditis following smallpox vaccination among vaccinia-naive US military personnel. JAMA 2003, 289, 3283–3289. [Google Scholar] [CrossRef]
- Eckart, R.E.; Love, S.S.; Atwood, J.E.; Arness, M.K.; Cassimatis, D.C.; Campbell, C.L.; Boyd, S.Y.; Murphy, J.G.; Swerdlow, D.L.; Collins, L.C. Incidence and follow-up of inflammatory cardiac complications after smallpox vaccination. J. Am. Coll. Cardiol. 2004, 44, 201–205. [Google Scholar] [CrossRef] [Green Version]
- Poland, G.A.; Grabenstein, J.D.; Neff, J.M. The US smallpox vaccination program: A review of a large modern era smallpox vaccination implementation program. Vaccine 2005, 23, 2078–2081. [Google Scholar] [CrossRef]
- Root-Bernstein, R.; Fairweather, D. Unresolved issues in theories of autoimmune disease using myocarditis as a framework. J. Biol. 2015, 375, 101–123. [Google Scholar] [CrossRef] [Green Version]
- Root-Bernstein, R.; Fairweather, D. Complexities in the relationship between infection and autoimmunity. Curr. Allergy Asthma Rep. 2014, 14, 407. [Google Scholar] [CrossRef] [Green Version]
- Fairweather, D.; Kaya, Z.; Shellam, G.; Berry, C.; Rose, N. From Infection to Autoimmunity. J. Autoimmun. 2001, 16, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Heidecker, B.; Williams, S.H.; Jain, K.; Oleynik, A.; Patriki, D.; Kottwitz, J.; Berg, J.; Garcia, J.A.; Baltensperger, N.; Lovrinovic, M. Virome Sequencing in Patients With Myocarditis. Circ. Heart Fail. 2020, 13, e007103. [Google Scholar] [CrossRef] [PubMed]
- Kühl, U.; Noutsias, M.; Seeberg, B.; Schultheiss, H. Immunohistological evidence for a chronic intramyocardial inflammatory process in dilated cardiomyopathy. Heart 1996, 75, 295–300. [Google Scholar] [CrossRef] [Green Version]
- Mahon, N.G.; Madden, B.P.; Caforio, A.L.; Elliott, P.M.; Haven, A.J.; Keogh, B.E.; Davies, M.J.; McKenna, W.J. Immunohistologic evidence of myocardial disease in apparently healthy relatives of patients with dilated cardiomyopathy. J. Am. Coll. Cardiol. 2002, 39, 455–462. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.S.; Judson, M.A.; Donnino, R.; Gold, M.; Cooper, L.T., Jr.; Prystowsky, E.N.; Prystowsky, S. Cardiac sarcoidosis. Am. Heart J. 2009, 157, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Biggs, R.; Patel, B.; Martinez, M.W.; McCambridge, M.; Kim, S. Cardiac Sarcoidosis Mimicking Arrhythmogenic Right Venticular Dysplasia. Heart Rhythm Case Rep. 2017, 3, 418–421. [Google Scholar]
- Blauwet, L.A.; Cooper, L.T. Idiopathic giant cell myocarditis and cardiac sarcoidosis. Heart Fail. Rev. 2013, 18, 733–746. [Google Scholar] [CrossRef]
- Baughman, R.P.; Teirstein, A.S.; Judson, M.A.; Rossman, M.D.; Yeager, H., Jr.; Bresnitz, E.A.; DePALO, L.; Hunninghake, G.; Iannuzzi, M.C.; Johns, C.J. Clinical characteristics of patients in a case control study of sarcoidosis. Am. J. Respir. Crit. Care Med. 2001, 164, 1885–1889. [Google Scholar] [CrossRef] [Green Version]
- Kandolin, R.; Lehtonen, J.; Airaksinen, J.; Vihinen, T.; Miettinen, H.; Ylitalo, K.; Kaikkonen, K.; Tuohinen, S.; Haataja, P.; Kerola, T. Cardiac sarcoidosis: Epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation 2015, 131, 624–632. [Google Scholar] [CrossRef]
