Abstract
We aim to review the literature for studies investigating the oncological outcomes of patients with penile cancer (PC) undergoing bilateral pelvic lymph node dissection (PLND) in the presence of inguinal lymph node metastasis (LNM) who are at risk of harboring pelvic metastasis. A search of English language literature was performed using the PubMed-MEDLINE database up to 3 December 2020 to identify articles addressing bilateral PLND in PC patients. Eight articles investigating bilateral PLND met our inclusion criteria. Patients with pelvic LNM have a dismal prognosis and, therefore, PLND has an important role in both the staging and treatment of PC patients. Ipsilateral PLND is recommended in the presence of ≥2 positive inguinal nodes and/or extranodal extension (ENE). Significant survival improvements were observed with a higher pelvic lymph node yield, in patients with pN2 disease, and in men treated with bilateral PLND as opposed to ipsilateral PLND. Nevertheless, the role of bilateral PLND for unilateral inguinal LNM remains unclear. Although the EAU guidelines state that pelvic nodal disease does not occur without ipsilateral inguinal LNM, metastatic spread from one inguinal side to the contralateral pelvic side has been reported in a number of studies. Further studies are needed to clarify the disseminative pattern of LNM, in order to establish PLND templates according to patients’ risk profiles and to investigate the benefit of performing bilateral PLND for unilateral inguinal disease.
1. Introduction
The development of lymph node metastases (LNM) in penile cancer (PC) follows the anatomical loco-regional drainage route and is characterized by a stepwise disseminative pattern [1]. The presence and extent of LNM is the single most important prognostic factor in determining long-term survival in men with invasive PC [2]. The European Association of Urology (EAU) and the National Comprehensive Cancer Network (NCCN) guidelines recommend performing an inguinal lymph node dissection (ILND) in patients with a Union for International Cancer Control TNM stage ≥ T1bG2 [3,4]. The number, diameter, and extracapsular nodal extension (ENE) of inguinal metastases have been established as independent risk factors for pelvic lymph node involvement, which carries a poor prognosis. Furthermore, the proportion of pelvic LNM was shown to increase from 0% to 57.1% in cases with no risk factors and when all three are present, respectively [5]. Therefore, ipsilateral pelvic lymph node dissection (PLND) is recommended as an adjunct to ILND in patients with ≥2 inguinal LNM on one side and/or ENE for proper lymphatic assessment and staging [3].
Nevertheless, controversy exists regarding the therapeutic role of PLND since the proportion of patients with pelvic LNM among those with inguinal LNM is highly variable, and it has been reported that only a fraction of patients with pelvic LNM may benefit from PLND alone [6]. Other studies suggest that PLND has a curative role only in patients with a single microscopic focus in the pelvic specimen [7]. A further polemic is whether PLND should be performed ipsilaterally only or bilaterally in patients with unilateral inguinal LNM since crossover from inguinal to contralateral pelvic nodes has not been well studied [8]. The rarity of PC and the lack of randomized studies preclude high-level evidence recommendations in the management of pelvic lymph nodes, and its benefit in low-risk patients remains to be confirmed.
This study aimed to retrieve articles addressing the oncological outcomes of patients with penile cancer (PC) undergoing bilateral PLND for inguinal LNM at risk of harboring pelvic LNM.
2. Materials and Methods
2.1. Search Strategy
A search was conducted using the PubMed-MEDLINE database up to 3 December 2020 with the following search string: (((“Penile Neoplasms”[MeSH]) OR (penile cancer)) OR (penile carcinoma)) AND (((((lymph node excision [MeSH]) OR (lymph node dissection)) OR (lymphadenectomy)) OR (pelvic lymph node dissection)) OR (pelvic lymphadenectomy)).
