Management of Lupus Nephritis
Abstract
:1. Introduction
2. Pathophysiology of Lupus Nephritis
3. Clinical and Pathological Diagnosis of Lupus Nephritis
4. Treatment of Lupus Nephritis
4.1. Global Therapeutic Strategy
4.2. Current Therapeutic Recommendations
5. How to Improve the Prognosis of Lupus Nephritis?
5.1. Treat-to-Target Approach
5.1.1. Clinical Target
5.1.2. Pathological and Immunological Target
5.2. Combination Therapy
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rovin, B.H.; Stillman, I. Kidney. In Systemic Lupus Erythematosus, 5th ed.; Lahita, R., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 769–814. [Google Scholar]
- Wang, H.; Ren, Y.L.; Chang, J.; Gu, L.; Sun, L.Y. A systematic review and meta-analysis of prevalence of biopsy-proven lupus nephritis. Arch. Rheumatol. 2017, 33, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Seligman, V.A.; Suarez, C.; Lum, R.; Inda, S.E.; Lin, D.; Li, H.; Olson, J.L.; Seldin, M.F.; Criswell, L.A. The Fcgamma receptor IIIA-158F allele is a major risk factor for the development of lupus nephritis among Caucasians but not non-Caucasians. Arthritis Rheum. 2001, 44, 618–625. [Google Scholar] [CrossRef]
- Lech, M.; Anders, H.J. The pathogenesis of lupus nephritis. J. Am. Soc. Nephrol. 2013, 24, 1357–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tojo, T.; Friou, G.J. Lupus nephritis: Varying complement-fixing properties of immunoglobulin G antibodies to antigens of cell nuclei. Science 1968, 161, 904–906. [Google Scholar] [CrossRef]
- Kramers, C.; Hylkema, M.N.; van Bruggen, M.C.; van de Lagemaat, R.; Dijkman, H.B.; Assmann, K.J.; Smeenk, R.J.; Berden, J.H. Anti-nucleosome antibodies complexed to nucleosomal antigens show anti-DNA reactivity and bind to rat glomerular basement membrane in vivo. J. Clin. Investig. 1994, 94, 568–577. [Google Scholar] [CrossRef]
- Mjelle, J.E.; Rekvig, O.P.; Van Der Vlag, J.; Fenton, K.A. Nephritogenic antibodies bind in glomeruli through interaction with exposed chromatin fragments and not with renal cross-reactive antigens. Autoimmunity 2011, 44, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Hahn, B.H. Antibodies to DNA. N. Engl. J. Med. 1998, 338, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Weening, J.J.; D’Agati, V.D.; Schwartz, M.M.; Seshan, S.V.; Alpers, C.E.; Appel, G.B.; Balow, J.E.; Bruijn, J.A.; Cook, T.; Ferrario, F.; et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004, 65, 521–530. [Google Scholar] [CrossRef] [Green Version]
- Chang, A.; Henderson, S.G.; Brandt, D.; Liu, N.; Guttikonda, R.; Hsieh, C.; Kaverina, N.; Utset, T.O.; Meehan, S.M.; Quigg, R.J.; et al. In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J. Immunol. 2011, 186, 1849–1860. [Google Scholar] [CrossRef] [Green Version]
- Maria, N.I.; Davidson, A. Protecting the kidney in systemic lupus erythematosus: From diagnosis to therapy. Nat. Rev. Rheumatol. 2020, 16, 255–267. [Google Scholar] [CrossRef]
- Christopher-Stine, L.; Siedner, M.; Lin, J.; Haas, M.; Parekh, H.; Petri, M.; Fine, D.M. Renal biopsy in lupus patients with low levels of proteinuria. J. Rheumatol. 2007, 34, 332–335. [Google Scholar]