- Brigden, W.; Bywaters, E.G.; Lessof, M.H.; Ross, I.P. The heart in systemic lupus erythematosus. Br. Heart J. 1960, 22, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Dubois, E.L.; Tuffanelli, D.L. Clinical Manifestations of Systemic Lupus Erythematosus: Computer Analysis of 520 Cases. JAMA 1964, 190, 104–111. [Google Scholar] [CrossRef]
- Hejtmancik, M.R.; Wright, J.C.; Quint, R.; Jennings, F.L. The cardiovascular manifestations of systemic lupus erythematosus. Am. Heart J. 1964, 68, 119–130. [Google Scholar] [CrossRef]
- Badui, E.; Garcia-Rubi, D.; Robles, E.; Jimenez, J.; Juan, L.; Deleze, M.; Diaz, A.; Mintz, G. Cardiovascular Manifestations in Systemic Lupus Erythematosus. Prospective Study of 100 Patients. Angiology 1985, 36, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Griffith, G.C.; Vural, I.L. Acute and subacute disseminated lupus erythematosus; A correlation of clinical and postmortem findings in eighteen cases. Circulation 1951, 3, 492–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, A.M.; Shulman, L.E.; Tumulty, P.A.; Conley, C.L.; Schoenrich, E.H. Systemic lupus erythematosus: Review of the literature and clinical analysis of 138 cases. Medicine 1954, 33, 291–437. [Google Scholar] [CrossRef] [Green Version]
- Kong, T.Q.; Kellum, R.E.; Haserick, J.R. Clinical diagnosis of cardiac involvement in systemic lupus erythematosus. A correlation of clinical and autopsy findings in thirty patients. Circulation 1962, 26, 7–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amador-Patarroyo, M.J.; Rodriguez-Rodriguez, A.; Montoya-Ortiz, G. How does age at onset influence the outcome of autoimmune diseases? Autoimmune Dis. 2012, 2012, 251730. [Google Scholar] [CrossRef] [PubMed]
- Hersh, A.O.; von Scheven, E.; Yazdany, J.; Panopalis, P.; Trupin, L.; Julian, L.; Katz, P.; Criswell, L.A.; Yelin, E. Differences in long-term disease activity and treatment of adult patients with childhood-and adult-onset systemic lupus erythematosus. Arthritis Care Res. 2009, 61, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Alonso, M.; Martinez-Vazquez, F.; de Teran, T.D.; Miranda-Filloy, J.; Dierssen, T.; Blanco, R.; Gonzalez-Juanatey, C.; Llorca, J.; Gonzalez-Gay, M. Late-onset systemic lupus erythematosus in Northwestern Spain: Differences with early-onset systemic lupus erythematosus and literature review. Lupus 2012, 21, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- Merola, J.F.; Bermas, B.; Lu, B.; Karlson, E.W.; Massarotti, E.; Schur, P.H.; Costenbader, K.H. Clinical manifestations and survival among adults with (SLE) according to age at diagnosis. Lupus 2014, 23, 778–784. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.C.; Xiao, R.; Mercer-Rosa, L.; Knight, A.M.; Weiss, P.F. Child-onset systemic lupus erythematosus is associated with a higher incidence of myopericardial manifestations compared to adult-onset disease. Lupus 2018, 27, 2146–2154. [Google Scholar] [CrossRef]
- Levin, M.-D.; Zoet-Nugteren, S.; Markusse, H. Myocarditis and primary Sjogren’s syndrome. Lancet 1999, 354, 128–129. [Google Scholar] [CrossRef]
- Busteed, S.; Sparrow, P.; Molloy, C.; Molloy, M. Myocarditis as a prognostic indicator in systemic lupus erythematosus. Postgrad. Med. J. 2004, 80, 366–367. [Google Scholar] [CrossRef] [Green Version]
- Cihakova, D.; Rose, N.R. Pathogenesis of myocarditis and dilated cardiomyopathy. Adv. Immunol. 2008, 99, 95–114. [Google Scholar]