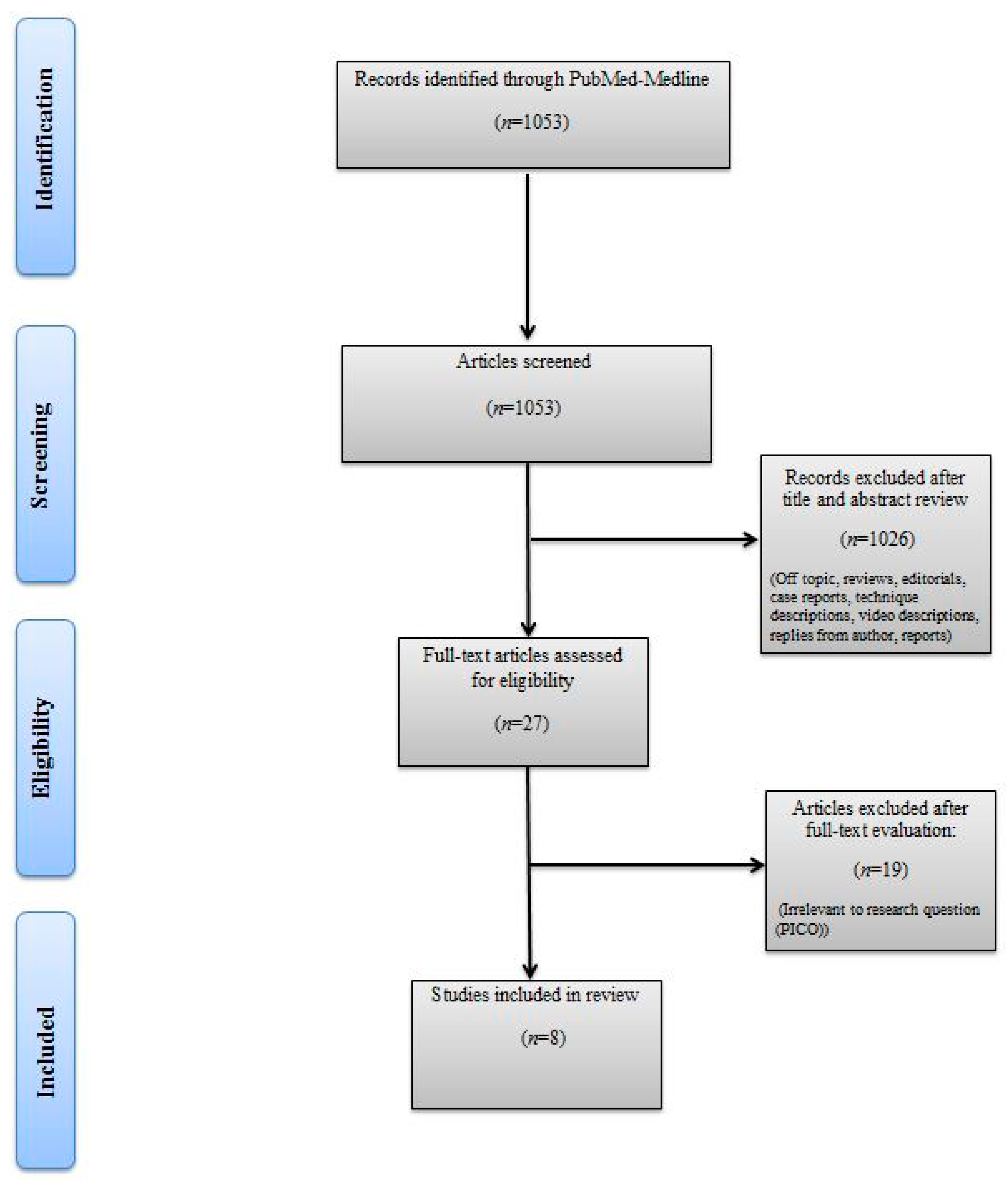
The titles and abstracts of identified articles were retrieved for evaluation. The full text of potentially eligible articles was independently screened against the study selection criteria by two independent reviewers (M.B. and R.S.). Disagreement was resolved by discussion; if no agreement was reached, a third independent party acted as an arbiter (A.M). Figure 1 shows the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram detailing the search strategy and identification of studies used in the evidence synthesis.
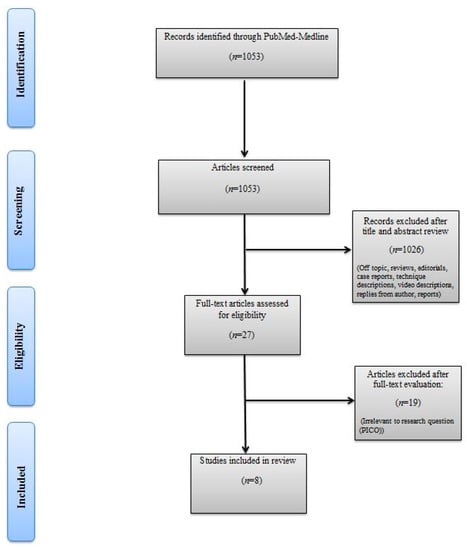
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow chart.