- De Rosa, M.; Rocha, A.S.; De Rosa, G.; Dubinsky, D.; Almaani, S.J.; Rovin, B.H. Low-grade proteinuria does not exclude significant kidney injury in lupus nephritis. Kidney Int. Rep. 2020, 5, 1066–1068. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, C.; Hajjar, Y.; Mueller, K.; Waldherr, R.; Ho, A.D.; Andrassy, K. Improved clinical outcome of lupus nephritis during the past decade: Importance of early diagnosis and treatment. Ann. Rheum. Dis. 2003, 62, 435–439. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Ucci, A.; Madias, N.E. Predominant tubulointerstitial lupus nephritis. Am. J. Kidney Dis. 1996, 27, 273–278. [Google Scholar] [CrossRef]
- Leatherwood, C.; Speyer, C.B.; Feldman, C.H.; D’Silva, K.; Gómez-Puerta, J.A.; Hoover, P.J.; Waikar, S.S.; McMahon, G.M.; Rennke, H.G.; Costenbader, K.H. Clinical characteristics and renal prognosis associated with interstitial fibrosis and tubular atrophy (IFTA) and vascular injury in lupus nephritis biopsies. Semin. Arthritis Rheum. 2019, 49, 396–404. [Google Scholar] [CrossRef]
- Vandepapelière, J.; Aydin, S.; Cosyns, J.P.; Depresseux, G.; Jadoul, M.; Houssiau, F.A. Prognosis of proliferative lupus nephritis subsets in the Louvain Lupus Nephritis inception Cohort. Lupus 2014, 23, 159–165. [Google Scholar] [CrossRef]
- Hsieh, C.; Chang, A.; Brandt, D.; Guttikonda, R.; Utset, T.O.; Clark, M.R. Predicting outcomes of lupus nephritis with tubulointerstitial inflammation and scarring. Arthritis Care Res. 2011, 63, 865–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajema, I.M.; Wilhelmus, S.; Alpers, C.E.; Bruijn, J.A.; Colvin, R.B.; Cook, H.T.; D’Agati, V.D.; Ferrario, F.; Haas, M.; Jennette, J.C.; et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: Clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018, 93, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Fanouriakis, A.; Kostopoulou, M.; Cheema, K.; Anders, H.J.; Aringer, M.; Bajema, I.; Boletis, J.; Frangou, E.; Houssiau, F.A.; Hollis, J.; et al. 2019 Update of the Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann. Rheum. Dis. 2020, 79, 713–723. [Google Scholar] [CrossRef] [Green Version]
- Houssiau, F.A.; Vasconcelos, C.; D’Cruz, D.; Sebastiani, G.D.; Garrido Ed, E.; Danieli, M.G.; Abramovicz, D.; Blockmans, D.; Mathieu, A.; Direskeneli, H.; et al. Immunosuppressive therapy in lupus nephritis: The Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum. 2002, 46, 2121–2131. [Google Scholar] [CrossRef]
- Austin, H.A., III; Klippel, J.H.; Balow, J.E.; le Riche, N.G.; Steinberg, A.D.; Plotz, P.H.; Decker, J.L. Therapy of lupus nephritis. Controlled trial of prednisone and cytotoxic drugs. N. Engl. J. Med. 1986, 314, 614–619. [Google Scholar] [CrossRef]
- Zeher, M.; Doria, A.; Lan, J.; Aroca, G.; Jayne, D.; Boletis, I.; Hiepe, F.; Prestele, H.; Bernhardt, P.; Amoura, Z. Efficacy and safety of enteric-coated mycophenolate sodium in combination with two glucocorticoid regimens for the treatment of active lupus nephritis. Lupus 2011, 20, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Lightstone, L.; Doria, A.; Wilson, H.; Ward, F.L.; Larosa, M.; Bargman, J.M. Can we manage lupus nephritis without chronic corticosteroids administration? Autoimmun. Rev. 2018, 17, 4–10. [Google Scholar] [CrossRef]
