Staphylococcus aureus Keratitis: Incidence, Pathophysiology, Risk Factors and Novel Strategies for Treatment
Abstract
1. Introduction
2. Staphylococcus aureus Keratitis—Incidence
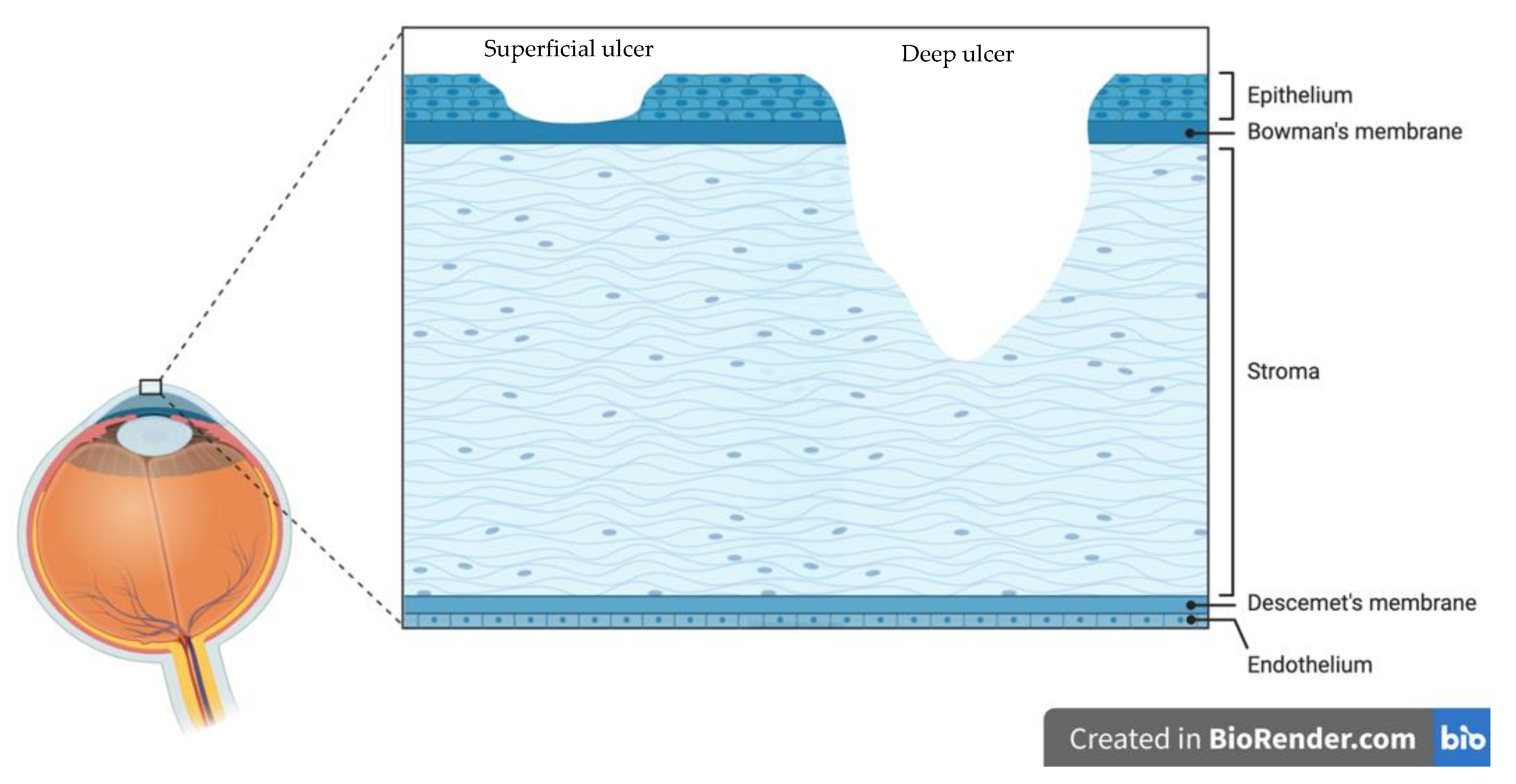
3. Staphylococcus Aureus Keratitis—Pathophysiology
3.1. Overcoming the Tear Film and Adhesion to the Cornea
3.2. Alpha-Haemolysin Toxin
3.3. Biofilm Formation and Contact Lenses
3.4. MRSA and Panton–Valentine Leukocidin
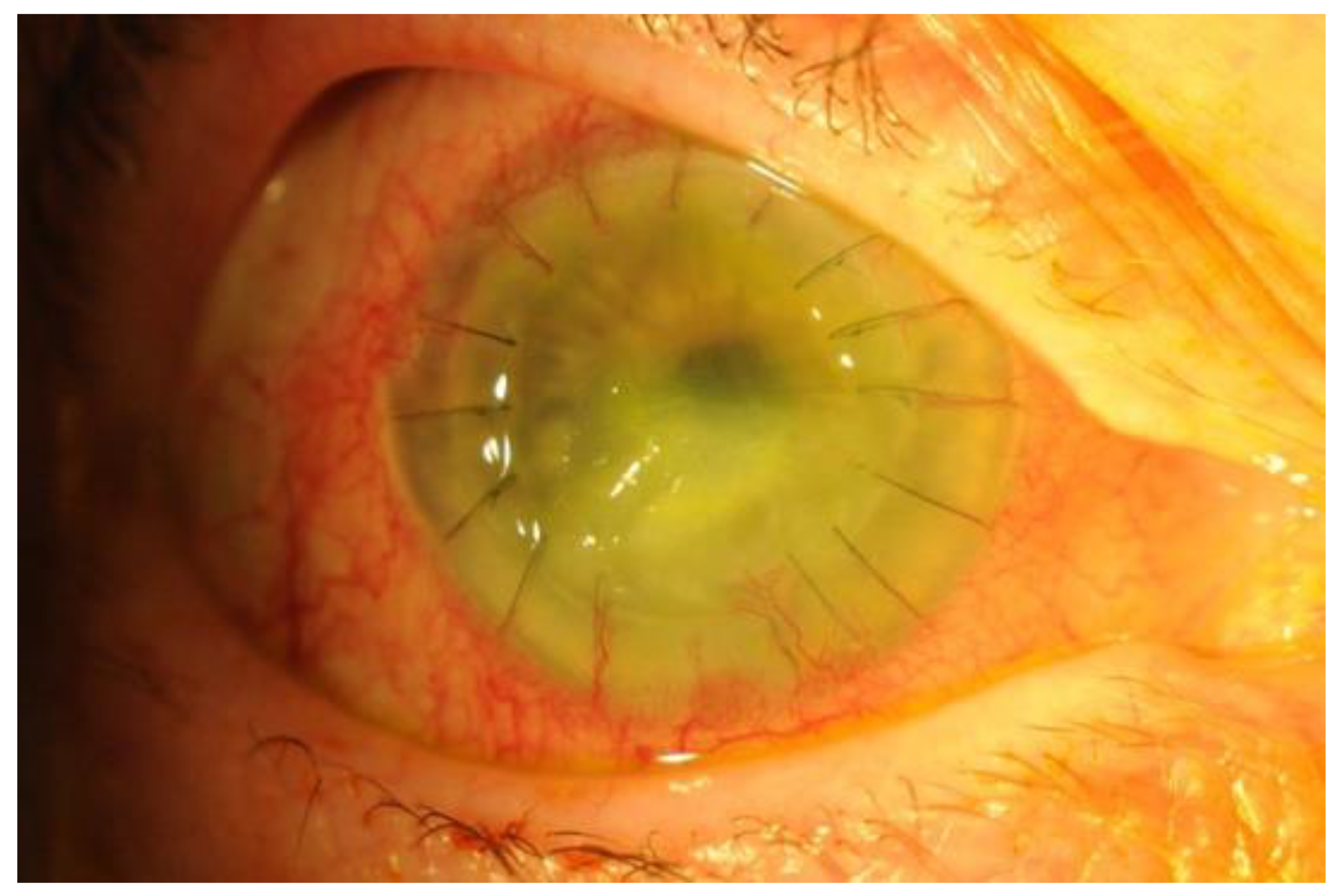
4. Risk Factors, Clinical Presentation and Diagnosis
5. Current Recommendations in Management and Antibiotic Resistance
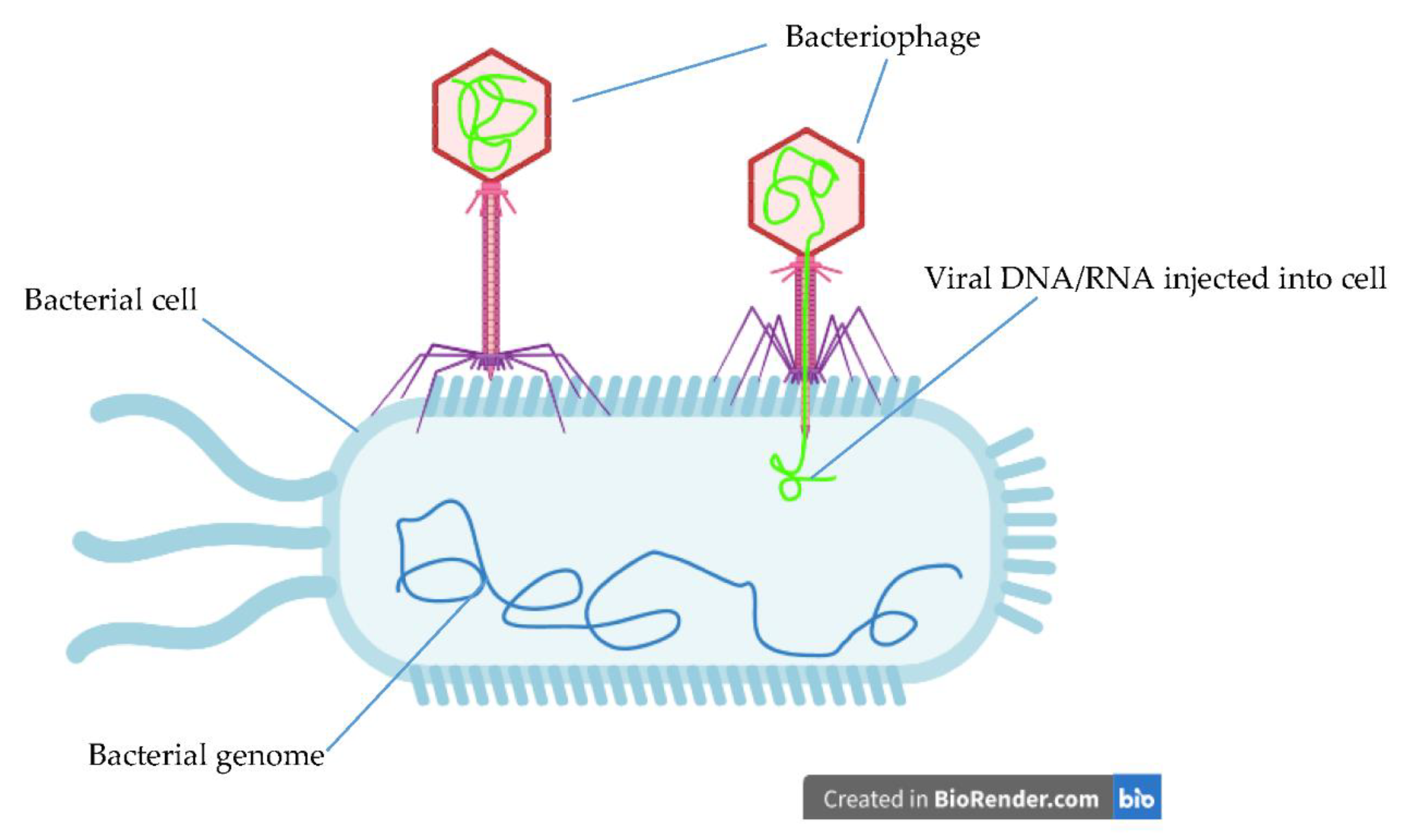
6. Novel Therapeutics
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bourcier, T.; Thomas, F.; Borderie, V.; Chaumeil, C.; Laroche, L. Bacterial keratitis: Predisposing factors, clinical and microbiological review of 300 cases. Br. J. Ophthalmol. 2003, 87, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Martines, E.; Brun, P.; Brun, P.; Cavazzana, R.; Deligianni, V.; Leonardi, A.; Tarricone, E.; Zuin, M. Towards a plasma treatment of corneal infections. Clin. Plasma Med. 2013, 1, 17–24. [Google Scholar] [CrossRef]
- Bartimote, C.; Foster, J.; Watson, S. The Spectrum of Microbial Keratitis: An Updated Review. Open Ophthalmol. J. 2019, 13, 100–130. [Google Scholar] [CrossRef]
- Chang, V.S.; Dhaliwal, D.K.; Raju, L.; Kowalski, R.P. Antibiotic Resistance in the Treatment of Staphylococcus aureus Keratitis_a 20-Year Review. Cornea 2015, 34, 698–703. [Google Scholar] [CrossRef]
- Fernandes, M.; Vira, D.; Medikonda, R.; Kumar, N. Extensively and pan-drug resistant Pseudomonas aeruginosa keratitis: Clinical features, risk factors, and outcome. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Vazirani, J.; Wurity, S.; Ali, M.H. Multidrug-Resistant Pseudomonas aeruginosa Keratitis: Risk Factors, Clinical Characteristics, and Outcomes. Ophthalmology 2015, 122, 2110–2114. [Google Scholar] [CrossRef]