2.2. Inclusion and Exclusion Criteria
According to the PRISMA statements, the PICOS: Population (P), Intervention (I), Comparator (C), Outcomes (O), and Study design (S), approach was used to specify the eligibility criteria. Therefore, studies were considered eligible if PC patients (P) were managed by bilateral PLND for inguinal LNM (I) as a single-arm or compared to patients in whom unilateral PLND or no PLND was performed (C) and if their oncological outcomes (O) were assessed in retrospective or prospective studies (S). After article selection and according to the eligibility criteria, the following types of studies were excluded: articles not describing bilateral PLND, articles not written in English, review articles, conference abstracts, and case reports.
2.3. Data Extraction and Analysis
A data extraction form was developed to collect information on author and journal, study design and period, purpose of investigation, number of patients included, patient age, type of intervention, TNM stage, tumor differentiation and lymphovascular invasion, ENE, PLND template, complications, local, regional, or systemic recurrences, neo/adjuvant treatment, follow-up period, lymph node yield and positivity, and survival analysis. Two reviewers independently extracted data for further assessment of qualitative and quantitative evidence synthesis. Descriptive statistics were used for baseline data and a narrative synthesis of the evidence was performed.
3. Results
Table 1 summarizes the demographic, baseline characteristics, and perioperative data of studies which included patients treated with bilateral PLND for PC. Eight studies were included that reported on the oncological outcomes of bilateral PLND in 619 patients with PC who underwent PLND, of which 420 were bilateral. All eight studies reported the anatomical boundaries of the PLND templates, of which three described the surgical technique for PLND in detail [7,9,10]. Seven studies were of a retrospective nature, while one study performed ilioinguinal lymph node dissections prospectively [10]. Table 2 summarizes the inguinal and pelvic lymph node status and survival analysis of patients treated for PC.

Table 1.
Summary of studies including patients treated with bilateral pelvic lymph node dissection for penile cancer.

Table 2.
Inguinal and pelvic lymph node status and survival analysis in patients treated for penile cancer.
3.1. Pelvic Lymph Node Yield Impacts Survival
Chipollini et al. performed PLND in 198 chemonaïve patients, of which 106 (53.5%) were bilateral dissections. The median number of lymph nodes (LN) and the median number of positive LN were 13 (8–19) and 2 (1–4), respectively. The survival analysis revealed that patients with a lymph node yield of ≥9 LN had better 5-year disease-specific survival (DSS) (64.2% vs. 47.2%), overall survival (OS) (60.3% vs. 39.8%), and recurrence-free survival (RFS) (60.3% vs. 43.2%). Moreover, a lymph node yield of ≥9 was found to be a predictor for RFS after adjustment for baseline characteristics. Although a distinction of DSS, OS, and RFS differences in patients that underwent unilateral as opposed to bilateral PLND was not undertaken, this study established an LN threshold that may be more readily obtained with bilateral dissections and that may provide a benefit in patients harboring pelvic LNM [11].
3.2. Prophylactic PLND
Djajadiningrat et al. prophylactically treated 79 chemonaïve PC patients with PLND, of which 23 (29%) were bilateral, when ≥2 positive inguinal LN or ENE were found. Tumor-positive pelvic nodes were found in 19 (24%) patients, and both inguinal ENE and ≥2 positive inguinal LN were identified as predictors of pelvic nodal involvement. A plausible explanation for ENE predicting pelvic LNM may lie in tumors’ aggressiveness and ability to penetrate the lymph node capsule. Moreover, patients with positive pelvic LNM had worse 5-year DSS compared to patients without pelvic LNM (17% vs. 62%). There was no comparison of outcomes between ipsilateral and bilateral PLND [7].
3.3. Bilateral PLND vs. No-PLND
In a multicenter study by Li et al., 69 patients underwent bilateral PLND and were compared to 121 patients in whom PLND was spared. Among the PLND group, 16 patients had unilateral and five patients had bilateral pelvic LNM, and no patient had crossover metastatic spread from one inguinal side to the contralateral pelvic side. The PLND group did not demonstrate higher 1- and 3-year DSS rates than the no-PLND group (65.7% and 39.0% vs. 65.4% and 39.6%, p = 0.796). However, propensity score matching among pN2 patients showed higher DSS in patients who underwent PLND compared to those who did not (83.3% and 83.3% vs. 69.0% and 50.2%, p = 0.030). Based on their analyses, a significant benefit was found for a subset of pN2 cases, while pN3 patients may not benefit from bilateral PLND [12].