- Malvar, A.; Pirruccio, P.; Alberton, V.; Lococo, B.; Recalde, C.; Fazini, B.; Nagaraja, H.; Indrakanti, D.; Rovin, B.H. Histologic versus clinical remission in proliferative lupus nephritis. Nephrol. Dial. Transplant. 2017, 32, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Dooley, M.A.; Jayne, D.; Ginzler, E.M.; Isenberg, D.; Olsen, N.J.; Wofsy, D.; Eitner, F.; Appel, G.B.; Contreras, G.; Lisk, L.; et al. Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N. Engl. J. Med. 2011, 365, 1886–1895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Hachmi, M.; Jadoul, M.; Lefèbvre, C.; Depresseux, G.; Houssiau, F.A. Relapses of lupus nephritis: Incidence, risk factors, serology and impact on outcome. Lupus 2003, 12, 692–696. [Google Scholar] [CrossRef]
- Parikh, S.V.; Nagaraja, H.N.; Hebert, L.; Rovin, B.H. Renal flare as a predictor of incident and progressive CKD in patients with lupus nephritis. Clin. J. Am. Soc. Nephrol. 2014, 9, 279–284. [Google Scholar] [CrossRef]
- Houssiau, F.A.; Vasconcelos, C.; D’Cruz, D.; Sebastiani, G.D.; de Ramon Garrido, E.; Danieli, M.G.; Abramovicz, D.; Blockmans, D.; Cauli, A.; Direskeneli, H.; et al. The 10-year follow-up data of the Euro-Lupus Nephritis Trial comparing low-dose and high-dose intravenous cyclophosphamide. Ann. Rheum. Dis. 2010, 69, 61–64. [Google Scholar] [CrossRef]
- Appel, G.B.; Contreras, G.; Dooley, M.A.; Ginzler, E.M.; Isenberg, D.; Jayne, D.; Li, L.S.; Mysler, E.; Sánchez-Guerrero, J.; Solomons, N.; et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J. Am. Soc. Nephrol. 2009, 20, 1103–1112. [Google Scholar] [CrossRef] [Green Version]
- Houssiau, F.A.; D’Cruz, D.; Sangle, S.; Remy, P.; Vasconcelos, C.; Petrovic, R.; Fiehn, C.; de Ramon Garrido, E.; Gilboe, I.M.; Tektonidou, M.; et al. Azathioprine versus mycophenolate mofetil for long-term immunosuppression in lupus nephritis: Results from the MAINTAIN Nephritis Trial. Ann. Rheum. Dis. 2010, 69, 2083–2089. [Google Scholar] [CrossRef] [PubMed]
- Tamirou, F.; D’Cruz, D.; Sangle, S.; Remy, P.; Vasconcelos, C.; Fiehn, C.; Ayala Guttierez, M.; Gilboe, I.M.; Tektonidou, M.; Blockmans, D.; et al. Long-term follow-up of the MAINTAIN Nephritis Trial, comparing azathioprine and mycophenolate mofetil as maintenance therapy of lupus nephritis. Ann. Rheum. Dis. 2016, 75, 526–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Zhang, H.; Liu, Z.; Xing, C.; Fu, P.; Ni, Z.; Chen, J.; Lin, H.; Liu, F.; He, Y.; et al. Multitarget therapy for induction treatment of lupus nephritis: A randomized trial. Ann. Intern. Med. 2015, 162, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Tamirou, F.; Lauwerys, B.R.; Dall’Era, M.; Mackay, M.; Rovin, B.; Cervera, R.; Houssiau, F.A.; MAINTAIN Nephritis Trial Investigators. A proteinuria cut-off level of 0.7 g/day after 12 months of treatment best predicts long-term renal outcome in lupus nephritis: Data from the MAINTAIN Nephritis Trial. Lupus Sci. Med. 2015, 2, e000123. [Google Scholar] [CrossRef] [Green Version]