- Silva, C.N.D.; Silva, F.R.D.; Dourado, L.F.N.; Reis, P.; Silva, R.O.; Costa, B.L.D.; Nunes, P.S.; Amaral, F.A.; Santos, V.L.; de Lima, M.E.; et al. A New Topical Eye Drop Containing LyeTxI-b, A Synthetic Peptide Designed from A Lycosa erithrognata Venom Toxin, Was Effective to Treat Resistant Bacterial Keratitis. Toxins (Basel) 2019, 11, 203. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Settle, C.; Morgan, S.J.; Baylis, O.; Ghosh, S. A 10-year analysis of microbiological profiles of microbial keratitis: The North East England study. Eye 2018, 32, 1416–1417. [Google Scholar] [CrossRef]
- Tan, S.Z.; Walkden, A.; Au, L.; Fullwood, C.; Hamilton, A.; Qamruddin, A.; Armstrong, M.; Brahma, A.K.; Carley, F. Twelve-year analysis of microbial keratitis trends at a UK tertiary hospital. Eye (Lond) 2017, 31, 1229–1236. [Google Scholar] [CrossRef]
- Tavassoli, S.; Nayar, G.; Darcy, K.; Grzeda, M.; Luck, J.; Williams, O.M.; Tole, D. An 11-year analysis of microbial keratitis in the South West of England using brain-heart infusion broth. Eye (Lond) 2019, 33, 1619–1625. [Google Scholar] [CrossRef]
- Piette, A.; Verschraegen, G. Role of coagulase-negative staphylococci in human disease. Vet. Microbiol. 2009, 134, 45–54. [Google Scholar] [CrossRef]
- Mun, Y.; Kim, M.K.; Oh, J.Y. Ten-year analysis of microbiological profile and antibiotic sensitivity for bacterial keratitis in Korea. PLoS ONE 2019, 14, e0213103. [Google Scholar] [CrossRef] [PubMed]
- Politis, M.; Wajnsztajn, D.; Rosin, B.; Block, C.; Solomon, A. Trends of Bacterial Keratitis Culture Isolates in Jerusalem; a 13-Years Analysis. PLoS ONE 2016, 11, e0165223. [Google Scholar] [CrossRef]
- Shaikh, F.; Lohano, M.K.; Memon, I. Pattern of Microbes Associated to Keratitis in Patients Presenting at Liaquat University Hospital. JLUMHS 2013, 12, 145–150. [Google Scholar]
