The Outcome of Chemotherapy for Metastatic Extramammary Paget’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Follow-Up
2.3. Statistical Analysis
3. Results
3.1. Clinicopathological Data of the Patients
3.2. Differences in Patient Characteristics According to the Receipt of Chemotherapy
3.3. Treatment and Outcomes
3.4. Multivariate Analyses
3.5. Adverse Events of Chemotherapy and Targeted Therapys
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cocker, H.R. Paget’s disease affecting the scrotum and penis. Trans. Pathol. Soc. Lond. 1888, 40, 187–191. [Google Scholar]
- Kanitakis, J. Mammary and extramammary Paget’s disease. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, V.; Davidson, E.J.; Davies-Humphreys, J. Extramammary Paget’s disease. BJOG 2005, 112, 273–279. [Google Scholar] [CrossRef]
- Simonds, R.M.; Segal, R.J.; Sharma, A. Extramammary Paget’s disease: A review of the literature. Int. J. Dermatol. 2019, 58, 871–879. [Google Scholar] [CrossRef]
- Ohara, K.; Fujisawa, Y.; Yoshino, K.; Kiyohara, Y.; Kadono, T.; Murata, Y.; Uhara, H.; Hatta, N.; Uchi, H.; Matsushita, S.; et al. A proposal for a TNM staging system for extramammary disease: Retrospective analysis of 301 patients with invasive primary tumors. J. Dermatol. Sci. 2016, 83, 234–239. [Google Scholar] [CrossRef]
- Ito, T.; Kaku-Ito, Y.; Furue, M. The diagnosis and management of extramammary Paget’s disease. Expert Rev. Anticancer Ther. 2018, 18, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Herrel, L.A.; Weiss, A.D.; Goodman, M.; Johnson, T.V.; Osunkoya, A.O.; Delman, K.A.; Master, V.A. Extramammary Paget’s disease in males: Survival outcomes in 495 patients. Ann. Surg. Oncol. 2015, 22, 1625–1630. [Google Scholar] [CrossRef]
- Weng, S.; Zhu, N.; Li, D.; Chen, Y.; Tan, Y.; Chen, J.; Yuan, Y. Clinical characteristics, treatment, and prognostic factors of patients with primary extramammary Paget’s disease (EMPD): A retrospective analysis of 44 patients from a single center and an analysis of data from the surveillance, epidemiology, and end results (SEER) database. Front. Oncol. 2020, 10, 1114. [Google Scholar] [PubMed]
