Changes in Plasma Glial Fibrillary Acidic Protein in Children Receiving Sevoflurane Anesthesia: A Preliminary Randomized Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design and Ethics
2.2. Participants
2.3. Interventions
2.4. Blood Sampling and Analysis
2.5. Outcomes
2.6. Sample Size Calculation
2.7. Randomization and Blinding
2.8. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, Y.; Hu, D.; Rodgers, E.L.; Katusic, S.K.; Gleich, S.J.; Hanson, A.C.; Schroeder, D.R.; Flick, R.P.; Warner, D.O. Epidemiology of general anesthesia prior to age 3 in a population-based birth cohort. Paediatr. Anaesth. 2018, 28, 513–519. [Google Scholar] [CrossRef]
- Cote, C.J.; Wilson, S. Guidelines for Monitoring and Management of Pediatric Patients Before, During, and After Sedation for Diagnostic and Therapeutic Procedures. Pediatrics 2019, 41, 26E–52E. [Google Scholar] [CrossRef]
- DeFrances, C.J.; Cullen, K.A.; Kozak, L.J. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat. 2007, 13, 1–209. [Google Scholar]
- Andropoulos, D.B.; Greene, M.F. Anesthesia and Developing Brains—Implications of the FDA Warning. N. Engl. J. Med. 2017, 376, 905–907. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Evaluation of Automatic Class III Designation for Banyan Brain Trauma Indicator. Available online: https://www. accessdata.fda.gov/cdrh_docs/reviews/DEN170045.pdf (accessed on 1 July 2019).
- Yue, J.K.; Yuh, E.L.; Korley, F.K.; Winkler, E.A.; Sun, X.; Puffer, R.C.; Deng, H.; Choy, W.; Chandra, A.; Taylor, S.R.; et al. Association between plasma GFAP concentrations and MRI abnormalities in patients with CT-negative traumatic brain injury in the TRACK-TBI cohort: A prospective multicentre study. Lancet Neurol. 2019, 18, 953–961. [Google Scholar] [CrossRef]
- Gill, J.; Latour, L.; Diaz-Arrastia, R.; Motamedi, V.; Turtzo, C.; Shahim, P.; Mondello, S.; DeVoto, C.; Veras, E.; Hanlon, D.; et al. Glial fibrillary acidic protein elevations relate to neuroimaging abnormalities after mild TBI. Neurology 2018, 91, e1385–e1389. [Google Scholar] [CrossRef] [PubMed]
- Vedovelli, L.; Padalino, M.; Suppiej, A.; Sartori, S.; Falasco, G.; Simonato, M.; Carnielli, V.P.; Stellin, G.; Cogo, P. Cardiopulmonary-Bypass Glial Fibrillary Acidic Protein Correlates with Neurocognitive Skills. Ann. Thorac. Surg. 2018, 106, 792–798. [Google Scholar] [CrossRef]
- Hossain, I.; Mohammadian, M.; Takala, R.S.K.; Tenovuo, O.; Lagerstedt, L.; Ala-Seppala, H.; Frantzén, J.; van Gils, M.; Hutchinson, P.; Katila, A.J.; et al. Early Levels of Glial Fibrillary Acidic Protein and Neurofilament Light Protein in Predicting the Outcome of Mild Traumatic Brain Injury. J. Neurotrauma 2019, 36, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Shemilt, M.; Boutin, A.; Lauzier, F.; Zarychanski, R.; Moore, L.; McIntyre, L.A.; Nadeau, L.; Fergusson, D.A.; Mercier, E.; Archambault, P.; et al. Prognostic Value of Glial Fibrillary Acidic Protein in Patients With Moderate and Severe Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Crit. Care Med. 2019, 47, e522–e529. [Google Scholar] [CrossRef]