- Peng, M.Y.; Cevallos, V.; McLeod, S.D.; Lietman, T.M.; Rose-Nussbaumer, J. Bacterial Keratitis: Isolated Organisms and Antibiotic Resistance Patterns in San Francisco. Cornea 2018, 37, 84–87. [Google Scholar] [CrossRef]
- Siddiqui, R.; Lakhundi, S.; Khan, N.A. Status of the effectiveness of contact lens solutions against keratitis-causing pathogens. Cont. Lens Anterior Eye 2015, 38, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.; Cabrera-Aguas, M.; Khoo, P.; Pratama, R.; Gatus, B.J.; Gulholm, T.; El-Nasser, J.; Lahra, M.M. Keratitis antimicrobial resistance surveillance program, Sydney, Australia: 2016 Annual Report. Clin. Exp. Ophthalmol. 2019, 47, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Aguas, M.; Khoo, P.; George, C.R.R.; Lahra, M.M.; Watson, S.L. Antimicrobial resistance trends in bacterial keratitis over 5 years in Sydney, Australia. Clin. Exp. Ophthalmol. 2020, 48, 183–191. [Google Scholar] [CrossRef]
- Liu, H.Y.; Chu, H.S.; Wang, I.J.; Chen, W.L.; Hu, F.R. Microbial Keratitis in Taiwan: A 20-Year Update. Am. J. Ophthalmol. 2019, 205, 74–81. [Google Scholar] [CrossRef]
- Lalitha, P.; Manoharan, G.; Karpagam, R.; Prajna, N.V.; Srinivasan, M.; Mascarenhas, J.; Das, M.; Porco, T.C.; Lietman, T.M.; Cevallos, V.; et al. Trends in antibiotic resistance in bacterial keratitis isolates from South India. Br. J. Ophthalmol. 2017, 101, 108–113. [Google Scholar] [CrossRef]
- Dalmon, C.; Porco, T.C.; Lietman, T.M.; Prajna, N.V.; Prajna, L.; Das, M.R.; Kumar, J.A.; Mascarenhas, J.; Margolis, T.P.; Whitcher, J.P.; et al. The clinical differentiation of bacterial and fungal keratitis: A photographic survey. Invest. Ophthalmol. Vis Sci. 2012, 53, 1787–1791. [Google Scholar] [CrossRef] [PubMed]
- Astley, R.; Miller, F.C.; Mursalin, M.H.; Coburn, P.S.; Callegan, M.C. An Eye on Staphylococcus aureus Toxins: Roles in Ocular Damage and Inflammation. Toxins (Basel) 2019, 11, 365. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, A.; Diep, B.A.; Mai, T.T.; Vo, N.H.; Warrener, P.; Suzich, J.; Stover, C.K.; Sellman, B.R. Differential expression and roles of Staphylococcus aureus virulence determinants during colonization and disease. mBio 2015, 6, e02272-14. [Google Scholar] [CrossRef]
