Higher Blood Pressure is Associated with Greater White Matter Lesions and Brain Atrophy: A Systematic Review with Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
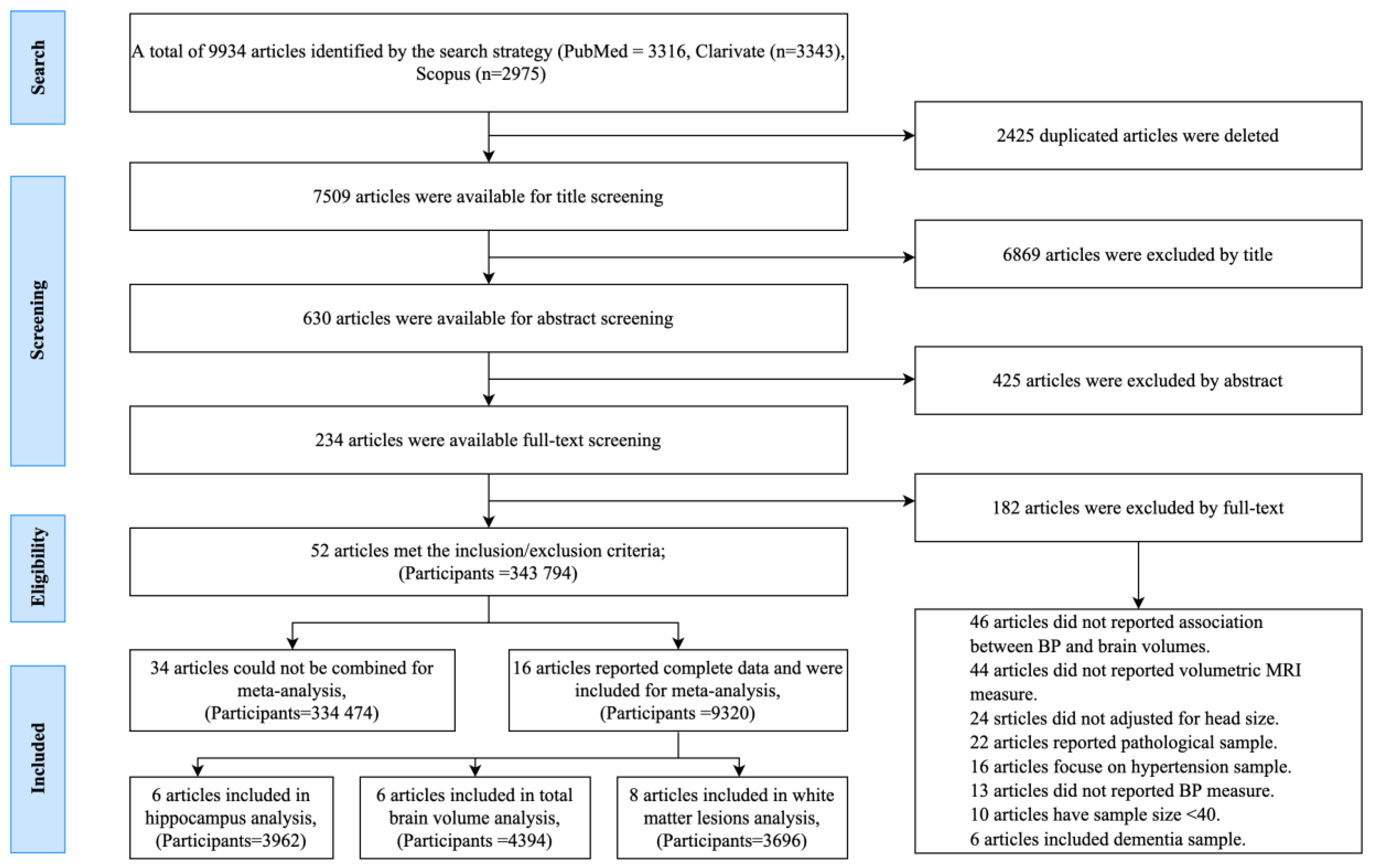
2.2. Screening
2.3. Selection Criteria and Study Screening
2.4. Data Extraction
2.5. Exposure
2.6. Outcome Measure
2.7. Meta-Analysis
2.8. Quality Assessment
3. Results
3.1. BP Assessment
3.2. Magnetic Resonance Imaging
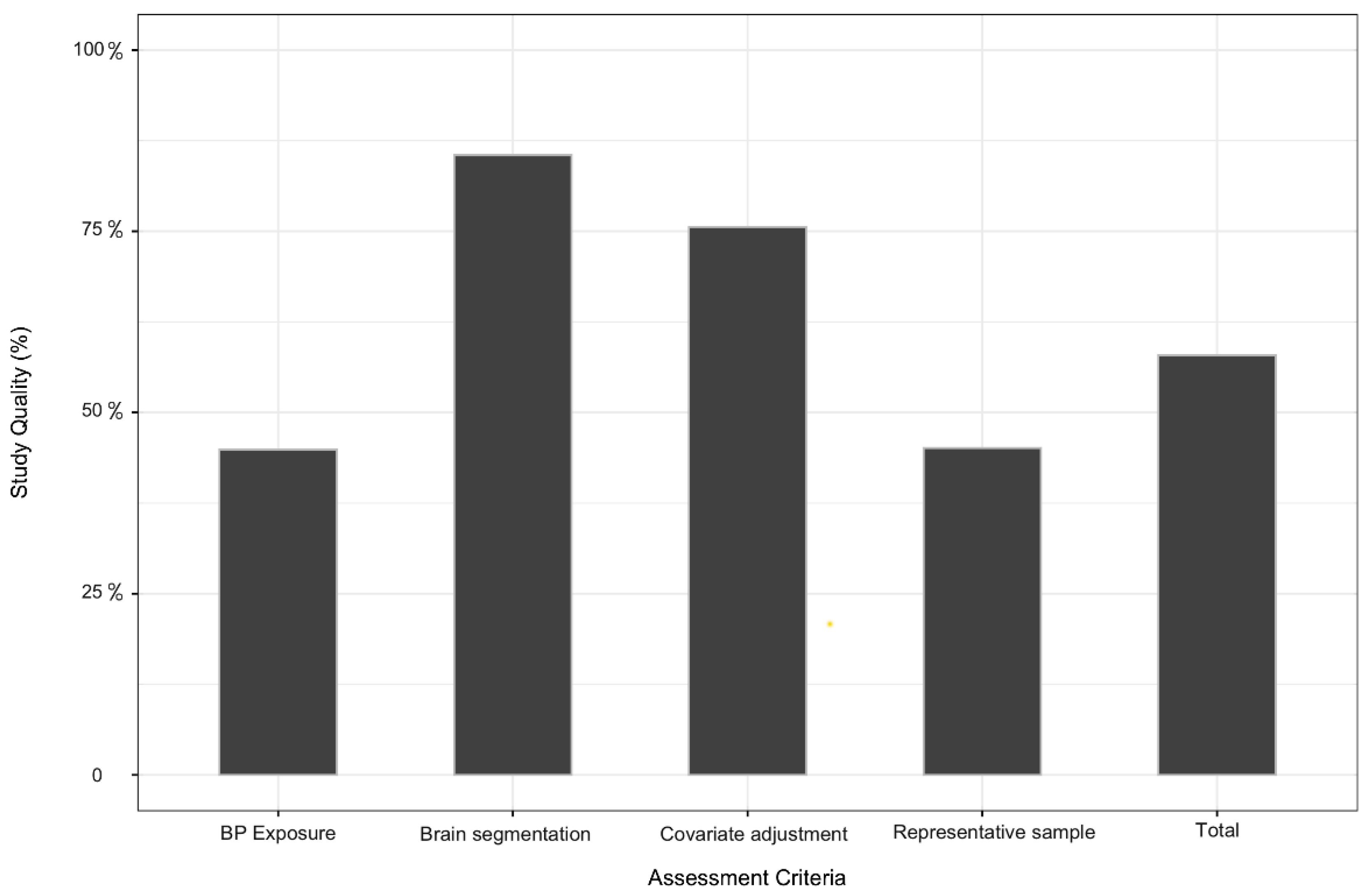
3.3. Quality Assessment
3.4. Publication Bias and Heterogeneity
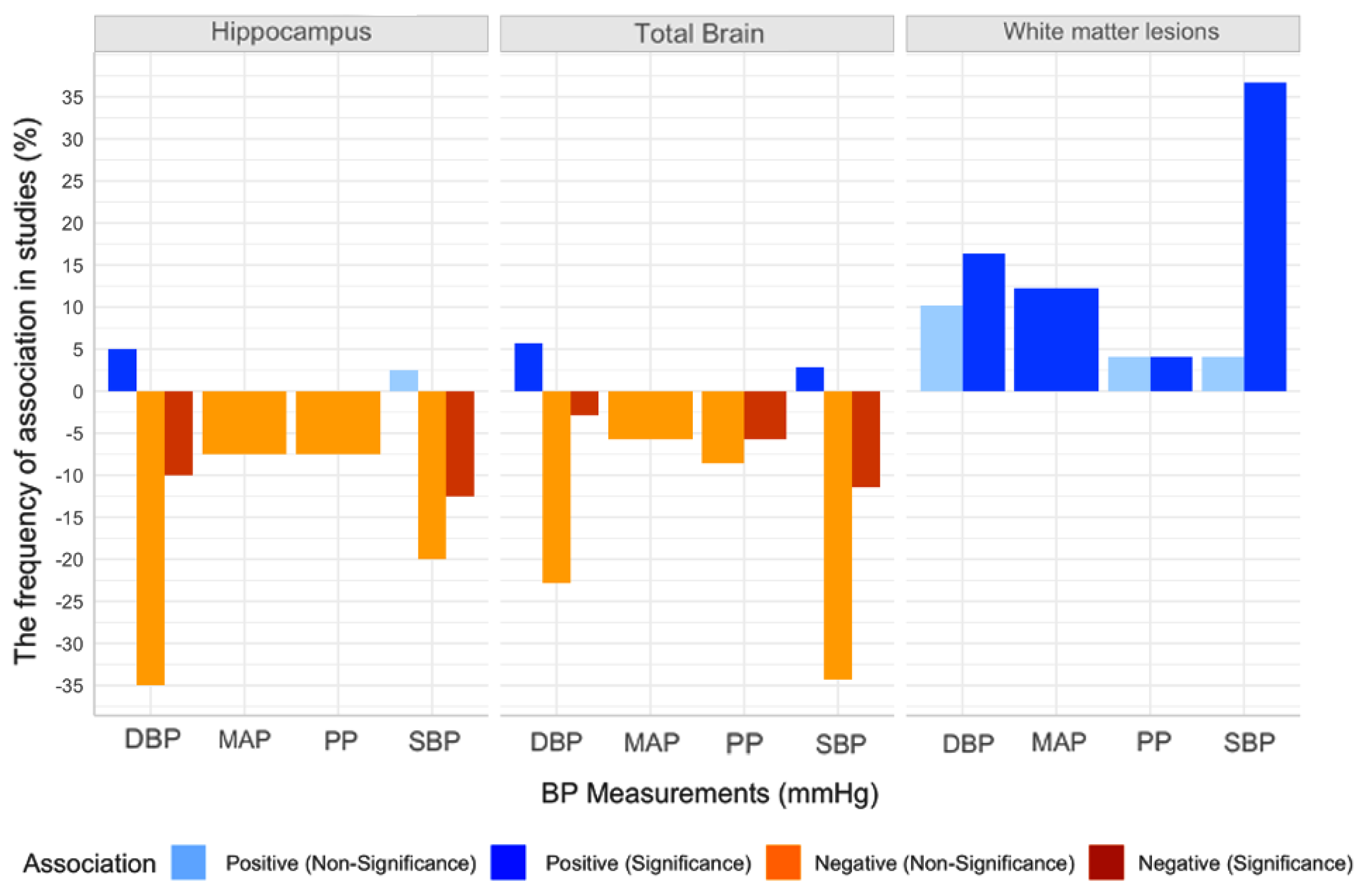