- Dall’Era, M.; Cisternas, M.G.; Smilek, D.E.; Straub, L.; Houssiau, F.A.; Cervera, R.; Rovin, B.H.; Mackay, M. Predictors of long-term renal outcome in lupus nephritis trials: Lessons learned from the Euro-Lupus Nephritis cohort. Arthritis Rheumatol. 2015, 67, 1305–1313. [Google Scholar] [CrossRef]
- Ugolini-Lopes, M.R.; Seguro, L.; Castro, M.; Daffre, D.; Lopes, A.C.; Borba, E.F.; Bonfá, E. Early proteinuria response: A valid real-life situation predictor of long-term lupus renal outcome in an ethnically diverse group with severe biopsy-proven nephritis? Lupus Sci. Med. 2017, 4, e000213. [Google Scholar] [CrossRef]
- Costedoat-Chalumeau, N.; Amoura, Z.; Hulot, J.S.; Aymard, G.; Leroux, G.; Marra, D.; Lechat, P.; Piette, J.C. Very low blood hydroxychloroquine concentration as an objective marker of poor adherence to treatment of systemic lupus erythematosus. Ann. Rheum. Dis. 2007, 66, 821–824. [Google Scholar] [CrossRef]
- Reyes-Thomas, J.; Blanco, I.; Putterman, C. Urinary biomarkers in lupus nephritis. Clin. Rev. Allergy Immunol. 2011, 40, 138–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korte, E.A.; Gaffney, P.M.; Powell, D.W. Contributions of mass spectrometry-based proteomics to defining cellular mechanisms and diagnostic markers for systemic lupus erythematosus. Arthritis Res. Ther. 2012, 14, 204. [Google Scholar] [CrossRef] [Green Version]
- Rubinstein, T.; Pitashny, M.; Levine, B.; Schwartz, N.; Schwartzman, J.; Weinstein, E.; Pego-Reigosa, J.M.; Lu, T.Y.; Isenberg, D.; Rahman, A.; et al. Urinary neutrophil gelatinase-associated lipocalin as a novel biomarker for disease activity in lupus nephritis. Rheumatology. 2010, 49, 960–971. [Google Scholar] [CrossRef] [Green Version]
- Torres-Salido, M.T.; Cortés-Hernández, J.; Vidal, X.; Pedrosa, A.; Vilardell-Tarrés, M.; Ordi-Ros, J. Neutrophil gelatinase-associated lipocalin as a biomarker for lupus nephritis. Nephrol. Dial. Transplant. 2014, 29, 1740–1749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa, R.F.; Takei, K.; Araújo, N.C.; Loduca, S.M.; Szajubok, J.C.; Chahade, W.H. Monocyte chemoattractant-1 as a urinary biomarker for the diagnosis of activity of lupus nephritis in Brazilian patients. J. Rheumatol. 2012, 39, 1948–1954. [Google Scholar] [CrossRef]
- Schwartz, N.; Rubinstein, T.; Burkly, L.C.; Collins, C.E.; Blanco, I.; Su, L.; Hojaili, B.; Mackay, M.; Aranow, C.; Stohl, W.; et al. Urinary TWEAK as a biomarker of lupus nephritis: A multicenter cohort study. Arthritis Res. Ther. 2009, 11, R143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somparn, P.; Hirankarn, N.; Leelahavanichkul, A.; Khovidhunkit, W.; Thongboonkerd, V.; Avihingsanon, Y. Urinary proteomics revealed prostaglandin H(2)D-isomerase, not Zn-α2-glycoprotein, as a biomarker for active lupus nephritis. J. Proteom. 2012, 75, 3240–3247. [Google Scholar] [CrossRef]
- Gupta, R.; Yadav, A.; Misra, R.; Aggarwal, A. Urinary prostaglandin D synthase as biomarker in lupus nephritis: A longitudinal study. Clin. Exp. Rheumatol. 2015, 33, 694–698. [Google Scholar] [PubMed]