- O’Callaghan, R.J. The Pathogenesis of Staphylococcus aureus Eye Infections. Pathogens 2018, 7, 9. [Google Scholar] [CrossRef]
- Zhang, Z.; Abdel-Razek, O.; Hawgood, S.; Wang, G. Protective Role of Surfactant Protein D in Ocular Staphylococcus aureus Infection. PLoS ONE 2015, 10, e0138597. [Google Scholar] [CrossRef]
- Hume, E.B.; Cole, N.; Khan, S.; Walsh, B.J.; Willcox, M.D. The role of staphopain a in Staphylococcus aureus keratitis. Exp. Eye Res. 2020, 193, 107994. [Google Scholar] [CrossRef]
- Putra, I.; Rabiee, B.; Anwar, K.N.; Gidfar, S.; Shen, X.; Babalooee, M.; Ghassemi, M.; Afsharkhamseh, N.; Bakhsh, S.; Missiakas, D.; et al. Staphylococcus aureus alpha-hemolysin impairs corneal epithelial wound healing and promotes intracellular bacterial invasion. Exp. Eye Res. 2019, 181, 263–270. [Google Scholar] [CrossRef] [PubMed]
- McCormick, C.C.; Caballero, A.R.; Balzli, C.L.; Tang, A.; O’Callaghan, R.J. Chemical inhibition of alpha-toxin, a key corneal virulence factor of Staphylococcus aureus. Invest. Ophthalmol. Vis Sci. 2009, 50, 2848–2854. [Google Scholar] [CrossRef]
- Hazlett, L.; Suvas, S.; McClellan, S.; Ekanayaka, S. Challenges of corneal infections. Expert Rev. Ophthalmol. 2016, 11, 285–297. [Google Scholar] [CrossRef]
- Heidari, H.; Hadadi, M.; Sedigh Ebrahim-Saraie, H.; Mirzaei, A.; Taji, A.; Hosseini, S.R.; Motamedifar, M. Characterization of virulence factors, antimicrobial resistance patterns and biofilm formation of Pseudomonas aeruginosa and Staphylococcus spp. strains isolated from corneal infection. J. Fr. Ophtalmol. 2018, 41, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Rossos, A.K.; Banti, C.N.; Kalampounias, A.G.; Papachristodoulou, C.; Kordatos, K.; Zoumpoulakis, P.; Mavromoustakos, T.; Kourkoumelis, N.; Hadjikakou, S.K. pHEMA@AGMNA-1: A novel material for the development of antibacterial contact lens. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110770. [Google Scholar] [CrossRef] [PubMed]