3.4. Bilateral vs. Unilateral PLND
In another multicentric study, Zargar-Shoshtari et al. investigated criteria predicting bilateral pelvic LNM in patients with pathologically confirmed inguinal nodes. In a cohort of 140 patients, 83 had both bilateral inguinal and pelvic LNM, and bilateral PLND was performed in 64 (77%) patients. Of note, 27 (32.5%) patients received neoadjuvant chemotherapy (NAC). The presence of ≥4 positive inguinal nodes had a sensitivity of 95% and an area under the curve (AUC) of 0.76 for predicting bilateral pelvic LNM, which was present in only one patient with <4 inguinal LNM. In a separate analysis which excluded patients who received NAC, ≥4 inguinal LNM was still the strongest predictor for bilateral disease; however, it did not reach statistical significance. The rate of regional failure was higher for patients who only had ipsilateral PLND compared to bilateral PLND (47% at 5.53 vs. 20% at 12.8 months, p = 0.02). Additionally, there was a trend for improved OS in men treated with bilateral PLND compared to unilateral (11.8 vs. 10.9 mo., p = 0.10), and OS was significantly lower with ≥4 positive inguinal LNM (8.7 vs. 15.4 months, p = 0.04) [13].
In a subsequent multicentric study, the same authors assessed the oncological outcomes of patients treated with bilateral PLND in the presence of unilateral pelvic LNM. In 51 patients with unilateral inguinal LNM and positive pelvic nodes, 38 (75%) had ipsilateral and 13 (25%) bilateral PLND. In the 13 patients with bilateral PLND, metastases were only found in ipsilateral pelvic nodes. It is important to note that 86% of the cohort received preoperative and postoperative chemotherapy, radiotherapy, or both, and a higher proportion of adjuvant radiotherapy was utilized in the unilateral PLND patients (50% vs. 8%, p = 0.01). Despite this, the median OS was significantly longer in bilateral PLND patients compared to unilateral (21.7 vs. 13.1 mo., p = 0.05) at a median 13.3 months follow-up. Cancer-specific survival (CSS) was statistically similar in bilateral and ipsilateral PLND, but regression analysis showed that bilateral PLND, adjusted for additional therapies and presence of multiple pelvic LNM, improved CSS [14].
3.5. Predicting the Disseminative Pattern of Pelvic LNM
Zhu et al. evaluated the value of computerized tomography, Cloquet’s node, and inguinal LN disease burden in predicting pelvic LNM. A cohort of 73 patients underwent bilateral ILND, of which 33 had inguinal LNM and 16 pelvic LNM. Cloquet’s node had a sensitivity of 30% and a specificity of 94.1% in pelvic CT-negative groin basins. The number of positive inguinal LNs, lymph node ratio (number of positive LNs/total number removed), ENE, and p53 expression were significantly associated with pelvic LNM. Prognostic factors for pelvic LNM were ≥3 enlarged inguinal LNs in preoperative CT imaging and lymph node size of ≥3.5 cm as the maximum diameter. Importantly, no patient developed pelvic LNM in the absence of inguinal LNM and contralateral pelvic LNM was not observed in unilateral inguinal LNM [9].
Zhu et al. further investigated the disseminative pattern of LNM in 46 chemonaïve patients that underwent bilateral ILND. In those with ≥1 positive inguinal LNM, bilateral iliac LND was performed. Among these patients, 21 had unilateral inguinal LNM and three bilateral LNM. The positive and negative predictive values of Cloquet’s node for predicting iliac LNM were 80% and 86%, respectively. Of 48 ILND, 21 had no LNM, and in the 21 groin basins, ilioinguinal LND did not reveal iliac LNM in the absence of inguinal LN involvement [10].
3.6. Lymph Node Mapping
Recently, Yao et al. provided an accurate dissemination map of LNM and aimed to determine the extent of PLND. One hundred and twenty-eight chemo-radio-naive patients received bilateral ILND, 111 received bilateral PLND, and 17 received unilateral PLND. Pelvic LNM was present in 57 (44.5%) patients and bilateral LNM was found in 13 patients. The median number of pelvic LNs removed was 18 (IQR: 10–30). All 57 patients with PLNM had inguinal LNM. As opposed to other studies where crossover was not found [9,10,12], two patients with unilateral inguinal LNM had bilateral PLNM, suggesting crossover from inguinal nodes to contralateral pelvic nodes. Lopes et al. similarly documented metastasis to the pelvic nodes with no inguinal involvement [15].