- Cooper, L.T., Jr.; Berry, G.J.; Shabetai, R. Idiopathic giant-cell myocarditis-natural history and treatment. Multicenter Giant Cell Myocarditis Study Group Investigators. N. Engl. J. Med. 1997, 336, 1860–1866. [Google Scholar] [CrossRef] [PubMed]
- Okada, R.; Wakafuji, S. Myocarditis in autopsy. Heart Vessel. 1985, 1, 23–29. [Google Scholar] [CrossRef]
- Wakafuji, S.; Okada, R. Twenty Year Autopsy Statistics of Myocarditis Incidence in Japan: The 10th conference on the 10th conference on prevention for rheumatic fever and rheumatic heart disease. Jpn. Circ. J. 1986, 50, 1288–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bracamonte-Baran, W.; Čiháková, D. Cardiac Autoimmunity: Myocarditis. In The Immunology of Cardiovascular Homeostasis and Pathology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 187–221. [Google Scholar]
- Aggarwal, H.K.; Jain, D.; Kaverappa, V.; Jain, P.; Kumar, A.; Yadav, S. Idiopathic hypereosinophilic syndrome presenting as severe Loeffler’s endocarditis. Arq Bras. Cardiol. 2013, 100, e43–e46. [Google Scholar] [CrossRef] [PubMed]
- Blauwet, L.A.; Cooper, L.T. Myocarditis. Prog. Cardiovasc. Dis. 2010, 52, 274–288. [Google Scholar] [CrossRef] [Green Version]
- Patriki, D.; Kottwitz, J.; Berg, J.; Landmesser, U.; Lüscher, T.F.; Heidecker, B. Clinical Presentation and Laboratory Findings in Men Versus Women with Myocarditis. J. Womens Health (Larchmt) 2020, 29, 193–199. [Google Scholar] [CrossRef]
- Caforio, A.L.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef]
- Dai, H.; Lotan, D.; Much, A.A.; Younis, A.; Lu, Y.; Bragazzi, N.L.; Wu, J. Global, regional, and national burden of myocarditis and cardiomyopathy, 1990–2017. medRxiv 2020. [Google Scholar] [CrossRef]
- Chow, L.C.; Dittrich, H.C.; Shabetai, R. Endomyocardial biopsy in patients with unexplained congestive heart failure. Ann. Intern. Med. 1988, 109, 535–539. [Google Scholar] [CrossRef]
- Vignola, P.A.; Aonuma, K.; Swaye, P.S.; Rozanski, J.J.; Blankstein, R.L.; Benson, J.; Gosselin, A.J.; Lister, J.W. Lymphocytic myocarditis presenting as unexplained ventricular arrhythmias: Diagnosis with endomyocardial biopsy and response to immunosuppression. J. Am. Coll. Cardiol. 1984, 4, 812–819. [Google Scholar] [CrossRef] [Green Version]
- Frustaci, A.; Caldarulo, M.; Buffon, A.; Bellocci, F.; Fenici, R.; Melina, D. Cardiac biopsy in patients with “primary” atrial fibrillation: Histologic evidence of occult myocardial diseases. Chest 1991, 100, 303–306. [Google Scholar] [CrossRef]
- Zee-Cheng, C.-S.; Tsai, C.C.; Palmer, D.C.; Codd, J.E.; Pennington, D.G.; Williams, G.A. High incidence of myocarditis by endomyocardial biopsy in patients with idiopathic congestive cardiomyopathy. J. Am. Coll. Cardiol. 1984, 3, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Latham, R.D.; Mulrow, J.P.; Virmani, R.; Robinowitz, M.; Moody, J.M. Recently diagnosed idiopathic dilated cardiomyopathy: Incidence of myocarditis and efficacy of prednisone therapy. Am. Heart J. 1989, 117, 876–882. [Google Scholar] [CrossRef]
- Cooper, L.T., Jr.; Keren, A.; Sliwa, K.; Matsumori, A.; Mensah, G.A. The global burden of myocarditis: Part 1: A systematic literature review for the Global Burden of Diseases, Injuries, and Risk Factors 2010 study. Glob. Heart 2014, 9, 121–129. [Google Scholar] [CrossRef] [Green Version]