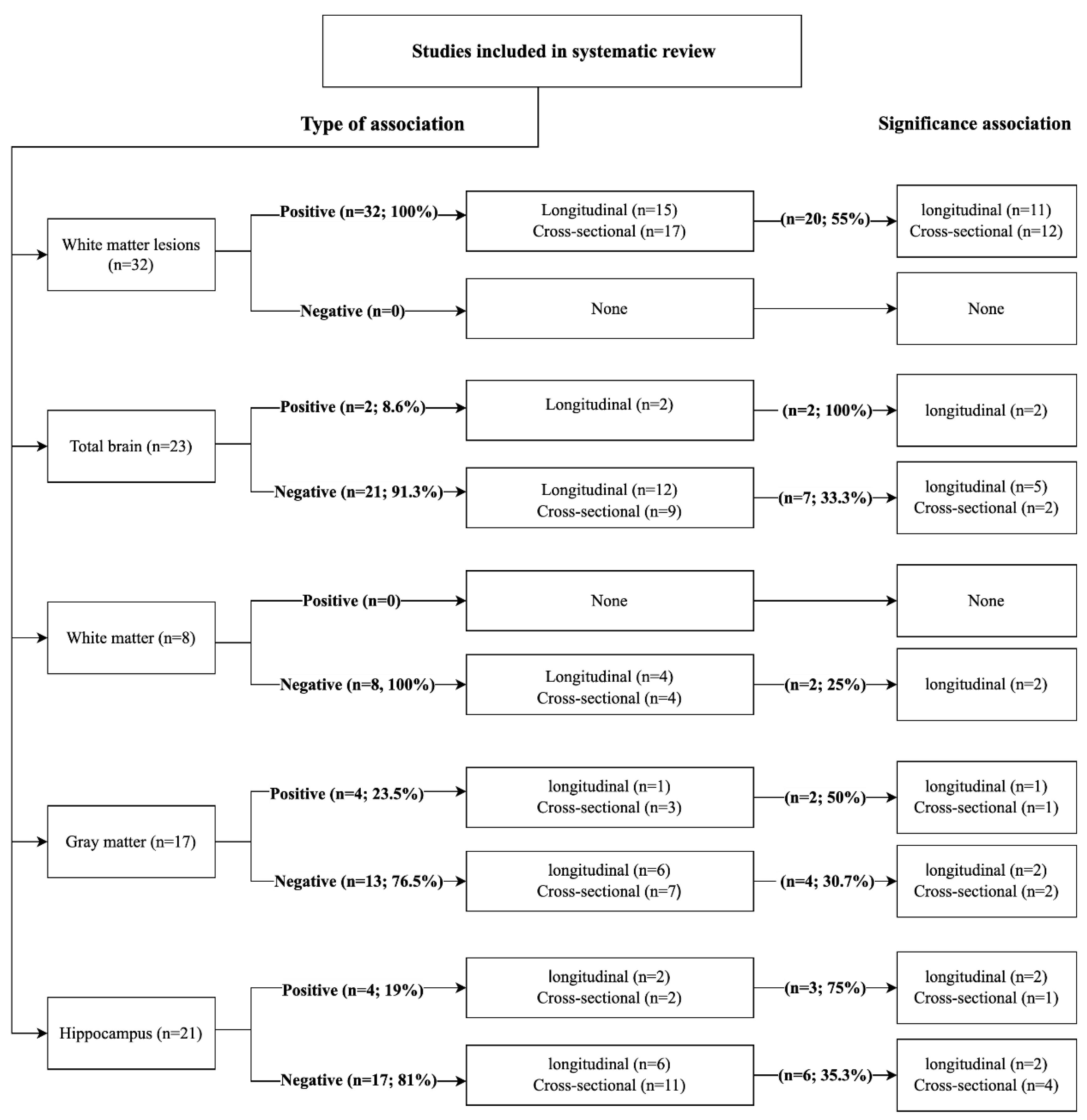
3.5. Association between Peripheral BP and Global and Regional Brain Volume
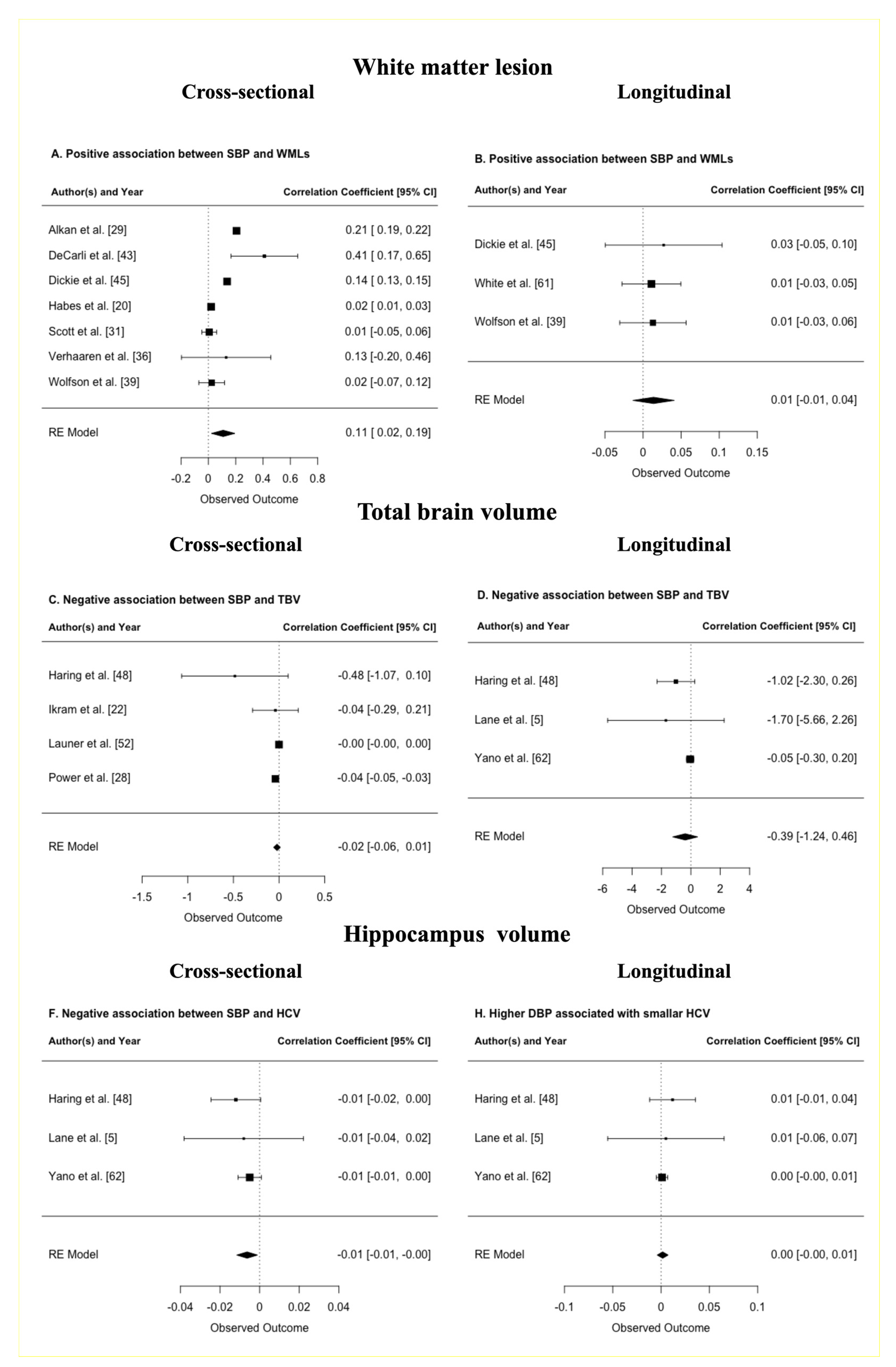
3.5.1. BP and White Matter Lesions
Positive Association with White Matter Lesions
Negative Association with WMLs
Meta-Analysis of BP and WMLs
Meta-Regression of BP and WMLs
3.5.2. BP and Total Brain Volume
Positive Association with TBV
Negative Association with TBV
Meta-Analysis of BP and TBV
3.5.3. BP and White Matter Volume
Positive Association with WM Volume
Negative Association with WM Volume
3.5.4. BP and Grey Matter (GM) Volume
Positive Association with GM Volume
Negative Association with GM Volume
3.5.5. BP and Hippocampal Volume
Positive Association with HCV
Negative Association with HCV
Meta-Analysis of BP and HCV
3.6. Association between Centeral BP and Global and Regional Brain Volume
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Population Reference Bureau. 2018 World Population Data Sheet with Focus on Changing Age Structures. In PRB Project 2.3 Billion More People Living on Earth by 2050. Available online: www.worldpopdata.org (accessed on 25 April 2020).