- Vanarsa, K.; Soomro, S.; Zhang, T.; Strachan, B.; Pedroza, C.; Nidhi, M.; Cicalese, P.; Gidley, C.; Dasari, S.; Mohan, S.; et al. Quantitative planar array screen of 1000 proteins uncovers novel urinary protein biomarkers of lupus nephritis. Ann. Rheum. Dis. 2020, 79, 1349–1361. [Google Scholar] [CrossRef]
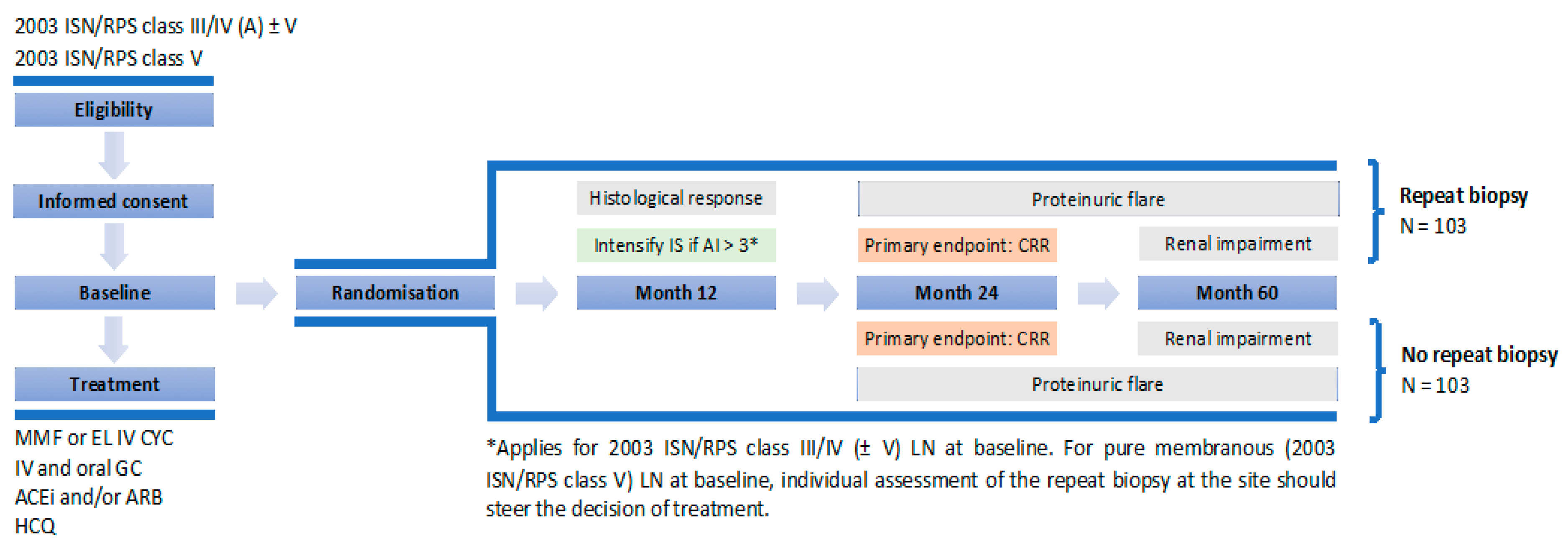
- Alvarado, A.S.; Malvar, A.; Lococo, B.; Alberton, V.; Toniolo, F.; Nagaraja, H.N.; Rovin, B.H. The value of repeat kidney biopsy in quiescent Argentinian lupus nephritis patients. Lupus 2014, 23, 840–847. [Google Scholar] [CrossRef]
- Alsuwaida, A.; Husain, S.; Alghonaim, M.; AlOudah, N.; Alwakeel, J.; Ullah, A.; Kfoury, H. Strategy for second kidney biopsy in patients with lupus nephritis. Nephrol. Dial. Transplant. 2012, 27, 1472–1478. [Google Scholar] [CrossRef]
- Alsuwaida, A.O. The clinical significance of serial kidney biopsies in lupus nephritis. Mod. Rheumatol. 2014, 24, 453–456. [Google Scholar] [CrossRef]
- Zickert, A.; Sundelin, B.; Svenungsson, E.; Gunnarsson, I. Role of early repeated renal biopsies in lupus nephritis. Lupus Sci. Med. 2014, 1, e000018. [Google Scholar] [CrossRef] [Green Version]
- Parodis, I.; Adamichou, C.; Aydin, S.; Gomez, A.; Demoulin, N.; Weinmann-Menke, J.; Houssiau, F.A.; Tamirou, F. Per-protocol repeat kidney biopsy portends relapse and long-term outcome in incident cases of proliferative lupus nephritis. Rheumatology 2020, 59, 3424–3434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Z.; Zhou, M.; Liu, Z.; Chen, J.; Xing, C.; Lin, H.; Ni, Z.; Fu, P.; Liu, F.; et al. Multitarget Therapy for Maintenance Treatment of Lupus Nephritis. J. Am. Soc. Nephrol. 2017, 28, 3671–3678. [Google Scholar] [CrossRef]
- Rovin, B.H.; Solomons, N.; Pendergraft, W.F., III; Dooley, M.A.; Tumlin, J.; Romero-Diaz, J.; Lysenko, L.; Navarra, S.V.; Huizinga, R.B.; AURA-LV Study Group. A randomized, controlled double-blind study comparing the efficacy and safety of dose-ranging voclosporin with placebo in achieving remission in patients with active lupus nephritis. Kidney Int. 2019, 95, 219–231. [Google Scholar] [CrossRef]
- Arriens, C.; Polyakova, S.; Adzerikho, I.; Randhawa, S.; Solomons, N. OP0277 AURORA phase 3 study demonstrates voclosporin statistical superiority over standard of care in lupus nephritis. Ann. Rheum. Dis. 2020, 79, 172–173. [Google Scholar] [CrossRef]
- Dooley, M.A.; Pendergraft, W., III; Ginzler, E.M.; Olsen, N.J.; Tumlin, J.; Rovin, B.H.; Houssiau, F.A.; Wofsy, D.; Isenberg, D.A.; Solomons, N.; et al. Speed of remission with the use of voclosporin, MMF and low dose steroids: Results of a global lupus nephritis study (abstract). Arthritis Rheumatol. 2016, 68 (Suppl. 10). [Google Scholar]
- Aurinia. FDA Approves Aurinia Pharmaceuticals’ LUPKYNIS (Voclosporin) for Adult Patients with Active Lupus Nephritis [Press Release]. Available online: https://ir.auriniapharma.com/press-releases/detail/210/fda-approves-aurinia-pharmaceuticals-lupkynis (accessed on 22 January 2021).