- McNamara, D.M.; Starling, R.C.; Cooper, L.T.; Boehmer, J.P.; Mather, P.J.; Janosko, K.M.; Gorcsan, J.; Kip, K.E.; Dec, G.W.; Investigators, I. Clinical and demographic predictors of outcomes in recent onset dilated cardiomyopathy: Results of the IMAC (Intervention in Myocarditis and Acute Cardiomyopathy)-2 study. J. Am. Coll. Cardiol. 2011, 58, 1112–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairweather, D.; Frisancho-Kiss, S.; Rose, N.R. Viruses as adjuvants for autoimmunity: Evidence from Coxsackievirus-induced myocarditis. Rev. Med. Virol 2005, 15, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Frisancho-Kiss, S.; Nyland, J.F.; Davis, S.E.; Frisancho, J.A.; Barrett, M.A.; Rose, N.R.; Fairweather, D. Sex differences in coxsackievirus B3-induced myocarditis: IL-12Rβ1 signaling and IFN-γ increase inflammation in males independent from STAT4. Brain Res. 2006, 1126, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, D.; Cooper, L.T., Jr.; Blauwet, L.A. Sex and gender differences in myocarditis and dilated cardiomyopathy. Curr. Probl. Cardiol. 2013, 38, 7–46. [Google Scholar] [CrossRef]
- Esfandiarei, M.; McManus, B.M. Molecular biology and pathogenesis of viral myocarditis. Annu. Rev. Pathol. Mech. Dis. 2008, 3, 127–155. [Google Scholar] [CrossRef] [PubMed]
- Frisancho-Kiss, S.; Nyland, J.F.; Davis, S.E.; Barrett, M.A.; Gatewood, S.J.; Njoku, D.B.; Cihakova, D.; Silbergeld, E.K.; Rose, N.R.; Fairweather, D. Cutting edge: T cell Ig mucin-3 reduces inflammatory heart disease by increasing CTLA-4 during innate immunity. J. Immunol. 2006, 176, 6411–6415. [Google Scholar] [CrossRef] [Green Version]
- Frisancho-Kiss, S.; Davis, S.E.; Nyland, J.F.; Frisancho, J.A.; Cihakova, D.; Barrett, M.A.; Rose, N.R.; Fairweather, D. Cutting edge: Cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J. Immunol. 2007, 178, 6710–6714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M.; Saidali, A.; Bakhtiar, A.; Arya, L. Diphtheria in Afghanistan—review of 155 cases. J. Trop. Med. Hyg. 1985, 88, 373–376. [Google Scholar]
- Salaki, A.; Samik, W.M.; Sugeng, B. Electrocardiographic pattern in children suffering from typhoid fever. Paediatr. Indones. 1985, 25, 131. [Google Scholar]
- Paul, F.M.; Yin-Murphy, M. Coxsackie B. virus infection in Singapore children. J. Trop. Pediatr. 1985, 31, 96–100. [Google Scholar] [CrossRef]
- Matsumori, A. Hepatitis C virus infection and cardiomyopathies. Am. Heart Assoc. 2005, 96, 144–147. [Google Scholar] [CrossRef] [Green Version]
- Matsumori, A.; Ohashi, N.; Hasegawa, K.; Sasayama, S.; Eto, T.; Imaizumi, T.; Izumi, T.; Kawamura, K.; Kawana, M.; Kimura, A. Hepatitis C virus infection and heart diseases. Jpn. Circ. J. 1998, 62, 389–391. [Google Scholar] [CrossRef] [Green Version]
- Matsumori, A.; Yutani, C.; Ikeda, Y.; Kawai, S.; Sasayama, S. Hepatitis C virus from the hearts of patients with myocarditis and cardiomyopathy. Lab. Investig. 2000, 80, 1137–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craig, M.; Vale, T.; Robertson, P.; Rawlinson, W.; Gould, B. Enterovirus 71 infection in Australian expatriate children following an outbreak in Malaysia. J. Paediatr. Child. Health 1999, 35, 107–108. [Google Scholar]
- Daley, A.; Isaacs, D.; Dwyer, D.; Gilbert, G. A cluster of cases of neonatal coxsackievirus B meningitis and myocarditis. J. Paediatr. Child. Health 1998, 34, 196–198. [Google Scholar] [CrossRef]