- Hilliam, Y.; Kaye, S.; Winstanley, C. Pseudomonas aeruginosa and microbial keratitis. J. Med. Microbiol. 2020, 69, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Sun, X.; Wang, Z.; Zhang, Y. Biofilm-forming capacity of Staphylococcus epidermidis, Staphylococcus aureus, and Pseudomonas aeruginosa from ocular infections. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5624–5631. [Google Scholar] [CrossRef]
- Zaidi, T.; Zaidi, T.; Yoong, P.; Pier, G.B. Staphylococcus aureus corneal infections: Effect of the Panton-Valentine leukocidin (PVL) and antibody to PVL on virulence and pathology. Invest. Ophthalmol. Vis. Sci. 2013, 54, 4430–4438. [Google Scholar] [CrossRef]
- Sueke, H.; Shankar, J.; Neal, T.; Winstanley, C.; Tuft, S.; Coates, R.; Horsburgh, M.J.; Kaye, S. lukSF-PV in Staphylococcus aureus keratitis isolates and association with clinical outcome. Invest. Ophthalmol. Vis. Sci. 2013, 54, 3410–3416. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Parker, W.T.; Law, N.W.; Clarke, C.L.; Gisseman, J.D.; Pflugfelder, S.C.; Wang, L.; Al-Mohtaseb, Z.N.. Evolving risk factors and antibiotic sensitivity patterns for microbial keratitis at a large county hospital. Br. J. Ophthalmol. 2017, 101, 1483–1487. [Google Scholar] [CrossRef] [PubMed]
- Durrani, A.F.; Atta, S.; Bhat, A.; Mammen, A.; Dhaliwal, D.; Kowalski, R.P.; Jhanji, V. Methicillin-resistant Staphylococcal aureus keratitis: Initial treatment, risk factors, clinical features, and treatment outcomes. Am. J. Ophthalmol. 2020, 214, 119–126. [Google Scholar] [CrossRef]