- Hatta, N.; Yamada, M.; Hirano, T.; Fujimoto, A.; Morita, R. Extramammary Paget’s disease: Treatment, prognostic factors and outcome in 76 patients. Br. J. Dermatol. 2008, 158, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Padrnos, L.; Karlin, N.; Halfdanarson, T.R. Mayo Clinic Cancer Center experience of metastatic extramammary Paget disease 1998–2012. Rare Tumors 2016, 8, 6804. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, K.; Fujisawa, Y.; Kiyohara, Y.; Kadono, T.; Murata, Y.; Uhara, H.; Hatta, N.; Uchi, H.; Matsushita, S.; Takenouchi, T.; et al. Usefulness of docetaxel as first-line chemotherapy for metastatic extramammary Paget’s disease. J. Dermatol. 2016, 43, 633–637. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tanese, K.; Hirai, I. Weekly docetaxel monotherapy for metastatic extramammary Paget’s disease: Retrospective single-institute analysis. J. Dermatol. 2020, 47, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Yoshino, K.; Maeda, T.; Nagai, K.; Oaku, S.; Hiura, A.; Fujisawa, Y. Single-agent taxane is useful in palliative chemotherapy for advanced extramammary Paget disease: A case series. Br. J. Dermatol. 2019, 181, 831–832. [Google Scholar] [CrossRef]
- Tokuda, Y.; Arakura, F.; Uhara, H. Combination chemotherapy of low-dose 5-fluorouracil and cisplatin for advanced extramammary Paget’s disease. Int. J. Clin. Oncol. 2015, 20, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Kariya, K.; Tsuji, T.; Schwartz, R.A. Trial of low-dose 5-fluorouracil/cisplatin therapy for advanced extramammary Paget’s disease. Dermatol. Surg. 2004, 30, 341–344. [Google Scholar] [CrossRef]
- Yamazaki, N.; Yamamoto, A.; Wada, T.; Ishikawa, M.; Moriya, Y.; Nakanishi, Y. A case of metastatic extramammary Paget’s disease that responded to combination chemotherapy. J. Dermatol. 1999, 26, 311–316. [Google Scholar] [CrossRef]
- Oashi, K.; Tsutsumida, A.; Namikawa, K.; Tanaka, R.; Omata, W.; Yamamoto, Y.; Yamazaki, N. Combination chemotherapy for metastatic extramammary Paget disease. Br. J. Dermatol. 2014, 170, 1354–1357. [Google Scholar] [CrossRef]
- Matsushita, S.; Yonekura, K.; Mera, K.; Kawai, K.; Kanekura, T. Successful treatment of metastatic extramammary Paget’s disease with S-1 and docetaxel combination chemotherapy. J. Dermatol. 2011, 38, 996–998. [Google Scholar] [CrossRef]
- Egashira, S.; Kajihara, I.; Kanemaru, H.; Uemura-Kiyohara, M.; Yamada-Kanazawa, S.; Nakahara, S.; Nagamoto, E.; Fukushima, S.; Jinnin, M.; Inoue, Y.; et al. Achieved good response of S-1 and docetaxel combination chemotherapy in two patients with metastatic extramammary Paget’s disease. J. Dermatol. 2017, 44, e103–e104. [Google Scholar] [CrossRef] [Green Version]