- Hori, D.; Everett, A.D.; Lee, J.K.; Ono, M.; Brown, C.H.; Shah, A.S.; Mandal, K.; Price, J.E.; Lester, L.C.; Hogue, C.W. Rewarming Rate During Cardiopulmonary Bypass Is Associated With Release of Glial Fibrillary Acidic Protein. Ann. Thorac Surg. 2015, 100, 1353–1358. [Google Scholar] [CrossRef]
- Fink, E.L.; Berger, R.P.; Clark, R.S.; Watson, R.S.; Angus, D.C.; Panigrahy, A.; Richichi, R.; Callaway, C.W.; Bell, M.J.; Mondello, S.; et al. Exploratory study of serum ubiquitin carboxyl-terminal esterase L1 and glial fibrillary acidic protein for outcome prognostication after pediatric cardiac arrest. Resuscitation 2016, 101, 65–70. [Google Scholar] [CrossRef] [PubMed]
- McKenney, S.L.; Mansouri, F.F.; Everett, A.D.; Graham, E.M.; Burd, I.; Sekar, P. Glial fibrillary acidic protein as a biomarker for brain injury in neonatal CHD. Cardiol. Young. 2016, 26, 1282–1289. [Google Scholar] [CrossRef][Green Version]
- Rappold, T.; Laflam, A.; Hori, D.; Brown, C.; Brandt, J.; Mintz, C.D.; Sieber, F.; Gottschalk, A.; Yenokyan, G.; Everett, A.; et al. Evidence of an association between brain cellular injury and cognitive decline after non-cardiac surgery. Br. J. Anaesth. 2016, 116, 83–89. [Google Scholar] [CrossRef]
- Wu, C.Y.; Lin, Y.S.; Tseng, H.M.; Cheng, H.L.; Lee, T.S.; Lin, P.L.; Chou, W.H.; Cheng, Y.J. Comparison of two stroke volume variation-based goal-directed fluid therapies for supratentorial brain tumour resection: A randomized controlled trial. Br. J. Anaesth. 2017, 119, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Deiner, S.; Baxter, M.G.; Mincer, J.S.; Sano, M.; Hall, J.; Mohammed, I.; O’Bryant, D.; Zetterberg, H.; Blennow, K.; Eckenhoff, R. Human plasma biomarker responses to inhalational general anaesthesia without surgery. Br. J. Anaesth. 2020, 125, 282–290. [Google Scholar] [CrossRef]
- Vakkuri, A.; Yli-Hankala, A.; Särkelä, M.; Lindgren, L.; Mennander, S.; Korttila, K.; Saarnivaara, L.; Jäntti, V. Sevoflurane mask induction of anaesthesia is associated with epileptiform EEG in children. Acta Anaesthesiol. Scand. 2001, 45, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Stasiowski, M.J.; Marciniak, R.; Duława, A.; Krawczyk, L.; Jałowiecki, P. Epileptiform EEG patterns during different techniques of induction of general anaesthesia with sevoflurane and propofol: A randomised trial. Anaesthesiol. Intensive Ther. 2019, 51, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Tallach, R.E.; Ball, D.R.; Jefferson, P. Monitoring seizures with the bispectral index. Anaesthesia 2004, 59, 1033–1034. [Google Scholar] [CrossRef]