- Somerville, T.F.; Shankar, J.; Aldwinckle, S.; Sueke, H.; Neal, T.; Horsburgh, M.J.; Kaye, S.B. Recurrent microbial keratitis and endogenous site Staphylococcus aureus colonisation. Sci. Rep. 2020, 10, 18559. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.J.; Huang, Y.C.; Tan, H.Y.; Ma, D.H.; Lin, H.C.; Yeh, L.K.; Chen, P.Y.; Chen, H.C.; Chuang, C.C.; Chang, C.J.; et al. Staphylococcus aureus keratitis: A review of hospital cases. PLoS ONE 2013, 8, e80119. [Google Scholar] [CrossRef]
- Tzamalis, A.; Romano, V.; Cheeseman, R.; Vinciguerra, R.; Batterbury, M.; Willoughby, C.; Neal, T.; Ahmad, S.; Kaye, S. Bandage contact lens and topical steroids are risk factors for the development of microbial keratitis after epithelium-off CXL. BMJ Open Ophthalmol. 2019, 4, e000231. [Google Scholar] [CrossRef]
- Al-Mujaini, A.; Al-Kharusi, N.; Thakral, A.; Wali, U.P. Bacterial Keratitis- Perspective on Epidemiology, Clinico-Pathogenesis, Diagnosis and Treatment. SQU Med. J. 2009, 9, 184–195. [Google Scholar]
- Ong, H.S.; Corbett, M.C. Corneal infections in the 21st century. Postgrad. Med. J. 2015, 91, 565–571. [Google Scholar] [CrossRef]
- Kaye, R.; Kaye, A.; Sueke, H.; Neal, T.; Winstanley, C.; Horsburgh, M.; Kaye, S. Recurrent bacterial keratitis. Invest. Ophthalmol. Vis. Sci. 2013, 54, 4136–4139. [Google Scholar] [CrossRef]
- Cole, N.; Hume, E.B.; Khan, S.; Garthwaite, L.; Conibear, T.C.; Willcox, M.D. The role of CXC chemokine receptor 2 in Staphylococcus aureus keratitis. Exp. Eye Res. 2014, 127, 184–189. [Google Scholar] [CrossRef]
- Nouwen, J.; Boelens, H.; van Belkum, A.; Verbrugh, H. Human factor in Staphylococcus aureus nasal carriage. Infect. Immun. 2004, 72, 6685–6688. [Google Scholar] [CrossRef] [PubMed]
- Borroni, D.; Romano, V.; Kaye, S.B.; Somerville, T.; Napoli, L.; Fasolo, A.; Gallon, P.; Ponzin, D.; Esposito, A.; Ferrari, S. Metagenomics in ophthalmology: Current findings and future prospectives. BMJ Open Ophthalmol. 2019, 4, e000248. [Google Scholar] [CrossRef]