- Ogata, D.; Hokama, Y.; Tsuchida, T. Successful treatment of bilateral multiple lymph node metastases in extramammary Paget’s disease with surgery and sequential chemotherapy of S-1 and docetaxel. J. Dermatol. 2015, 42, 1193–1194. [Google Scholar] [CrossRef]
- Kato, J.; Hida, T.; Yamashita, T.; Kamiya, S.; Horimoto, K.; Sato, S.; Takahashi, H.; Sawada, M.; Yamada, M.; Uhara, H. Successful TS-1 monotherapy as the second-line treatment for advanced extramammary Paget’s disease: A report of two cases. J. Dermatol. 2018, 45, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Mikoshiba, Y.; Uhara, H.; Kubo, H.; Okuyama, R. S-1 induced a durable response in metastatic extramammary Paget’s disease. J. Dermatol. 2013, 40, 664–665. [Google Scholar] [CrossRef]
- Hirai, I.; Funakoshi, T. Modified weekly regimen of cisplatin, epirubicin and paclitaxel induced a durable response in two cases of metastatic extramammary Paget’s disease. J. Dermatol. 2017, 44, 1148–1151. [Google Scholar] [CrossRef] [PubMed]
- Hirai, I.; Tanese, K.; Nakamura, Y.; Ishii, M.; Kawakami, Y.; Funakoshi, T. Combination Cisplatin-Epirubicin-Paclitaxel Therapy for Metastatic Extramammary Paget’s Disease. Oncologist 2019, 24, e394–e396. [Google Scholar] [CrossRef] [Green Version]
- Hanawa, F.; Inozume, T.; Harada, K.; Kawamura, T.; Shibagaki, N.; Shimada, S. A case of metastatic extramammary Paget’s disease responding to trastuzumab plus paclitaxel combination therapy. Case Rep. Dermatol. 2011, 3, 223–227. [Google Scholar] [CrossRef]
- Takahagi, S.; Noda, H.; Kamegashira, A.; Madokoro, N.; Hori, I.; Shindo, H.; Mihara, S.; Hide, M. Metastatic extramammary Paget’s disease treated with paclitaxel and trastuzumab combination chemotherapy. J. Dermatol. 2009, 36, 457–461. [Google Scholar] [CrossRef]
- Shin, D.S.; Sherry, T.; Kallen, M.E.; Wong, S.; Drakaki, A. Human epidermal growth factor receptor 2 (HER-2/neu)-directed therapy for rare metastatic epithelial tumors with HER-2 amplification. Case Rep. Oncol. 2016, 9, 298–304. [Google Scholar] [CrossRef]
- Gershenwald, J.E.; Scolyer, R.A. Melanoma staging: American Joint Committee on Cancer (AJCC) 8th edition and beyond. Ann. Surg. Oncol. 2018, 25, 2105–2110. [Google Scholar] [CrossRef]
- Fukuda, K.; Funakoshi, T. Metastatic Extramammary Paget’s Disease: Pathogenesis and Novel Therapeutic Approach. Front. Oncol. 2018, 8, 38. [Google Scholar] [CrossRef] [Green Version]
- Morris, C.R.; Hurst, E.A. Extramammary Paget’s disease: A review of the literature Part II: Treatment and prognosis. Dermatol Surg. 2020, 46, 305–311. [Google Scholar] [CrossRef]