OS was significantly longer in patients that underwent bilateral PLND than those with unilateral (30 vs. 18 mo., p = 0.004). Among the 57 patients with pelvic LNM, the estimated median OS was higher in the bilateral than in the unilateral group (16 vs. 11 mo., p = 0.07). Moreover, the prevalence of pelvic LNM was similar in patients with ENE and without ENE (42.9% vs. 45.8%, p = 0.74), which questions the validity of ENE for predicting pelvic LNM, as has been reported by a multitude of studies [8].
4. Conclusions
There is a paucity of data on the disseminative pattern of pelvic LNM in patients with PC. Moreover, there are scarce data comparing the outcomes of patients treated with bilateral vs. unilateral PLND in the presence of unilateral inguinal disease. Although the EAU guidelines state that crossover metastatic spread from one groin to the contralateral pelvis has never been reported [3], a number of studies have found metastases to pelvic nodes without ipsilateral inguinal involvement [8,12,15]. Interestingly, despite the rarely found crossover, data gathered in the current review suggest that bilateral PLND is beneficial, but the ideal candidate is yet to be determined.
Among the justifications for performing bilateral PLND is the improved survival observed compared to patients treated unilaterally, and particularly in the pN2 subset of patients, both of which may correlate with the treatment of occult micrometastatic disease. Moreover, a possible explanation for the observation of minimal crossover metastatic spread to contralateral pelvic LNs in patients with unilateral inguinal LNM may be disease underestimation even when invasive nodal staging is performed.
Despite patients with invasive or high-grade PC seemingly benefiting from undergoing extensive lymph node dissection, both ILND and PLND are characteristically associated with considerable physical and psychological morbidity. However, only a single study reported data on postoperative complications in patients that underwent bilateral PLND, where 14 patients (18%) had some type of complication, 9 of these wound-related and 5 non-wound-related such as pneumonia, delirium, and ileus [7]. Modern series report complication rates of 42–57% for all patients undergoing ILND, the most common being wound necrosis and dehiscence, lymphocele, lymphoedema, and venous thromboembolism [16]. Several strategies have been recommended to prevent adverse effects of ILND such as prophylactic antibiotics until drain removal, early ambulation, debridement of non-viable skin, meticulous control of lymphatics, preservation of the saphenous vein, and antiembolism stockings, none of which have been studied in prospective trials [17]. Contemporary minimally invasive management strategies for LN involvement aim to minimize the morbidity associated with traditional radical inguinal and pelvic lymphadenectomy through appropriate risk stratification whilst optimizing oncological outcomes.
Due to inherent methodological limitations, false-negative rates of inguinal and pelvic lymphadenectomy remain unknown. The implementation of new imaging modalities such as 18FDG-PET/CT into clinical workflows may improve the diagnostic accuracy for the detection of LNM [18,19,20]. A recent Danish study by Jakobsen et al. found 18FDG-PET/CT to have a groin false-negative rate of 2.1% and a sensitivity of 85.4% per patient for assessing inguinal lymph node metastasis and distant metastasis compared to 47.5% for the conventional CT scan [21]. Graafland et al. reported a sensitivity of 91% and a specificity of 100% with PET/CT for pelvic staging [6]. In contrast, for dynamic sentinel-node biopsy, false-negative rates of up to 12–15% have been reported [22]. Consequently, these approaches might help to address challenges in adequately identifying patients that might benefit from bilateral PLND and assist in treatment decision making.
However, specific bilateral PLND indications and patient selection need to be better defined. Furthermore, studies are needed addressing the value of the LN yield as a surrogate of survival. Since retrospective studies cannot establish causality properly, ongoing prospective randomized trials, such as the International Penile Advanced Cancer Trial (InPACT), may help clarify the role of PLND and its integration with multimodal therapies. Studies are warranted to determine the benefit of bilateral PLND in patients with unilateral inguinal LNM suspected of harboring contralateral metastatic disease. This implies studies that help clarify the disseminative pattern of metastatic spread and the limits of dissection in a precise and personalized manner.