- Mayosi, B.M. Contemporary trends in the epidemiology and management of cardiomyopathy and pericarditis in sub-Saharan Africa. Heart 2007, 93, 1176–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poltera, A.; Cox, J.; Owor, R. Pancarditis affecting the conducting system and all valves in human African trypanosomiasis. Heart 1976, 38, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Schofield, C.J.; Jannin, J.; Salvatella, R. The future of Chagas disease control. Trends Parasitol. 2006, 22, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Mady, C.; Pereira-Barretto, A.C.; Ianni, B.M.; Lopes, E.A.; Pileggi, F. Right ventricular endomyocardial biopsy in undetermined form of Chagas’ disease. Angiology 1984, 35, 755–759. [Google Scholar] [CrossRef]
- Maguire, J. Trypanosoma. Infectious Diseases, 2nd ed.; Lippincott Williams Wilkins: Philadephia, PA, USA, 2004; pp. 2327–2334. [Google Scholar]
- Sartori, A.M.C.; Lopes, M.H.; Caramelli, B.; Duarte, M.I.S.; Pinto, P.L.d.S.; Neto, V.A.; Shikanai-Yasuda, M.A. Simultaneous occurrence of acute myocarditis and reactivated Chagas’ disease in a patient with AIDS. Clin. Infect. Dis. 1995, 21, 1297–1299. [Google Scholar] [CrossRef] [PubMed]
- Strasen, J.; Williams, T.; Ertl, G.; Zoller, T.; Stich, A.; Ritter, O. Epidemiology of Chagas disease in Europe: Many calculations, little knowledge. Clin. Res. Cardiol. 2014, 103, 1–10. [Google Scholar] [CrossRef]
- Basile, L.; Jansa, J.; Carlier, Y.; Salamanca, D.D.; Angheben, A.; Bartoloni, A.; Seixas, J.; Van Gool, T.; Cañavate, C.; Flores-Chávez, M. Chagas disease in European countries: The challenge of a surveillance system. Eurosurveillance 2011, 16, 19968. [Google Scholar] [CrossRef] [Green Version]
- Schmunis, G.A.; Yadon, Z.E. Chagas disease: A Latin American health problem becoming a world health problem. Acta Trop. 2010, 115, 14–21. [Google Scholar] [CrossRef]
- Schmunis, G.A. Epidemiology of Chagas disease in non endemic countries: The role of international migration. Memórias Do Inst. Oswaldo Cruz 2007, 102, 75–86. [Google Scholar] [CrossRef]
- Karjalainen, J.; Heikkilä, J.; Nieminen, M.S.; Jalanko, H.; Kleemola, M.; Lapinleimu, K.; Sahi, T. Etiology of mild acute infectious myocarditis: Relation to clinical features. Acta Med. Scand. 1983, 213, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson, K.J.; Taylor, A.J.; Fitzgerald, P.B.; Topliss, D.J.; Elsik, M.; McNeil, J.J. Diagnostic characteristics of clozapine-induced myocarditis identified by an analysis of 38 cases and 47 controls. J. Clin. Psychiatry 2010, 71, 976. [Google Scholar] [CrossRef] [Green Version]
- Kerroumi, A.; Majdoub, K.; Guennoun, Z.; Doghmi, N.; Cherti, M. Myocarditis Associated With Anabolic Steroid Abuse Report of Two Cases. IOSR J. Dent. Med Sci. 2019, 18, 55–61. [Google Scholar]
- Vasiljevic, J.D.; Kanjuh, V.; Seferovic, P.; Sesto, M.; Stojsic, D.; Olsen, E.G. The incidence of myocarditis in endomyocardial biopsy samples from patients with congestive heart failure. Am. Heart J. 1990, 120, 1370–1377. [Google Scholar] [CrossRef]
- Wilke, A.; Kaiser, A.; Ferency, I.; Maisch, B. Alcohol and myocarditis. Herz 1996, 21, 248–257. [Google Scholar] [PubMed]
- Manthey, J.; Rehm, J. Mortality from Alcoholic Cardiomyopathy: Exploring the Gap between Estimated and Civil Registry Data. J. Clin. Med. 2019, 8, 1137. [Google Scholar] [CrossRef] [Green Version]