- Barth, P.; Dulaimi Al-Saleem, E.; Edwards, K.W.; Millis, S.Z.; Wong, Y.-N.; Geynisman, D.M. Metastatic extramammary Paget’s disease of scrotum responds completely to single agent trastuzumab in a hemodialysis patient: Case report, molecular profiling and brief review of the literature. Case Rep. Oncol. Med. 2015, 2015, 895151. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, S.; Togawa, Y.; Yoneyama, K.; Suehiro, K.; Kambe, N.; Matsue, H. Dramatic clinical response of relapsed metastatic extramammary Paget’s disease to trastuzumab monotherapy. Case Rep. Dermatol. Med. 2012, 2012, 401362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Chen, Y.; Gao, F.; Chen, N.; Liu, J.-Y. Advanced scrotal extramammary Paget’s disease treated with apatinib: A case report. Clin. Genitourin. Cancer 2018, 16, e339–e342. [Google Scholar] [CrossRef]
- Ichiyama, T.; Gomi, D.; Fukushima, T.; Kobayashi, T.; Sekiguchi, N.; Sakamoto, A.; Sasaki, S.; Mamiya, K.; Koizumi, T.; Hama, Y. Successful and long-term response to trastuzumab plus paclitaxel combination therapy in human epidermal growth factor receptor 2-positive extramammary Paget’s disease: A case report and review of the literature. Mol. Clin. Oncol. 2017, 7, 763–766. [Google Scholar] [CrossRef]
| Parameter | N (%) |
|---|---|
| Sex | |
| Male | 15 (71.4) |
| Female | 6 (28.6) |
| Age at distant metastasis (years) | |
| Mean | 74.8 |
| Median (range) | 75 (58–93) |
| ECOG performance status | |
| 0 | 15 (71.4) |
| 1 | 6 (28.6) |
| Tumor site | |
| Genital area only | 17 (81.0) |
| Perianal area only | 1 (4.8) |
| Genital + perianal areas | 2 (9.5) |
| Genital + axillary + abdominal areas | 1 (4.8) |
| Clinical manifestation | |
| Erythematous plaque | 21 (100.0) |
| Nodule | 17 (81.0) |
| Erosion/ulceration | 13 (61.9) |
| Hypopigmentation | 8 (38.1) |
| Primary lesion size (cm2) | |
| <25 | 9 (42.9) |
| ≥25 | 12 (57.1) |
| TT (mm) | |
| In situ | 0 (0.0) |
| ≤4 | 9 (42.9) |
| >4 | 12 (57.1) |
| Site of distant metastasis | |
| Brain | 3 (14.3) |
| Lung | 8 (38.1) |
| Liver | 10 (47.6) |
| Bone | 7 (33.3) |
| Mediastinal LN | 3 (14.3) |
| Abdominal LN | 13 (61.9) |
| Pelvic LN † | 7 (33.3) |
| Distant visceral organ metastasis | |
| Present | 16 (76.2) |
| Absent (only distant LN metastasis) | 5 (23.8) |
| Number of metastatic sites | |
| 1 | 4 (19.0) |
| 2 | 8 (38.1) |
| 3 | 5 (23.8) |
| 4 | 4 (19.0) |