- Stevens, S.L.; Wood, S.; Koshiaris, C.; Law, K.; Glasziou, P.; Stevens, R.J.; McManus, R.J. Blood pressure variability and cardiovascular disease: Systematic review and meta-analysis. BMJ 2016, 354, 4098–4105. [Google Scholar] [CrossRef]
- Seshadri, S.; Wolf, P.A.; Beiser, A.; Vasan, R.S.; Wilson, P.W.F.; Kase, C.S.; Kelly-Hayes, M.; Kannel, W.B.; D’Agostino, R.B. Elevated midlife blood pressure increases stroke risk in elderly persons: The Framingham study. Arch. Intern. Med. 2001, 161, 2343–2350. [Google Scholar] [CrossRef]
- Sharp, S.I.; Aarsland, D.; Day, S.; Sønnesyn, H.; Ballard, C. Hypertension is a potential risk factor for vascular dementia: Systematic review. Int. J. Geriatr. Psychiatry 2011, 26, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Lane, C.A.; Barnes, J.; Nicholas, J.M.; Sudre, C.H.; Cash, D.M.; Parker, T.D.; Malone, I.B.; Lu, K.; James, S.-N.; Keshavan, A.; et al. Associations between blood pressure across adulthood and late-life brain structure and pathology in the neuroscience substudy of the 1946 British birth cohort (Insight 46): An epidemiological study. Lancet. Neurol. 2019, 18, 942–952. [Google Scholar] [CrossRef]
- Cherbuin, N.; Mortby, M.E.; Janke, A.L.; Sachdev, P.S.; Abhayaratna, W.P.; Anstey, K.J. Blood Pressure, Brain Structure, and Cognition: Opposite Associations in Men and Women. Am. J. Hypertens. 2015, 28, 225–231. [Google Scholar] [CrossRef]
- Duff, M.C.; Covington, N.V.; Hilverman, C.; Cohen, N.J. Semantic Memory and the Hippocampus: Revisiting, Reaffirming, and Extending the Reach of Their Critical Relationship. Front. Hum. Neurosci. 2020, 13, 471. [Google Scholar] [CrossRef]
- Jennings, J.R.; Muldoon, M.F.; Ryan, C.; Gach, H.M.; Heim, A.; Sheu, L.K.; Gianaros, P.J. Prehypertensive blood pressures and regional cerebral blood flow independently relate to cognitive performance in midlife. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Baker, S.E.; Limberg, J.K.; Dillon, G.A.; Curry, T.B.; Joyner, M.J.; Nicholson, W.T. Aging Alters the Relative Contributions of the Sympathetic and Parasympathetic Nervous System to Blood Pressure Control in Women. Hypertension 2018, 72, 1236–1242. [Google Scholar] [CrossRef]
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cífková, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A.; et al. Hypertension. Nat. Rev. Dis. Prim. 2018, 4, 18014. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Cherbuin, N.; Kim, S.; Anstey, K.J. Dementia risk estimates associated with measures of depression: A systematic review and meta-analysis. BMJ Open 2015, 5, e008853. [Google Scholar] [CrossRef]
- Kelly, R.; Fitchett, D. Noninvasive determination of aortic input impedance and external left ventricular power output: A validation and repeatability study of a new technique. J. Am. Coll. Cardiol. 1992, 20, 952–963. [Google Scholar] [CrossRef]
- Peterson, R.A.; Brown, S.P. On the Use of Beta Coefficients in Meta-Analysis. J. Appl. Psychol. 2005, 90, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar]
- Glodzik, L.; Rusinek, H.; Pirraglia, E.; McHugh, P.; Tsui, W.; Williams, S.; Cummings, M.; Li, Y.; Rich, K.; Randall, C.; et al. Blood pressure decrease correlates with tau pathology and memory decline in hypertensive elderly. Neurobiol. Aging 2014, 35, 64–71. [Google Scholar] [CrossRef]
- Habes, M.; Erus, G.; Toledo, J.B.; Zhang, T.; Bryan, N.; Launer, L.J.; Rosseel, Y.; Janowitz, D.; Doshi, J.; Van der Auwera, S.; et al. White matter hyperintensities and imaging patterns of brain ageing in the general population. Brain 2016, 139, 1164–1179. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, I.; Zhao, P.; Alsop, D.; Abduljalil, A.; Selim, M.; Novak, P.; Novak, V. Association of blood pressure elevation and nocturnal dipping with brain atrophy Perfusion and Functional Measures in Stroke and Nonstroke Individuals. Am. J. Hypertens. 2010, 23, 17–23. [Google Scholar] [CrossRef]
- Ikram, M.A.; Vrooman, H.A.; Vernooij, M.W.; van der Lijn, F.; Hofman, A.; van der Lugt, A.; Niessen, W.J.; Breteler, M.M.B. Brain tissue volumes in the general elderly population. The Rotterdam Scan Study. Neurobiol. Aging 2008, 29, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Kern, K.C.; Wright, C.B.; Bergfield, K.L.; Fitzhugh, M.C.; Sacco, R.L.; Stern, Y.; Decarli, C.S.; Alexander, G.E. Blood Pressure Control in Aging Predicts Cerebral Atrophy Related to Small-Vessel White Matter Lesions. Front. Aging Neurosci. 2017, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kobuch, S.; Fatouleh, R.H.; Macefield, J.M.; Henderson, L.A.; Macefield, V.G. Differences in regional grey matter volume of the brain are related to mean blood pressure and muscle sympathetic nerve activity in normotensive humans. J. Hypertens. 2020, 38, 303–313. [Google Scholar] [CrossRef]
- Launer, L.J.; Lewis, C.E.; Schreiner, P.J.; Sidney, S.; Battapady, H.; Jacobs, D.R.; Lim, K.O.; D’Esposito, M.; Zhang, Q.; Reis, J.; et al. Vascular factors and multiple measures of early brain health: CARDIA Brain MRI Study. PLoS ONE 2015, 10, e0122138. [Google Scholar] [CrossRef] [PubMed]
- McNeil, C.J.; Myint, P.K.; Sandu, A.L.; Potter, J.F.; Staff, R.; Whalley, L.J.; Murray, A.D. Increased diastolic blood pressure is associated with MRI biomarkers of dementia-related brain pathology in normative ageing. Age Ageing 2018, 47, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Pase, M.P.; Beiser, A.; Aparicio, H.; DeCarli, C.; Vasan, R.S.; Murabito, J.; Seshadri, S. Interarm differences in systolic blood pressure and the risk of dementia and subclinical brain injury. Alzheimers. Dement. 2016, 12, 438–445. [Google Scholar] [CrossRef]