- Grisaru, D.; Rachmilewitz, E.A.; Mosseri, M.; Gotsman, M.; Lafair, J.S.; Okon, E.; Goldfarb, A.; Hasin, Y. Cardiopulmonary assessment in beta-thalassemia major. Chest 1990, 98, 1138–1142. [Google Scholar] [CrossRef] [PubMed]
- Kremastinos, D.T.; Tiniakos, G.; Theodorakis, G.N.; Katritsis, D.G.; Toutouzas, P.K. Myocarditis in β-thalassemia major: A cause of heart failure. Circulation 1995, 91, 66–71. [Google Scholar] [CrossRef]
- Kremastinos, D.; Toutouzas, P.; Vyssoulis, G.; Venetis, C.; Avgoustakis, D. Iron overload and left ventricular performance in beta thalassemia. Acta Cardiol. 1984, 39, 29–40. [Google Scholar]
- Bawaskar, H.; Bawaskar, P. Scorpion sting. J. Assoc. Physicians India 1998, 46, 388–392. [Google Scholar] [CrossRef]
- Rahav, G.; Weiss, A.T. Scorpion sting-induced pulmonary edema: Scintigraphic evidence of cardiac dysfunction. Chest 1990, 97, 1478–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Members, W.C.; Hundley, W.G.; Bluemke, D.A.; Finn, J.P.; Flamm, S.D.; Fogel, M.A.; Friedrich, M.G.; Ho, V.B.; Jerosch-Herold, M.; Kramer, C.M. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: A report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation 2010, 121, 2462–2508. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: Expert recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef] [PubMed]
- Gräni, C.; Eichhorn, C.; Bière, L.; Murthy, V.L.; Agarwal, V.; Kaneko, K.; Cuddy, S.; Aghayev, A.; Steigner, M.; Blankstein, R. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J. Am. Coll. Cardiol. 2017, 70, 1964–1976. [Google Scholar] [CrossRef] [PubMed]
- Pasupathy, S.; Air, T.; Dreyer, R.P.; Tavella, R.; Beltrame, J.F. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation 2015, 131, 861–870. [Google Scholar] [CrossRef] [Green Version]
- Maddox, T.M.; Ho, P.M.; Roe, M.; Dai, D.; Tsai, T.T.; Rumsfeld, J.S. Utilization of secondary prevention therapies in patients with nonobstructive coronary artery disease identified during cardiac catheterization: Insights from the National Cardiovascular Data Registry Cath-PCI Registry. Circ. Cardiovasc. Qual. Outcomes 2010, 3, 632–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heidecker, B.; Ruedi, G.; Baltensperger, N.; Gresser, E.; Kottwitz, J.; Berg, J.; Manka, R.; Landmesser, U.; Lüscher, T.F.; Patriki, D. Systematic use of cardiac magnetic resonance imaging in MINOCA led to a five-fold increase in the detection rate of myocarditis: A retrospective study. Swiss Med. Wkly. 2019, 149, w20098. [Google Scholar] [CrossRef] [Green Version]
- Patriki, D.; Gresser, E.; Manka, R.; Emmert, M.Y.; Lüscher, T.F.; Heidecker, B. Approximation of the incidence of myocarditis by systematic screening with cardiac magnetic resonance imaging. JACC Heart Fail. 2018, 6, 573–579. [Google Scholar] [CrossRef]
- Gerbaud, E.; Harcaut, E.; Coste, P.; Erickson, M.; Lederlin, M.; Labèque, J.N.; Perron, J.M.; Cochet, H.; Dos Santos, P.; Durrieu-Jaïs, C. Cardiac magnetic resonance imaging for the diagnosis of patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Int. J. Cardiovasc. Imaging 2012, 28, 783–794. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Harden, S.; Abid, N.; Peebles, C.; Nicholas, Z.; Jones, T.; Mckenzie, D.; Curzen, N. Troponin-positive chest pain with unobstructed coronary arteries: Definitive differential diagnosis using cardiac MRI. Br. J. Radiol. 2012, 85, e461–e466. [Google Scholar] [CrossRef] [Green Version]