- Yue, H.; Han, J.; Liu, L.; Wang, K.; Li, J. Effect of rocuronium on the bispectral index under anesthesia and tracheal intubation. Exp. Ther. Med. 2016, 12, 3785–3789. [Google Scholar] [CrossRef]
- Dahaba, A.A.; Mattweber, M.; Fuchs, A.; Zenz, W.; Rehak, P.H.; List, W.F.; Metzler, H. The effect of different stages of neuromuscular block on the bispectral index and the bispectral index-XP under remifentanil/propofol anesthesia. Anesth Analg. 2004, 99, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Bazarian, J.J.; Biberthaler, P.; Welch, R.D.; Lewis, L.M.; Barzo, P.; Bogner-Flatz, V.; Gunnar Brolinson, P.; Büki, A.; Chen, J.Y.; Christenson, R.H.; et al. Serum GFAP and UCH-L1 for prediction of absence of intracranial injuries on head CT (ALERT-TBI): A multicentre observational study. Lancet Neurol. 2018, 17, 782–789. [Google Scholar] [CrossRef]
- Fraser, D.D.; Close, T.E.; Rose, K.L.; Ward, R.; Mehl, M.; Farrell, C.; Lacroix, J.; Creery, D.; Kesselman, M.; Stanimirovic, D.; et al. Severe traumatic brain injury in children elevates glial fibrillary acidic protein in cerebrospinal fluid and serum. Pediatr Crit Care Med. 2011, 12, 319–324. [Google Scholar] [CrossRef]
- Bembea, M.M.; Savage, W.; Strouse, J.J.; Schwartz, J.M.; Graham, E.; Thompson, C.B.; Everett, A. Glial fibrillary acidic protein as a brain injury biomarker in children undergoing extracorporeal membrane oxygenation. Pediatric Crit. Care Med. 2011, 12, 572–579. [Google Scholar] [CrossRef]
- Ren, X.; Ma, H.; Zuo, Z. Dexmedetomidine Postconditioning Reduces Brain Injury after Brain Hypoxia-Ischemia in Neonatal Rats. J. Neuroimmune Pharmacol. 2016, 11, 238–247. [Google Scholar] [CrossRef]
- Wang, Y.; Han, R.; Zuo, Z. Dexmedetomidine post-treatment induces neuroprotection via activation of extracellular signal-regulated kinase in rats with subarachnoid haemorrhage. Br. J. Anaesth. 2016, 116, 384–392. [Google Scholar] [CrossRef]
- Dahmani, S.; Rouelle, D.; Gressens, P.; Mantz, J. Characterization of the postconditioning effect of dexmedetomidine in mouse organotypic hippocampal slice cultures exposed to oxygen and glucose deprivation. Anesthesiology 2010, 112, 373–383. [Google Scholar] [CrossRef]
- Schoeler, M.; Loetscher, P.D.; Rossaint, R.; Fahlenkamp, A.V.; Eberhardt, G.; Rex, S.; Weis, J.; Coburn, M. Dexmedetomidine is neuroprotective in an in vitro model for traumatic brain injury. BMC Neurol. 2012, 12, 20. [Google Scholar] [CrossRef]
- Sanders, R.D.; Xu, J.; Shu, Y.; Januszewski, A.; Halder, S.; Fidalgo, A.; Sun, P.; Hossain, M.; Ma, D.; Maze, M. Dexmedetomidine attenuates isoflurane-induced neurocognitive impairment in neonatal rats. Anesthesiology 2009, 110, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Pancaro, C.; Segal, B.S.; Sikes, R.W.; Almeer, Z.; Schumann, R.; Azocar, R.J.; Marchand, J.E. Dexmedetomidine and ketamine show distinct patterns of cell degeneration and apoptosis in the developing rat neonatal brain. J. Matern. Fetal Neonatal Med. 2016, 29, 3827–3833. [Google Scholar] [CrossRef]