- Power, M.C.; Schneider, A.L.C.; Wruck, L.; Griswold, M.; Coker, L.H.; Alonso, A.; Jack, C.R.; Knopman, D.; Mosley, T.H.; Gottesman, R.F. Life-course blood pressure in relation to brain volumes. Alzheimer’s Dement. 2016, 12, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Alkan, E.; Taporoski, T.P.; Sterr, A.; von Schantz, M.; Vallada, H.; Krieger, J.E.; Pereira, A.C.; Alvim, R.; Horimoto, A.R.V.R.; Pompéia, S.; et al. Metabolic syndrome alters relationships between cardiometabolic variables, cognition and white matter hyperintensity load. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Schaare, H.L.; Masouleh, S.K.; Beyer, F.; Kumral, D.; Uhlig, M.; Reinelt, J.D.; Reiter, A.M.F.; Lampe, L.; Babayan, A.; Al, E. Association of peripheral blood pressure with gray matter volume in 19- to 40-year-old adults. Neurology 2019, 92, e758–e773. [Google Scholar] [CrossRef]
- Scott, J.A.; Braskie, M.N.; Tosun, D.; Thompson, P.M.; Weiner, M.; DeCarli, C.; Carmichael, O.T. Cerebral amyloid and hypertension are independently associated with white matter lesions in elderly. Front. Aging Neurosci. 2015, 7, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Gao, H.; Bai, W.; Evangelou, E.; Glocker, B.; O’Regan, D.P.; Elliott, P.; Matthews, P.M. Abnormal brain white matter microstructure is associated with both pre-hypertension and hypertension. PLoS ONE 2017, 12, e0187600. [Google Scholar] [CrossRef]
- Taki, Y.; Goto, R.; Evans, A.; Zijdenbos, A.; Neelin, P.; Lerch, J.; Sato, K.; Ono, S.; Kinomura, S.; Nakagawa, M.; et al. Voxel-based morphometry of human brain with age and cerebrovascular risk factors. Neurobiol. Aging 2004, 25, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Trotman, G.P.; Williams, S.E.; Ginty, A.T.; Gianaros, P.J. Increased stressor—Evoked cardiovascular reactivity is associated with reduced amygdala and hippocampus volume. Psychophysiology 2019, 56, e13277. [Google Scholar] [CrossRef] [PubMed]
- van Velsen, E.F.S.; Vernooij, M.W.; Vrooman, H.A.; van der Lugt, A.; Breteler, M.M.B.; Hofman, A.; Niessen, W.J.; Ikram, M.A. Brain cortical thickness in the general elderly population: The Rotterdam Scan Study. Neurosci. Lett. 2013, 550, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Verhaaren, B.F.J.; Vernooij, M.W.; De Boer, R.; Hofman, A.; Niessen, W.J.; Van Der Lugt, A.; Ikram, M.A. High blood pressure and cerebral white matter lesion progression in the general population. Hypertension 2013, 61, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Allerhand, M.; Doubal, F.N.; Hernandez, M.V.; Morris, Z.; Gow, A.J.; Bastin, M.; Starr, J.M.; Dennis, M.S.; Deary, I.J. Vascular risk factors, large-artery atheroma, and brain white matter hyperintensities. Neurology 2014, 82, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, R.M.; Saxby, B.K.; Burton, E.J.; Barber, R.; Ford, G.A.; Brien, J.T.O. Hippocampal atrophy, whole brain volume, and white matter lesions in older hypertensive subjects. Neurology 2004, 63, 1892–1897. [Google Scholar] [CrossRef]
- Wolfson, L.; Wakefield, D.B.; Moscufo, N.; Kaplan, R.F.; Hall, C.B.; Schmidt, J.A.; Guttmann, C.R.G.; White, W.B. Rapid buildup of brain white matter hyperintensities over 4 years linked to ambulatory blood pressure, mobility, cognition, and depression in old persons. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 1387–1394. [Google Scholar] [CrossRef]
- Bender, A.R.; Raz, N. Age-related differences in memory and executive functions in healthy APOE varepsilon4 carriers: The contribution of individual differences in prefrontal volumes and systolic blood pressure. Neuropsychologia 2012, 50, 704–714. [Google Scholar] [CrossRef][Green Version]
- Allan, C.L.; Zsoldos, E.; Filippini, N.; Sexton, C.E.; Topiwala, A.; Valkanova, V.; Singh-Manoux, A.; Tabák, A.G.; Shipley, M.J.; Mackay, C.; et al. Lifetime hypertension as a predictor of brain structure in older adults: Cohort study with a 28-year follow-up. Br. J. Psychiatry 2015, 206, 308–315. [Google Scholar] [CrossRef]
- Burns, J.M.; Church, J.A.; Johnson, D.K.; Xiong, C.; Marcus, D.; Fotenos, A.F.; Snyder, A.Z.; Morris, J.C.; Buckner, R.L. White matter lesions are prevalent but differentially related with cognition in aging and early Alzheimer disease. Arch. Neurol. 2005, 62, 1870–1876. [Google Scholar] [CrossRef] [PubMed]
- DeCarli, C.; Murphy, D.G.; Tranh, M.; Grady, C.L.; Haxby, J.V.; Gillette, J.A.; Salerno, J.A.; Gonzales-Aviles, A.; Horwitz, B.; Rapoport, S.I.; et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology 1995, 45, 2077–2084. [Google Scholar] [CrossRef]
- den Heijer, T.; Launer, L.J.; Prins, N.D.; van Dijk, E.J.; Vermeer, S.E.; Hofman, A.; Koudstaal, P.J.; Breteler, M.M. Association between blood pressure, white matter lesions, and atrophy of the medial temporal lobe. Neurology 2005, 64, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Dickie, D.A.; Ritchie, S.J.; Cox, S.R.; Sakka, E.; Royle, N.A.; Aribisala, B.S.; Hernández, M.D.C.V.; Maniega, S.M.; Pattie, A.; Corley, J.; et al. Vascular risk factors and progression of white matter hyperintensities in the Lothian Birth Cohort 1936. Neurobiol. Aging 2016, 42, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Firbank, M.J.; Wiseman, R.M.; Burton, E.J.; Saxby, B.K.; O’Brien, J.T.; Ford, G.A. Brain atrophy and white matter hyperintensity change in older adults and relationship to blood pressure. J. Neurol. 2007, 254, 713–734. [Google Scholar] [CrossRef]