- Panovský, R.; Borová, J.; Pleva, M.; Feitová, V.; Novotný, P.; Kincl, V.; Holeček, T.; Meluzín, J.; Sochor, O.; Štěpánová, R. The unique value of cardiovascular magnetic resonance in patients with suspected acute coronary syndrome and culprit-free coronary angiograms. BMC Cardiovasc. Disord. 2017, 17, 170. [Google Scholar] [CrossRef] [Green Version]
- Assomull, R.G.; Lyne, J.C.; Keenan, N.; Gulati, A.; Bunce, N.H.; Davies, S.W.; Pennell, D.J.; Prasad, S.K. The role of cardiovascular magnetic resonance in patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Eur. Heart J. 2007, 28, 1242–1249. [Google Scholar] [CrossRef] [Green Version]
- Kawecki, D.; Morawiec, B.; Monney, P.; Pellaton, C.; Wojciechowska, C.; Jojko, J.; Basiak, M.; Przywara-Chowaniec, B.; Fournier, S.; Nowalany-Kozielska, E.; et al. Diagnostic contribution of cardiac magnetic resonance in patients with acute coronary syndrome and culprit-free angiograms. Med. Sci. Monit 2015, 21, 171–180. [Google Scholar]
- Leurent, G.; Langella, B.; Fougerou, C.; Lentz, P.A.; Larralde, A.; Bedossa, M.; Boulmier, D.; Le Breton, H. Diagnostic contributions of cardiac magnetic resonance imaging in patients presenting with elevated troponin, acute chest pain syndrome and unobstructed coronary arteries. Arch. Cardiovasc. Dis. 2011, 104, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W. The Northwell COVID-19 Research Consortium. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–810. [Google Scholar] [CrossRef] [Green Version]
- Arentz, M.; Yim, E.; Klaff, L.; Lokhandwala, S.; Riedo, F.X.; Chong, M.; Lee, M. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA 2020, 323, 1612–1614. [Google Scholar] [CrossRef] [Green Version]
- Alhogbani, T. Acute myocarditis associated with novel Middle east respiratory syndrome coronavirus. Ann. Saudi Med. 2016, 36, 78–80. [Google Scholar] [CrossRef] [Green Version]
- Trogen, B.; Gonzalez, F.J.; Shust, G.F. COVID-19-associated myocarditis in an adolescent. Pediatric Infect. Dis. J. 2020, 39, e204–e205. [Google Scholar] [CrossRef]
- Doyen, D.; Moceri, P.; Ducreux, D.; Dellamonica, J. Myocarditis in a patient with COVID-19: A cause of raised troponin and ECG changes. Lancet 2020, 395, 1516. [Google Scholar] [CrossRef]
- Kim, I.-C.; Kim, J.Y.; Kim, H.A.; Han, S. COVID-19-related myocarditis in a 21-year-old female patient. Eur. Heart J. 2020, 41, 1859. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.-H.; Liu, Y.-X.; Yuan, J.; Wang, F.-X.; Wu, W.-B.; Li, J.-X.; Wang, L.-F.; Gao, H.; Wang, Y.; Dong, C.-F. First case of COVID-19 complicated with fulminant myocarditis: A case report and insights. Infection 2020, 48, 773–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Rajpal, S.; Tong, M.S.; Borchers, J.; Zareba, K.M.; Obarski, T.P.; Simonetti, O.P.; Daniels, C.J. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2020, 6, 116–118. [Google Scholar] [CrossRef]
- Halushka, M.K.; Vander Heide, R.S. Myocarditis is rare in COVID-19 autopsies: Cardiovascular findings across 277 post-mortem examinations. Cardiovasc. Pathol. 2020, 50, 107300. [Google Scholar] [CrossRef]