- Perez-Zoghbi, J.F.; Zhu, W.; Grafe, M.R.; Brambrink, A.M. Dexmedetomidine-mediated neuroprotection against sevoflurane-induced neurotoxicity extends to several brain regions in neonatal rats. Br. J. Anaesth. 2017, 119, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Song, A.Y.; Jones, S.E. Vineland social maturity scale norm examined—The Wisconsin experience with 0- to 3-year-old children. Am. J. Ment. Defic. 1982, 86, 428–431. [Google Scholar] [PubMed]
| Dexmedetomidine Group (n = 26) | Control Group (n = 29) | |
|---|---|---|
| Age (months) | 12.0 (4.8) | 13.7 (6.5) |
| Weight (kg) | 9.6 (1.8) | 10.1 (1.9) |
| Height (cm) | 76.2 (6.4) | 76.0 (8.3) |
| Gender (male, %) | 11 (42) | 20 (69) |
| Total anesthesia time (min.) | 285.7 (73.4) | 251.1 (69.1) |
| Type of operation (n) | ||
| Cochlear implantation | 19 | 14 |
| Penoplasty | 3 | 6 |
| Syndactyly division | 4 | 7 |
| Suturectomy | 0 | 2 |
| Fraction of expired sevoflurane (%) | 1.3 (0.3) | 2.5 (0.6) |
| End tidal carbon dioxide (mmHg) | 37 (2.1) | 38 (2.0) |
| Bispectral index | 61.6 (8.4) | 61.5 (4.5) |
| Duration of hypotension (%) | 2.1 (3.5) | 1.2 (3.0) |
| Presence of arrhythmia | None | None |
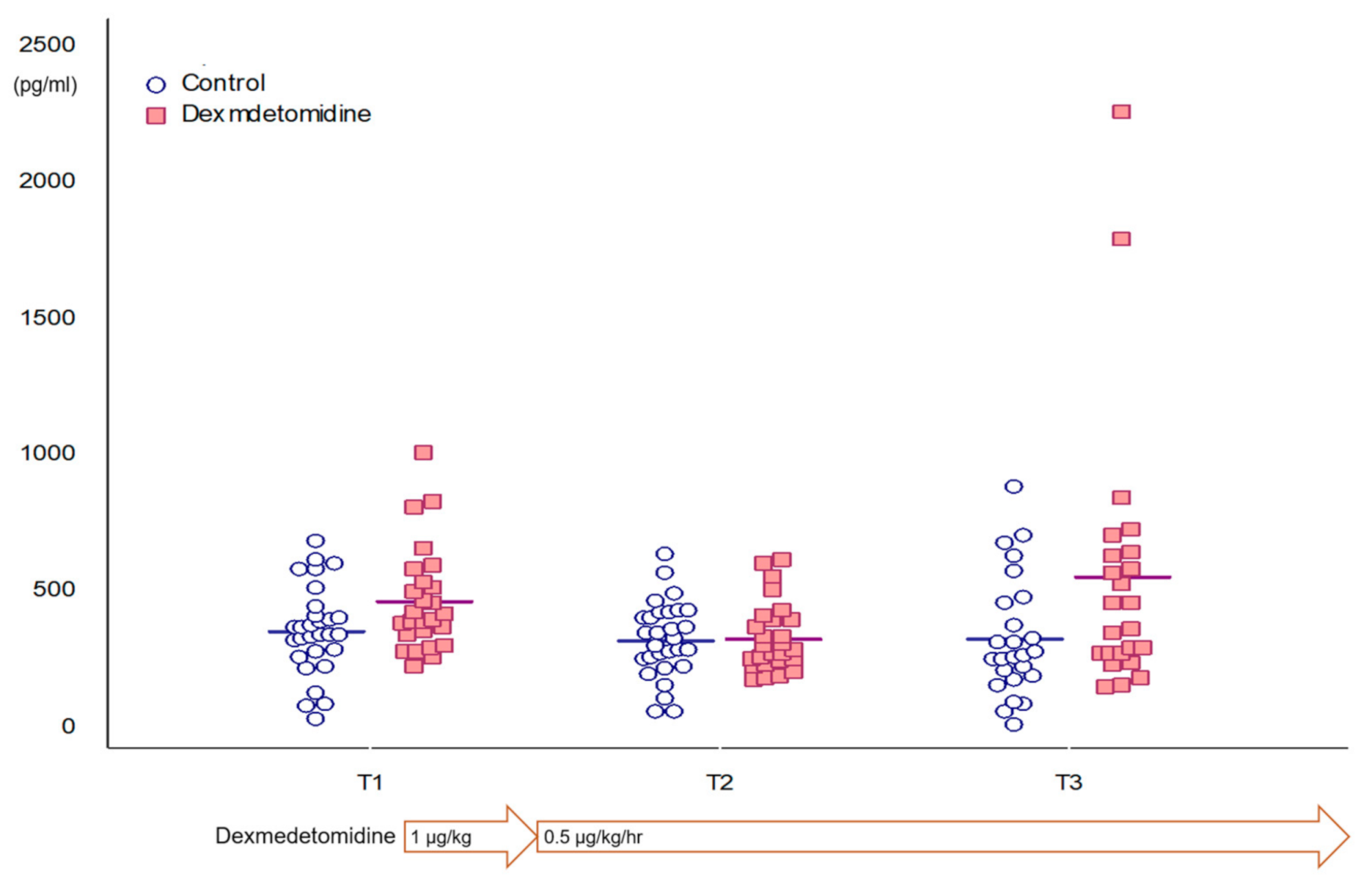
| T1 | T2 | T3 | p-Value | |
|---|---|---|---|---|
| All patients | 387.7 (298.9–510.8) | 329.1 (131.5) | 312.9 (233.8–576.2) | 0.072 |
| Control group | 358.1 (162.7) | 325.8 (138.3) | 332.0 (220.1) | 0.759 |
| Dexmedetomidine group | 406.7 (342.9–534.3) | 332.8 (126.1) | 412.4 (273.9–638.8) | 0.044 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.-H.; Jang, Y.-E.; Ji, S.-H.; Lee, J.-H.; Cho, S.-A.; Kim, J.-T.; Yoon, H.; Kim, H.-S. Changes in Plasma Glial Fibrillary Acidic Protein in Children Receiving Sevoflurane Anesthesia: A Preliminary Randomized Trial. J. Clin. Med. 2021, 10, 662. https://doi.org/10.3390/jcm10040662
Kim E-H, Jang Y-E, Ji S-H, Lee J-H, Cho S-A, Kim J-T, Yoon H, Kim H-S. Changes in Plasma Glial Fibrillary Acidic Protein in Children Receiving Sevoflurane Anesthesia: A Preliminary Randomized Trial. Journal of Clinical Medicine. 2021; 10(4):662. https://doi.org/10.3390/jcm10040662
Chicago/Turabian StyleKim, Eun-Hee, Young-Eun Jang, Sang-Hwan Ji, Ji-Hyun Lee, Sung-Ae Cho, Jin-Tae Kim, Hyunyee Yoon, and Hee-Soo Kim. 2021. "Changes in Plasma Glial Fibrillary Acidic Protein in Children Receiving Sevoflurane Anesthesia: A Preliminary Randomized Trial" Journal of Clinical Medicine 10, no. 4: 662. https://doi.org/10.3390/jcm10040662
APA StyleKim, E.-H., Jang, Y.-E., Ji, S.-H., Lee, J.-H., Cho, S.-A., Kim, J.-T., Yoon, H., & Kim, H.-S. (2021). Changes in Plasma Glial Fibrillary Acidic Protein in Children Receiving Sevoflurane Anesthesia: A Preliminary Randomized Trial. Journal of Clinical Medicine, 10(4), 662. https://doi.org/10.3390/jcm10040662