- Gattringer, T.; Enzinger, C.; Ropele, S.; Gorani, F.; Petrovic, K.E.; Schmidt, R.; Fazekas, F. Vascular risk factors, white matter hyperintensities and hippocampal volume in normal elderly individuals. Dement. Geriatr. Cogn. Disord. 2012, 33, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Haring, B.; Liu, J.; Salmoirago-Blotcher, E.; Hayden, K.M.; Sarto, G.; Roussouw, J.; Kuller, L.H.; Rapp, S.R.; Wassertheil-Smoller, S. Blood pressure variability and brain morphology in elderly women without cardiovascular disease. Neurology 2019, 92, E1284–E1297. [Google Scholar] [CrossRef] [PubMed]
- Hoogendam, Y.Y.; van der Geest, J.N.; van der Lijn, F.; van der Lugt, A.; Niessen, W.J.; Krestin, G.P.; Hofman, A.; Vernooij, M.W.; Breteler, M.M.; Ikram, M.A. Determinants of cerebellar and cerebral volume in the general elderly population. Neurobiol. Aging 2012, 33, 2774–2781. [Google Scholar] [CrossRef]
- Jeerakathil, T.; Wolf, P.A.; Beiser, A.; Massaro, J.; Seshadri, S.; D’Agostino, R.B.; DeCarli, C. Stroke risk profile predicts white matter hyperintensity volume-The Framingham Study. Stroke 2004, 35, 1857–1861. [Google Scholar] [CrossRef]
- Korf, E.S.; White, L.R.; Scheltens, P.; Launer, L.J. Midlife blood pressure and the risk of hippocampal atrophy-The Honolulu Asia Aging Study. Hypertension 2004, 44, 29–34. [Google Scholar] [CrossRef]
- Mahinrad, S.; Kurian, S.; Garner, C.R.; Sedaghat, S.; Nemeth, A.J.; Moscufo, N.; Higgins, J.P.; Jacobs, D.R.; Hausdorff, J.M.; Lloyd-Jones, D.M.; et al. Cumulative Blood Pressure Exposure During Young Adulthood and Mobility and Cognitive Function in Midlife. Circulation 2019, 141, 712–724. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; Sigurdsson, S.; Kjartansson, O.; Aspelund, T.; Lopez, O.L.; Jonnson, P.V.; Harris, T.B.; Van Buchem, M.; Gudnason, V.; Launer, L.J. Joint effect of mid- and late-life blood pressure on the brain: The AGES-Reykjavik Study. Neurology 2014, 82, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; Sigurdsson, S.; Kjartansson, O.; Gunnarsdottir, I.; Thorsdottir, I.; Harris, T.B.; van Buchem, M.; Gudnason, V.; Launer, L.J. Late-life brain volume: A life-course approach. The AGES-Reykjavik study. Neurobiol. Aging 2016, 41, 86–92. [Google Scholar] [CrossRef]
- Nation, D.A.; Preis, S.R.; Beiser, A.; Bangen, K.J.; Delano-Wood, L.; Lamar, M.; Libon, D.J.; Seshadri, S.; Wolf, P.A.; Au, R. Pulse Pressure Is Associated With Early Brain Atrophy and Cognitive Decline: Modifying Effects of APOE-epsilon4. Alzheimer Dis. Assoc. Disord. 2016, 30, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Paganini-Hill, A.; Bryant, N.; Corrada, M.M.; Greenia, D.E.; Fletcher, E.; Singh, B.; Floriolli, D.; Kawas, C.H.; Fisher, M.J. Blood Pressure Circadian Variation, Cognition and Brain Imaging in 90+ Year-Olds. Front. Aging Neurosci. 2019, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Sabayan, B.; Wijsman, L.W.; Foster-Dingley, J.C.; Stott, D.J.; Ford, I.; Buckley, B.M.; Sattar, N.; Jukema, J.W.; van Osch, M.J.; van der Grond, J.; et al. Association of visit-to-visit variability in blood pressure with cognitive function in old age: Prospective cohort study. BMJ Clin. Res. Ed 2013, 34, 1544–1550. [Google Scholar]
- Spartano, N.L.; Himali, J.J.; Beiser, A.S.; Lewis, G.D.; DeCarli, C.; Vasan, R.S.; Seshadri, S. Midlife exercise blood pressure, heart rate, and fitness relate to brain volume 2 decades later. Neurology 2016, 86, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Taki, Y.; Thyreau, B.; Kinomura, S.; Sato, K.; Goto, R.; Wu, K.; Kawashima, R.; Fukuda, H. A longitudinal study of age- and gender-related annual rate of volume changes in regional gray matter in healthy adults. Hum. Brain Mapp. 2013, 34, 2292–2301. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Himali, J.J.; Beiser, A.S.; Larson, M.G.; Decarli, C.; Mitchell, G.F. Association of arterial stiffness with progression of subclinical brain and cognitive disease. Neurology 2016, 7, 619–626. [Google Scholar] [CrossRef]
- White, W.B.; Wolfson, L.; Wakefield, D.B.; Hall, C.B.; Campbell, P.; Moscufo, N.; Schmidt, J.; Kaplan, R.F.; Pearlson, G.; Guttmann, C.R. Average daily blood pressure, not office blood pressure, is associated with progression of cerebrovascular disease and cognitive decline in older people. Circulation 2011, 124, 2312–2319. [Google Scholar] [CrossRef] [PubMed]
- Yano, Y.; Reis, J.P.; Levine, D.A.; Bryan, R.N.; Viera, A.J.; Shimbo, D.; Tedla, Y.G.; Allen, N.B.; Schreiner, P.J.; Bancks, M.P.; et al. Visit-to-Visit Blood Pressure Variability in Young Adulthood and Hippocampal Volume and Integrity at Middle Age. Hypertension 2017, 70, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Brickman, A.M.; Reitz, C.; Luchsinger, J.A.; Manly, J.J.; Schupf, N.; Muraskin, J.; DeCarli, C.; Brown, T.R.; Mayeux, R. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch. Neurol. 2010, 67, 564–569. [Google Scholar] [CrossRef] [PubMed]
- den Heijer, T.; van der Lijn, F.; Ikram, A.; Koudstaal, P.J.; Van Der Lugt, A.; Krestin, G.P.; Vrooman, H.A.; Hofman, A.; Niessen, W.J.; Breteler, M.M.B. Vascular risk factors, apolipoprotein E, and hippocampal decline on magnetic resonance imaging over a 10-year follow-up. Alzheimer’s Dement. 2012, 8, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Gianaros, P.J.; Greer, P.J.; Ryan, C.M.; Jennings, J.R. Higher blood pressure predicts lower regional grey matter volume: Consequences on short-term information processing. Neuroimage 2006, 31, 754–765. [Google Scholar] [CrossRef]