| Total | 21 (100.0) |
| Parameters | Chemotherapy/Targeted Therapy | p * | |
|---|---|---|---|
| Conducted (n = 14) | Not Conducted (n = 7) | ||
| Sex | 0.35 | ||
| Male | 11 (78.6%) | 4 (57.1%) | |
| Female | 3 (21.4%) | 3 (42.9%) | |
| Age at distant metastasis (years) | 0.057 | ||
| Mean | 71.7 | 80.9 | |
| Median (range) | 74 (58–82) | 87 (68–93) | |
| ECOG performance status | 0.12 | ||
| 0 | 12 (85.7%) | 3 (42.9%) | |
| 1 | 2 (14.3%) | 4 (57.1%) | |
| Tumor site | 0.26 | ||
| Genital only | 10 (71.4%) | 7 (100.0%) | |
| Others | 4 (28.6%) | 0 (0.0%) | |
| Nodule formation | 0.26 | ||
| Present | 10 (71.4%) | 7 (100.0%) | |
| Absent | 4 (28.6%) | 0 (0.0%) | |
| Size of primary lesion | 0.40 | ||
| <25 cm2 | 5 (35.7%) | 4 (57.1%) | |
| ≥25 cm2 | 9 (64.3%) | 3 (42.9%) | |
| TT (mm) | 0.64 | ||
| ≤4 | 7 (50.0%) | 2 (28.6%) | |
| >4 | 7 (50.0%) | 5 (71.4%) | |
| Distant visceral organ metastasis | 1.00 | ||
| Present | 11 (78.6%) | 5 (71.4%) | |
| Absent (only distant LN metastasis) | 3 (21.4%) | 2 (28.6%) | |
| Brain metastasis | 1.00 | ||
| Present | 2 (14.3%) | 1 (14.3%) | |
| Absent | 12 (85.7%) | 6 (85.7%) | |
| Lung metastasis | 0.17 | ||
| Present | 7 (50.0%) | 1 (14.3%) | |
| Absent | 7 (50.0%) | 6 (85.7%) | |
| Liver metastasis | 1.00 | ||
| Present | 7 (50.0%) | 3 (42.9%) | |
| Absent | 7 (50.0%) | 4 (57.1%) | |
| Number of metastatic sites | 0.81 | ||
| 1 | 2 (14.3%) | 2 (28.6%) | |
| 2 | 6 (42.9%) | 2 (28.6%) | |
| 3 | 3 (21.4%) | 2 (28.6%) | |
| 4 | 3 (21.4%) | 1 (14.3%) | |
| First-Line Regimen | Without Chemotherapy (n = 7) | ||||||
|---|---|---|---|---|---|---|---|
| Overall (n = 14) | DTX (n = 10) | DTX + Tegafur (n = 1) | DTX + Trastuzumab (n = 1) | PTX (n = 1) | Low-Dose FP (n = 1) | ||
| Sex | |||||||
| Male | 11 | 8 | 0 | 1 | 1 | 1 | 4 |
| Female | 3 | 2 | 1 | 0 | 0 | 0 | 3 |
| Age (years) | |||||||
| Median (range) | 74 (58–82) | 76 (58–82) | 76 | 73 | 61 | 65 | 87 (68–93) |
| Tumor site | |||||||
| Genital only | 10 | 7 | 0 | 1 | 1 | 1 | 7 |
| Other sites | 4 | 3 | 1 | 0 | 0 | 0 | 0 |
| Metastatic site | |||||||
| Brain | 2 | 2 | 0 | 0 | 0 | 0 | 1 |
| Lung | 7 | 6 | 0 | 0 | 1 | 0 | 1 |
| Liver | 7 | 4 | 1 | 1 | 0 | 1 | 3 |
| Bone | 3 | 2 | 0 | 1 | 0 | 0 | 4 |
| LN | 14 | 10 | 1 | 1 | 1 | 1 | 7 |
| Second-line regimen | Low-dose FP (n = 2) PTX (n = 1) | Low-dose FP (n = 2) PTX (n = 1) | None | None | None | None | - |
| Radiation therapy | |||||||
| Done | 4 | 4 | 0 | 0 | 0 | 0 | 2 |