- Navarra, S.V.; Guzmán, R.M.; Gallacher, A.E.; Hall, S.; Levy, R.A.; Jimenez, R.E.; Li, E.K.; Thomas, M.; Kim, H.Y.; León, M.G.; et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: A randomised, placebo-controlled, phase 3 trial. Lancet 2011, 377, 721–731. [Google Scholar] [CrossRef]
- Furie, R.; Petri, M.; Zamani, O.; Cervera, R.; Wallace, D.J.; Tegzová, D.; Sanchez-Guerrero, J.; Schwarting, A.; Merrill, J.T.; Chatham, W.W.; et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011, 63, 3918–3930. [Google Scholar] [CrossRef] [PubMed]
- Dooley, M.A.; Houssiau, F.; Aranow, C.; D’Cruz, D.P.; Askanase, A.; Roth, D.A.; Zhong, Z.J.; Cooper, S.; Freimuth, W.W.; Ginzler, E.M.; et al. Effect of belimumab treatment on renal outcomes: Results from the phase 3 belimumab clinical trials in patients with SLE. Lupus 2013, 22, 63–72. [Google Scholar] [CrossRef]
- Furie, R.; Rovin, B.H.; Houssiau, F.; Malvar, A.; Teng, Y.; Contreras, G.; Amoura, Z.; Yu, X.; Mok, C.C.; Santiago, M.B.; et al. Two-Year, Randomized, Controlled Trial of Belimumab in Lupus Nephritis. N. Engl. J. Med. 2020, 383, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Sjöwall, C.; Cöster, L. Belimumab may not prevent lupus nephritis in serologically active patients with ongoing non-renal disease activity. Scand. J. Rheumatol. 2014, 43, 428–430. [Google Scholar] [CrossRef]
- Hui-Yuen, J.S.; Reddy, A.; Taylor, J.; Li, X.; Eichenfield, A.H.; Bermudez, L.M.; Starr, A.J.; Imundo, L.F.; Buyon, J.; Furie, R.A.; et al. Safety and Efficacy of Belimumab to Treat Systemic Lupus Erythematosus in Academic Clinical Practices. J. Rheumatol. 2015, 42, 2288–2295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staveri, C.; Karokis, D.; Liossis, S.C. New onset of lupus nephritis in two patients with SLE shortly after initiation of treatment with belimumab. Semin. Arthritis Rheum. 2017, 46, 788–790. [Google Scholar] [CrossRef]
- Anjo, C.; Mascaró, J.M., Jr.; Espinosa, G.; Cervera, R. Effectiveness and safety of belimumab in patients with systemic lupus erythematosus in a real-world setting. Scand. J. Rheumatol. 2019, 48, 469–473. [Google Scholar] [CrossRef]
- Parodis, I.; Vital, E.M.; Hassan, S.U.; Jönsen, A.; Bengtsson, A.A.; Eriksson, P.; Leonard, D.; Gunnarsson, I.; Rönnblom, L.; Sjöwall, C. De novo lupus nephritis during treatment with belimumab. Rheumatology 2020, keaa796. [Google Scholar] [CrossRef]
- Reddy, V.; Klein, C.; Isenberg, D.A.; Glennie, M.J.; Cambridge, G.; Cragg, M.S.; Leandro, M.J. Obinutuzumab induces superior B-cell cytotoxicity to rituximab in rheumatoid arthritis and systemic lupus erythematosus patient samples. Rheumatology 2017, 56, 1227–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furie, R.; Aroca, G.; Alvarez, A.; Fragoso-Loyo, H.; Zuta Santillan, E.; Rovin, B.; Brunetta, P.; Schindler, T.; Hassan, I.; Cascino, M.; et al. Two-year results from a randomized; controlled study of obinutuzumab for proliferative lupus nephritis (abstract). Arthritis Rheumatol. 2020, 72 (Suppl. 10). [Google Scholar]