- Goldstein, I.B.; Bartzokis, G.; Guthrie, D.; Shapiro, D. Ambulatory blood pressure and brain atrophy in the healthy elderly. Neurology 2002, 59, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, I.B.; Bartzokis, G.; Guthrie, D.; Shapiro, D. Ambulatory blood pressure and the brain: A 5-year follow-up. Neurology 2005, 64, 1846–1852. [Google Scholar] [CrossRef] [PubMed]
- Cherbuin, N.; Sachdev, P.; Anstey, K.J. Higher normal fasting plasma glucose is associated with hippocampal atrophy: The PATH Study. Neurology 2012, 79, 1019–1026. [Google Scholar] [CrossRef]
- Allen, B.; Muldoon, M.F.; Gianaros, P.J.; Jennings, J.R. Higher Blood Pressure Partially Links Greater Adiposity to Reduced Brain White Matter Integrity. Am. J. Hypertens. 2016, 29, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- de Jong, L.W.; Forsberg, L.E.; Vidal, J.S.; Sigurdsson, S.; Zijdenbos, A.P.; Garcia, M.; Eiriksdottir, G.; Gudnason, V.; van Buchem, M.A.; Launer, L.J. Different susceptibility of medial temporal lobe and basal ganglia atrophy rates to vascular risk factors. Neurobiol. Aging 2014, 35, 72–78. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Franklin, S.S.; Gustin, W.; Wong, N.D.; Larson, M.G.; Weber, M.A.; Kannel, W.B.; Levy, D. Hemodynamic Patterns of Age-Related Changes in Blood Pressure. Circulation 1997, 96, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Fraser, M.A.; Shaw, M.E.; Cherbuin, N. A systematic review and meta-analysis of longitudinal hippocampal atrophy in healthy human ageing. Neuroimage 2015, 112, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei-Jafari, H.; Shaw, M.E.; Cherbuin, N. Cerebral atrophy in mild cognitive impairment: A systematic review with meta-analysis. Alzheimer’s Dement. Diagnosis, Assess. Dis. Monit. 2015, 11, 487–504. [Google Scholar] [CrossRef]
- Nosalski, R.; McGinnigle, E.; Siedlinski, M.; Guzik, T.J. Novel Immune Mechanisms in Hypertension and Cardiovascular Risk. Curr. Cardiovasc. Risk Rep. 2017, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Touyz, R.M. Oxidative Stress, Inflammation, and Vascular Aging in Hypertension. Hypertension 2017, 70, 660–667. [Google Scholar] [CrossRef]
- Mikolajczyk, T.P.; Nosalski, R.; Szczepaniak, P.; Budzyn, K.; Osmenda, G.; Skiba, D.; Sagan, A.; Wu, J.; Vinh, A.; Marvar, P.J.; et al. Role of chemokine RANTES in the regulation of perivascular inflammation, T-cell accumulation, and vascular dysfunction in hypertension. FASEB J. 2016, 30, 1987–1999. [Google Scholar] [CrossRef]
- Perry, V.H. The influence of systemic inflammation on inflammation in the brain: Implications for chronic neurodegenerative disease. Brain. Behav. Immun. 2004, 18, 407–413. [Google Scholar] [CrossRef]
- Hamilton, O.K.L.; Backhouse, E.V.; Janssen, E.; Jochems, A.C.C.; Maher, C.; Ritakari, T.E.; Stevenson, A.J.; Xia, L.; Deary, I.J.; Wardlaw, J.M. Cognitive impairment in sporadic cerebral small vessel disease: A systematic review and meta-analysis. Alzheimer’s Dement. 2020, 13, 1–21. [Google Scholar] [CrossRef]
- Ji, X.; Leng, X.-Y.; Dong, Y.; Ma, Y.-H.; Xu, W.; Cao, X.-P.; Hou, X.-H.; Dong, Q.; Tan, L.; Yu, J.-T. Modifiable risk factors for carotid atherosclerosis: A meta-analysis and systematic review. Ann. Transl. Med. 2019, 7, 632. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.A.; Power, M.C.; Gottesman, R.F. Defining the Relationship Between Hypertension, Cognitive Decline, and Dementia: A Review. Curr. Hypertens. Rep. 2017, 19, 24. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Small vessel disease: Mechanisms and clinical implications. Lancet Neurol. 2019, 18, 684–696. [Google Scholar] [CrossRef]
- Duering, M.; Righart, R.; Wollenweber, F.A.; Zietemann, V.; Gesierich, B.; Dichgans, M. Acute infarcts cause focal thinning in remote cortex via degeneration of connecting fiber tracts. Neurology 2015, 84, 1685–1692. [Google Scholar] [CrossRef] [PubMed]
- Liao, R.; Wang, L.; Li, J.; Sun, S.; Xiong, Y.; Li, Y.; Han, M.; Jiang, H.; Anil, M.; Su, B. Vascular calcification is associated with Wnt-signaling pathway and blood pressure variability in chronic kidney disease rats. Nephrology 2020, 25, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Honarmand, A.R.; Schnell, S.; Kuhn, R.; Schoeneman, S.E.; Ansari, S.A.; Carr, J.; Markl, M.; Shaibani, A. Age-related changes of normal cerebral and cardiac blood flow in children and adults aged 7 months to 61 years. J. Am. Heart Assoc. 2016, 5, e002657. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Neugebauer, H.; Li, F.; Lulé, D.; Müller, H.P.; Zhang, J.; Ludolph, A.C.; Huang, Y.; Kassubek, J.; Zhang, W. Clinical and neuroimaging disparity between Chinese and German patients with cerebral small vessel disease: A comparative study. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Beauchet, O.; Celle, S.; Roche, F.; Bartha, R.; Montero-Odasso, M.; Allali, G.; Annweiler, C. Blood pressure levels and brain volume reduction: A systematic review and meta-analysis. J. Hypertens. 2013, 31, 1502–1516. [Google Scholar] [CrossRef] [PubMed]
| Author, and year | Study Setting/Design | N | Age M (SD) | Sex (% female) | BP Methods | SBP M (SD) | DBP M (SD) | %HT | %AHT | Brain Region | Magnet/Segmentation | Covariates | Meta-Analysis of: |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alkan et al. 2019 [29] | The Baependi Heart Study/Cross-sectional | 164 | 60.1 (7.8) | 59.1 | Occasional | 129.6 (16.9) | 79.5 (19.2) | 54.5 | NR | WMLs | 1.5 T/Semi-automated | Age, education, BMI, WC, cholesterol, FBG, triglyceride, HDL-C, LDL-C, SBP, DBP, effect of sex and number of MetS | SBP and WMLs (cross-sectional) |