The clinical heterogeneity within studies and the limitations of the study need to be acknowledged. Firstly, the search strategy was not systematically performed, and therefore the acquisition of all bilateral PLND studies cannot be confirmed. Secondly, there was not enough information to propose a meta-analysis given the little convergence within studies, which would incur in a high risk of outcome measurement bias. Not all patients that underwent bilateral PLND had the same tumor stage, nor did they receive the same treatment regimen or LND templates, which precludes the comparison of outcomes. The different PLND templates, aims/outcomes of studies, the low patient number, interference of neo/adjuvant treatments, and the retrospective nature of studies contribute to the heterogeneity of data.
Although pelvic and inguinal LNM dissemination patterns are predominantly reported following the anatomical staged pathway, exceptions exist and further efforts and larger cohorts are needed to deepen the understanding of LNM spread, as they may provide insights for directing LND strategies. Moreover, data suggest that preoperative imaging and staging may play a role in identifying pelvic LN involvement. Therefore, further research in this field may contribute to better patient selection for either unilateral or bilateral PLND, while weighing the oncological benefits, perioperative complications, and patients’ quality of life.
Author Contributions
Project conceptualization: R.S.-I.; literature review and data extraction: R.S.-I. and M.B.-M.; writing—original draft: R.S.-I., M.B.-M., and A.S.; writing—review and editing: M.A. and A.S.; supervision: C.G. and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no funding.
Institutional Review Board Statement
Ethical review and approval were waived as a review article.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors abide to the journal´s ethical guidelines.
Acknowledgments
The article processing charge was funded by the Baden-Wuerttemberg Ministry of Science, Research and Art and the University of Freiburg in the funding program Open Access Publishing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Leijte, J.A.; Olmos, R.A.V.; Nieweg, O.E.; Horenblas, S. Anatomical Mapping of Lymphatic Drainage in Penile Carcinoma with SPECT-CT: Implications for the Extent of Inguinal Lymph Node Dissection. Eur. Urol. 2008, 54, 885–892. [Google Scholar] [CrossRef]
- Djajadiningrat, R.S.; Graafland, N.M.; Van Werkhoven, E.; Meinhardt, W.; Bex, A.; Van Der Poel, H.G.; Van Boven, H.H.; Olmos, R.A.V.; Horenblas, S. Contemporary Management of Regional Nodes in Penile Cancer—Improvement of Survival? J. Urol. 2014, 191, 68–73. [Google Scholar] [CrossRef]
- Hakenberg, O.W.; Compérat, E.M.; Minhas, S.; Necchi, A.; Protzel, C.; Watkin, N. EAU Guidelines on Penile Cancer: 2014 Update. Eur. Urol. 2015, 67, 142–150. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology. Penile Cancer Version 2.2019—May 13, 2019. Available online: https://www2.tri-kobe.org/nccn/guideline/urological/english/penile.pdf (accessed on 3 December 2020).
- Lughezzani, G.; Catanzaro, M.; Torelli, T.; Piva, L.; Biasoni, D.; Stagni, S.; Crestani, A.; Guttilla, A.; Raggi, D.; Giannatempo, P.; et al. The Relationship between Characteristics of Inguinal Lymph Nodes and Pelvic Lymph Node Involvement in Penile Squamous Cell Carcinoma: A Single Institution Experience. J. Urol. 2014, 191, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Graafland, N.M.; Van Boven, H.H.; Van Werkhoven, E.; Moonen, L.M.; Horenblas, S. Prognostic Significance of Extranodal Extension in Patients with Pathological Node Positive Penile Carcinoma. J. Urol. 2010, 184, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Djajadiningrat, R.S.; Van Werkhoven, E.; Horenblas, S. Prophylactic Pelvic Lymph Node Dissection in Patients with Penile Cancer. J. Urol. 2015, 193, 1976–1980. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Chen, Y.; Ye, Y.; Wu, Z.; Chen, D.; Han, H.; Li, Z.; Liu, Z.; Wang, Y.; Qin, Z.; et al. Lymph node mapping in paients with penile cancer undergoing pelvic lymph node dissection. J. Urol. 2021, 205, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, S.L.; Ye, D.W.; Yao, X.D.; Jiang, Z.X.; Zhou, X.Y. Predicting Pelvic Lymph Node Metastases in Penile Cancer Patients: A Comparison of Computed Tomography, Cloquet’s Node, and Disease Burden of Inguinal Lymph Nodes. Oncol. Res. Treat. 2008, 31, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, S.L.; Ye, D.W.; Yao, X.D.; Dai, B.; Zhang, H.L.; Shen, Y.J.; Zhu, Y.P.; Shi, G.H.; Ma, C.G. Prospectively packaged ilioinguinal lymphadenectomy for penile cancer: The disseminative pattern of lymph node metastasis. J. Urol. 2009, 181, 2103–2108. [Google Scholar] [CrossRef]
- Chipollini, J.; Azizi, M.; Vullo, S.L.; Mariani, L.; Zhu, Y.; Ye, D.W.; Ornellas, A.A.; Watkin, N.; Ager, M.; Hakenberg, O.; et al. Identifying an optimal lymph node yield for penile squamous cell carcinoma: Prognostic impact of surgical dissection. BJU Int. 2019, 125, 82–88. [Google Scholar] [CrossRef]
- Li, Z.-S.; Deng, C.-Z.; Yao, K.; Tang, Y.; Liu, N.; Chen, P.; Wang, B.; Li, X.; Chen, X.-F.; Liao, H.; et al. Bilateral pelvic lymph node dissection for Chinese patients with penile cancer: A multicenter collaboration study. J. Cancer Res. Clin. Oncol. 2016, 143, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Zargar-Shoshtari, K.; Djajadiningrat, R.; Sharma, P.; Catanzaro, M.; Zhu, Y.; Nicolai, N.; Horenblas, S.; Spiess, P.E. Establishing Criteria for Bilateral Pelvic Lymph Node Dissection in the Management of Penile Cancer: Lessons Learned from an International Multicenter Collaboration. J. Urol. 2015, 194, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Zargar-Shoshtari, K.; Sharma, P.; Djajadiningrat, R.S.; Catanzaro, M.; Ye, D.-W.; Zhu, Y.; Nicolai, N.; Horenblas, S.; Spiess, P.E. Extent of pelvic lymph node dissection in penile cancer may impact survival. World J. Urol. 2016, 34, 353–359. [Google Scholar] [CrossRef]
- Lopes, A.; Bezerra, A.L.R.; Serrano, S.V.; Hidalgo, G.S. Iliac nodal metastasis from carcinoma of the penis treated surgically. BJU Int. 2000, 86, 690–693. [Google Scholar] [CrossRef]
- Spiess, P.E.; Hernandez, M.S.; Pettaway, C.A. Contemporary inguinal lymph node dissection: Minimizing complications. World J. Urol. 2008, 27, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Diorio, G.J.; Pettaway, C.; Master, V.; Spiess, P.E. Contemporary management of patients with penile cancer and lymph node metastasis. Nat. Rev. Urol. 2017, 14, 335–347. [Google Scholar] [CrossRef]
- Rosevear, H.M.; Williams, H.; Collins, M.; Lightfoot, A.J.; Coleman, T.; Brown, J.A. Utility of 18F-FDG PET/CT in identifying penile squamous cell carcinoma metastatic lymph nodes. Urol. Oncol. Semin. Orig. Investig. 2012, 30, 723–726. [Google Scholar] [CrossRef]
- Schlenker, B.; Scher, B.; Tiling, R.; Siegert, S.; Hungerhuber, E.; Gratzke, C.; Tilki, D.; Reich, O.; Schneede, P.; Bartenstein, P.; et al. Detection of inguinal lymph node involvement in penile squamous cell carcinoma by 18F-fluorodeoxyglucose PET/CT: A prospective single-center study. Urol. Oncol. Semin. Orig. Investig. 2012, 30, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Souillac, I.; Rigaud, J.; Ansquer, C.; Marconnet, L.; Bouchot, O. Prospective Evaluation of 18 F-Fluorodeoxyglucose Positron Emission Tomography-Computerized Tomography to Assess Inguinal Lymph Node Status in Invasive Squamous Cell Carcinoma of the Penis. J. Urol. 2012, 187, 493–497. [Google Scholar] [CrossRef]
- Lopes, A.; Bezerra, A.L.; Serrano, S.V.; Hidalgo, G.S. DaPeCa-7: Comparative assessment of fluorodeoxyglucose positron emission tomography/computed tomography (CT) and conventional diagnostic CT in diagnosis of lymph node metastasis, distant metastases and incidental findings in patients with invasive penile cancer. BJU Int. 2021, 127, 254–262. [Google Scholar]
- Meijer, R.P.; Boon, T.A.; van Venrooij, G.E.; Wijburg, C.J. Long-term follow-up after laser therapy for penile carcinoma. Urology 2007, 69, 759–762. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).