- Rovin, B.H.; Furie, R.; Latinis, K.; Looney, R.J.; Fervenza, F.C.; Sanchez-Guerrero, J.; Maciuca, R.; Zhang, D.; Garg, J.P.; Brunetta, P.; et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: The Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum. 2012, 64, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Houssiau, F.A. Why will lupus nephritis trials not fail anymore? Rheumatology 2017, 56, 677–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Reference | Trial | N Patients | ISN/RPS Class of LN | Treatment | Follow Up | Primary Endpoint | Results |
|---|---|---|---|---|---|---|---|
| Austin HA, NEJM 1986 [22] | NIH | 107 | All | High dose GC vs. NIH IV CY | Median 7 years | Time to end-stage kidney disease (ESKD) | NIH IV CY > high dose GC |
| Houssiau FA, A&R 2002 [21] | ELNT | 90 | III, IV, Vc, Vd | EL IV CY vs. NIH IV CY as induction treatment | Median 41 months | Treatment failure regarding kidney function, proteinuria, relapses | No difference observed between EL IV CY and NIH IV CY |
| Mean 115 months (Houssiau, ARD 2010 [29]) | Time to major event: death, ESKD, doubling of serum creatinine | No difference observed between EL IV CY and NIH IV CY | |||||
| Appel GB, JASN 2009 [30] | 370 | III, IV et/ou V | MMF vs. IV CY as induction treatment | 24 weeks | Proteinuria decrease (UPC ratio) | No difference observed between MMF and IV CY | |
| Dooley MA, NEJM 2011 [26] | ALMS | 227 | III, IV ou V | AZA vs. MMF as maintenance treatment after induction by MMF or IV CY | 36 months | Time to first event: death, end-stage-kidney disease, renal relapse, need of a rescue therapy | MMF > AZA Relapses: 16.4% in MMF group vs. 32.4% in AZA group |
| Houssiau FA, ARD 2010 [31] | MAINTAIN | 105 | III, IV, Vc, Vd | AZA vs. MMF as maintenance treatment after induction by EL IV CY | Mean 48 months | Time to first renal relapse | No difference observed between MMF and AZA Relapses: 19% in MMF group vs. 25% in AZA group |
| 87 | Median 110 months (Tamirou F, ARD 2016 [32]) | Time to renal flare | No difference observed between MMF and AZA Relapses: 45% in MMF group vs. 49% in AZA group | ||||
| Liu Z, AIM 2015 [33] | 310 | III–IV ± V, V | Tacrolimus + MMF vs. NIH IV CY | 24 weeks | Complete remission | Tacrolimus + MMF (45.9%) > NIH IV CY (25.6%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamirou, F.; Houssiau, F.A. Management of Lupus Nephritis. J. Clin. Med. 2021, 10, 670. https://doi.org/10.3390/jcm10040670
Tamirou F, Houssiau FA. Management of Lupus Nephritis. Journal of Clinical Medicine. 2021; 10(4):670. https://doi.org/10.3390/jcm10040670
Chicago/Turabian StyleTamirou, Farah, and Frédéric A. Houssiau. 2021. "Management of Lupus Nephritis" Journal of Clinical Medicine 10, no. 4: 670. https://doi.org/10.3390/jcm10040670
APA StyleTamirou, F., & Houssiau, F. A. (2021). Management of Lupus Nephritis. Journal of Clinical Medicine, 10(4), 670. https://doi.org/10.3390/jcm10040670