| Not done | 10 | 6 | 1 | 1 | 1 | 1 | 5 |
| Overall response † | |||||||
| CR | 0 | 0 | 0 | 0 | 0 | 0 | - |
| PR | 7 | 5 | 0 | 1 | 0 | 1 | - |
| SD | 2 | 2 | 0 | 0 | 0 | 0 | - |
| PD | 5 | 3 | 1 | 0 | 1 | 0 | - |
| FU, mo ‡ | |||||||
| Median (range) | 18.0 (1.3–63.3) | 23.4 (1.3–63.3) | 12.7 | 16.6 | 4.4 | 41.0 | 8.9 (2.6–43.0) |
| Status at last FU | |||||||
| Alive | 3 | 2 | 0 | 1 | 0 | 0 | 2 |
| DPD | 11 | 8 | 1 | 0 | 1 | 1 | 5 |
| Patients | One-Year PFS (%) | p * | One-Year OS (%) | p * | |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 11 | 63.6 | 0.049 | 72.7 | 0.11 |
| Female | 3 | 33.3 | 66.7 | ||
| Age (years) | |||||
| <75 | 8 | 50.0 | 0.50 | 75.0 | 0.86 |
| ≥75 | 6 | 66.7 | 66.7 | ||
| Tumor site | |||||
| Genital only | 10 | 60.0 | 0.23 | 70.0 | 0.19 |
| Other sites | 4 | 50.0 | 66.7 | ||
| Distant visceral organ metastasis | |||||
| Present | 11 | 54.6 | 0.24 | 72.7 | 0.45 |
| Absent (only distant LN metastasis) | 3 | 33.3 | 66.7 | ||
| Lung metastasis | |||||
| Present | 7 | 42.9 | 0.16 | 71.4 | 0.41 |
| Absent | 7 | 71.4 | 71.4 | ||
| Liver metastasis | |||||
| Present | 7 | 57.1 | 0.54 | 71.4 | 0.44 |
| Absent | 7 | 57.1 | 68.6 | ||
| Number of metastatic sites | |||||
| 1 or 2 | 8 | 62.5 | 0.35 | 75.0 | 0.31 |
| ≥3 | 6 | 50.0 | 62.5 |
| Variable | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Sex, male | 0.38 | 0.13–1.12 | 0.079 | 0.66 | 0.16–2.75 | 0.56 |
| Age (years) † | 1.01 | 0.95–1.07 | 0.79 | 0.99 | 0.93–1.06 | 0.84 |
| Perianal lesion | 0.78 | 0.22–2.82 | 0.71 | 1.96 | 0.28–13.76 | 0.50 |
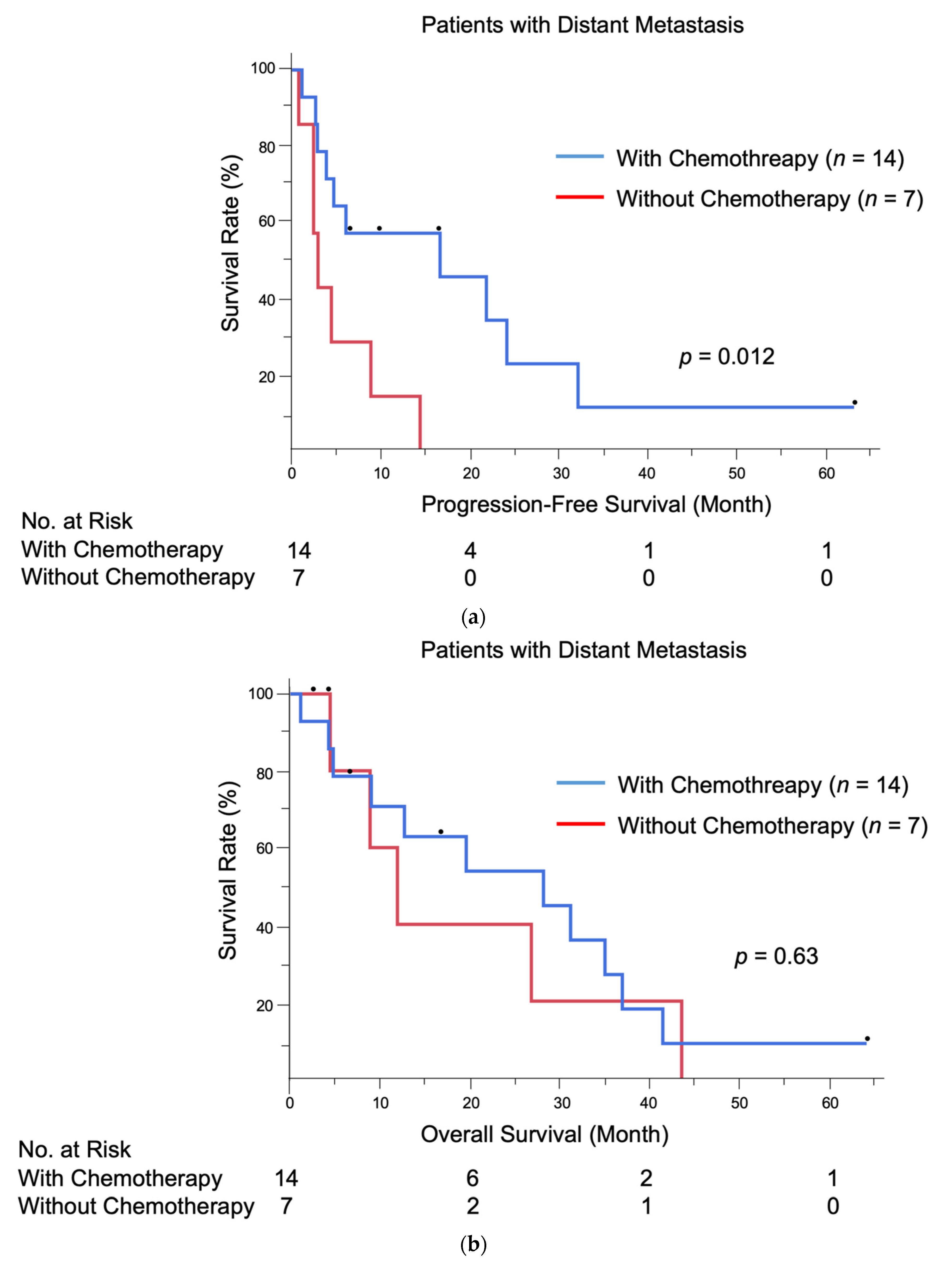
| Chemotherapy, conducted | 0.27 | 0.089–0.81 | 0.020 | 0.22 | 0.052–0.92 | 0.038 |
| Radiation therapy for metastatic sites, conducted | 0.58 | 0.19–1.79 | 0.34 | 0.49 | 0.15–1.57 | 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashimoto, H.; Kaku-Ito, Y.; Furue, M.; Ito, T. The Outcome of Chemotherapy for Metastatic Extramammary Paget’s Disease. J. Clin. Med. 2021, 10, 739. https://doi.org/10.3390/jcm10040739
Hashimoto H, Kaku-Ito Y, Furue M, Ito T. The Outcome of Chemotherapy for Metastatic Extramammary Paget’s Disease. Journal of Clinical Medicine. 2021; 10(4):739. https://doi.org/10.3390/jcm10040739
Chicago/Turabian StyleHashimoto, Hiroki, Yumiko Kaku-Ito, Masutaka Furue, and Takamichi Ito. 2021. "The Outcome of Chemotherapy for Metastatic Extramammary Paget’s Disease" Journal of Clinical Medicine 10, no. 4: 739. https://doi.org/10.3390/jcm10040739