| DeCarli et al. 1995 [43] | Cross-sectional/National Institute on Aging | 51 | 52 (20) | 49 | Occasional | 124 (14) | 78 (9) | 0 | NR | WMLs | 0.5 T/NR | Age and education | SBP and WMLs (cross-sectional) |
| Den Heijer et al. 2005 [44] | Rotterdam Study/Cross-sectional and Longitudinal | 511 | 73.4 (8) | 49.1 | Occasional | 145.8 (20.3) | 76.5 (11.6) | NR | 38.9 | HCV, Amygdala | 1.5 T/Manual | Age, sex and CVD factors | DBP and HCV (cross-sectional) |
| Dickie et al. 2016 [45] | Community dwelling/Cross-sectional and longitudinal (~2 years) | 681 | 72.7 (0.7) | 47 | Occasional | 146 (18) | 79 (9) | 48.2 | NR | WMLs | 1.5 T/Semi-automated | Sex, BMI, and CVD history | SBP and WMLs (cross-sectional and longitudinal) |
| Habes et al. 2016 [20] | SHIP study/Cross-sectional | 2367 | 52.4 (13.7) | 56.7 | Occasional | 127.3 (17.6) | NR | NR | 32.7 | WMLs | NR/Semi-automated | Age, sex and education | SBP and WMLs (cross-sectional) |
| Haring et al. 2019 [48] | Women’s Health Initiative/Longitudinal (~ 8 years) | 558 | 78.3 (3.6) | 100 | Variability | 122 (1) | 73 (7) | 48 | NR | Regional GM | 3 T/Semi-automated | Age, education, APOE4 allele | (SBP, DBP) and TBV/ (SBP, DBP) and HCV (longitudinal) |
| Ikram et al. 2008 [22] | Rotterdam study/Cross-sectional | 490 | 73.4 (7.9) | 50.8 | Occasional | NR | NR | 51 | 0 | TBV, GM, WM | 1.5 T/Manual: TR was blinded to information | Age and sex. | (SBP, DBP) and TBV (cross-sectional) |
| Lane et al. 2019 [5] | NSHD/Cross-sectional and Longitudinal | 441 | 36 | 49 | Occasional and changes | 120.2 (13.7) | 78.4 (9.5) | 16 | 2 | TBV, HCV WMLs, | automated 3 T/Semi-automated | Sex, APOE ε4 status, AHT medication, and BP at 69 years of age. | SBP, DBP) and TBV/(SBP, DBP) and HCV (Cross-sectional and Longitudinal) |
| 441 | 43 | 49 | Occasional and changes | 123.5 (13.7) | 80 (9.3) | 52 | 28 | ||||||
| 441 | 53 | 49 | Occasional and changes | 133.5 (19) | 83.1 (11.8) | 46 | 12 | ||||||
| 441 | 60-64 | 49 | Occasional and changes | 124.9 (16.9) | 77.4 (9.5) | 22 | 2 | ||||||
| 441 | 69 | 49 | Occasional and changes | 120.2 (13.7) | 78.4 (9.5) | 16 | 2 | ||||||
| Launer et al. 2015 [52] | CARDIA/Cross-sectional | 680 | 50.3 (3.5) | 52.2 | Occasional | 139.9 (1.5) | 79.5 (0.9) | 32.2 | NR | TBV | 3 T/Semi-automated | Age, sex, and race. | (SBP, DBP) and TBV (Cross-sectional) |
| McNeil et al. 2018 [26] | Aberdeen 1936 Birth Cohort/Cross-sectional | 227 | 64.5 (0.8) | 52 | Occasional | 139.9 (1.5) | 79.5 (0.9) | NR | 45 | HCV | 1.5 T/Semi-automated | Age, sex, and AHT medication | SBP, DBP and HCV (Cross-sectional) |
| Power et al. 2016 [28] | ARIC study/Cross-sectional and Longitudinal (~15 and ~24 years) | 1678 | 52.0 | 61 | Occasional | 130 (5.9) | 66 (3.6) | 23.0 | 72.0 | TBV, HCV, brain lobes | 3 T/Semi-automated | Age, sex, race, education, ICV, BMI, DM, cholesterol, and smoking status | (SBP, DBP) and TBV /(SBP, DBP) and HCV (Cross-sectional and Longitudinal) |
| Scott et al. 2015 [31] | ADNI/Cross-sectional | 150 | 73.7 (6.3) | 48.7 | Occasional | 136 (16) | 75 (10) | 44.0 | NR | WMLs | 3 T/NR | Age | SBP and WMLs (Cross-sectional) |
| Verhaaren et al. 2013 [36] | Rotterdam Study/Cross-sectional and longitudinal | 665 | 61.6 (5) | 52 | Occasional | 138 (19) | 78 (10) | 25.9 | 22 | WMLs | 1.5 T/Semi-automated | Age, sex, and ICV, CVD factors | SBP and WMLs (Cross-sectional) |
| White et al. 2011 [61] | Community dwelling/Longitudinal (~2 years) | 72 | 82.1 (3.9) | 56.9 | Occasional | 122 (1.3) | 73 (7) | 70 | 64.0 | WMLs | 3 T/Semi-automated | Age and LDL cholesterol levels, | SBP and WMLs (longitudinal) |
| Wolfson et al. 2013 [39] | Community dwelling/Cross-sectional and Longitudinal (~2 years) | 67 | 81.7 (3.9) | 61.0 | ASBP | 138 (14) | 69 (7) | NR | 69.0 | WMLs | 3 T/Semi-automated | Age, sex, and BMI or education | SBP and WMLs (Cross-sectional and longitudinal) |
| Yano et al. 2017 [62] | CARDIA/Longi-tudinal (~2 years) | 547 | 25.6 (3.4) | 53.9 | Variability | 123.2 (12.2) | 73.4 (8.5) | 51.8 | 21.2 | TBV, GM, WM, HCV | 3 T/Semi-automated | Age, sex, ICV, AHT medications, education, fasting glucose, smoking, and physical activity and BMI | SBP and HCV (longitudinal) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alateeq, K.; Walsh, E.I.; Cherbuin, N. Higher Blood Pressure is Associated with Greater White Matter Lesions and Brain Atrophy: A Systematic Review with Meta-Analysis. J. Clin. Med. 2021, 10, 637. https://doi.org/10.3390/jcm10040637
Alateeq K, Walsh EI, Cherbuin N. Higher Blood Pressure is Associated with Greater White Matter Lesions and Brain Atrophy: A Systematic Review with Meta-Analysis. Journal of Clinical Medicine. 2021; 10(4):637. https://doi.org/10.3390/jcm10040637
Chicago/Turabian StyleAlateeq, Khawlah, Erin I. Walsh, and Nicolas Cherbuin. 2021. "Higher Blood Pressure is Associated with Greater White Matter Lesions and Brain Atrophy: A Systematic Review with Meta-Analysis" Journal of Clinical Medicine 10, no. 4: 637. https://doi.org/10.3390/jcm10040637
APA StyleAlateeq, K., Walsh, E. I., & Cherbuin, N. (2021). Higher Blood Pressure is Associated with Greater White Matter Lesions and Brain Atrophy: A Systematic Review with Meta-Analysis. Journal of Clinical Medicine, 10(4), 637. https://doi.org/10.3390/jcm10040637