- Babapoor-Farrokhran, S.; Gill, D.; Walker, J.; Rasekhi, R.T.; Bozorgnia, B.; Amanullah, A. Myocardial injury and COVID-19: Possible mechanisms. Life Sci. 2020, 253, 117723. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef] [Green Version]
- Murthy, S.; Gomersall, C.D.; Fowler, R.A. Care for critically ill patients with COVID-19. JAMA 2020, 323, 1499–1500. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Trump, S.; Lukassen, S.; Anker, M.S.; Chua, R.L.; Liebig, J.; Thürmann, L.; Corman, V.M.; Binder, M.; Loske, J.; Klasa, C.; et al. Hypertension delays viral clearance and exacerbates airway hyperinflammation in patients with COVID-19. Nat. Biotechnol. 2020. [Google Scholar] [CrossRef]
- Li, B.; Yang, J.; Zhao, F.; Zhi, L.; Wang, X.; Liu, L.; Bi, Z.; Zhao, Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020, 109, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Bottio, T.; Bagozzi, L.; Fiocco, A.; Nadali, M.; Caraffa, R.; Bifulco, O.; Ponzoni, M.; Lombardi, C.M.; Metra, M.; Russo, C.F.; et al. COVID-19 in Heart Transplant Recipients: A Multicenter Analysis of the Northern Italian Outbreak. JACC Heart Fail. 2021, 9, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing Long COVID in an International Cohort: 7 Months of Symptoms and Their Impact. medRxiv 2020. [Google Scholar] [CrossRef]
- Dec, G.W., Jr.; Palacios, I.F.; Fallon, J.T.; Aretz, H.T.; Mills, J.; Lee, D.C.; Johnson, R.A. Active myocarditis in the spectrum of acute dilated cardiomyopathies: Clinical features, histologic correlates, and clinical outcome. N. Engl. J. Med. 1985, 312, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.W.; O’connell, J.B.; Herskowitz, A.; Rose, N.R.; McManus, B.M.; Billingham, M.E.; Moon, T.E.; Investigators, M.T.T. A clinical trial of immunosuppressive therapy for myocarditis. N. Engl. J. Med. 1995, 333, 269–275. [Google Scholar] [CrossRef]
- McCarthy, R.E.; Boehmer, J.P.; Hruban, R.H.; Hutchins, G.M.; Kasper, E.K.; Hare, J.M.; Baughman, K.L. Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N. Engl. J. Med. 2000, 342, 690–695. [Google Scholar] [CrossRef] [Green Version]
- Grogan, M.; Redfield, M.M.; Bailey, K.R.; Reeder, G.S.; Gersh, B.J.; Edwards, W.D.; Rodeheffer, R.J. Long-term outcome of patients with biopsy-proved myocarditis: Comparison with idiopathic dilated cardiomyopathy. J. Am. Coll. Cardiol. 1995, 26, 80–84. [Google Scholar] [CrossRef] [Green Version]
- MH, F.G.; Fonarow, G.; Givertz, M.; Hollenberg, S.; Lindenfeld, J.; Masoudi, F.; McBride, P. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. Circulation 2017, 136, e137–e161. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golpour, A.; Patriki, D.; Hanson, P.J.; McManus, B.; Heidecker, B. Epidemiological Impact of Myocarditis. J. Clin. Med. 2021, 10, 603. https://doi.org/10.3390/jcm10040603
Golpour A, Patriki D, Hanson PJ, McManus B, Heidecker B. Epidemiological Impact of Myocarditis. Journal of Clinical Medicine. 2021; 10(4):603. https://doi.org/10.3390/jcm10040603
Chicago/Turabian StyleGolpour, Ainoosh, Dimitri Patriki, Paul J. Hanson, Bruce McManus, and Bettina Heidecker. 2021. "Epidemiological Impact of Myocarditis" Journal of Clinical Medicine 10, no. 4: 603. https://doi.org/10.3390/jcm10040603
APA StyleGolpour, A., Patriki, D., Hanson, P. J., McManus, B., & Heidecker, B. (2021). Epidemiological Impact of Myocarditis. Journal of Clinical Medicine, 10(4), 603. https://doi.org/10.3390/jcm10040603