- Kaye, S.; Sueke, H.; Romano, V.; Chen, J.Y.; Carnt, N.; Tuft, S.; Neal, T. Impression membrane for the diagnosis of microbial keratitis. Br. J. Ophthalmol. 2016, 100, 607–610. [Google Scholar] [CrossRef]
- Parekh, M.; Borroni, D.; Romano, V.; Kaye, S.B.; Camposampiero, D.; Ponzin, D.; Ferrari, S. Next-generation sequencing for the detection of microorganisms present in human donor corneal preservation medium. BMJ Open Ophthalmol. 2019, 4, e000246. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Miller, S.; Chiu, C.Y. Clinical Metagenomic Next-Generation Sequencing for Pathogen Detection. Annu. Rev. Pathol. 2019, 14, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Egrilmez, S.; Yildirim-Theveny, S. Treatment-Resistant Bacterial Keratitis: Challenges and Solutions. Clin. Ophthalmol. 2020, 14, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Mah, F.S.; Davidson, R.; Holland, E.J.; Hovanesian, J.; John, T.; Kanellopoulos, J.; Shamie, N.; Starr, C.; Vroman, D.; Kim, T. Current knowledge about and recommendations for ocular methicillin-resistant Staphylococcus aureus. J. Cataract Refract. Surg. 2014, 40, 1894–1908. [Google Scholar] [CrossRef]
- Srinivasan, M.; Mascarenhas, J.; Rajaraman, R.; Ravindran, M.; Lalitha, P.; Glidden, D.V.; Ray, K.J.; Hong, K.C.; Oldenburg, C.E.; Lee, S.M.; et al. Corticosteroids for bacterial keratitis: The Steroids for Corneal Ulcers Trial (SCUT). Arch. Ophthalmol. 2012, 130, 143–150. [Google Scholar] [CrossRef]
- Cong, Y.; Yang, S.; Rao, X. Vancomycin resistant Staphylococcus aureus infections: A review of case updating and clinical features. J. Adv. Res. 2020, 21, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.C.; Hsiao, C.H.; Tan, H.Y.; Ma, D.H.; Lin, K.K.; Chang, C.J.; Huang, Y.C. Staphylococcus aureus ocular infection: Methicillin-resistance, clinical features, and antibiotic susceptibilities. PLoS ONE 2012, 8, e42437. [Google Scholar]
- Chuang, C.C.; Hsiao, C.H.; Tan, H.Y.; Ma, D.H.; Lin, K.K.; Chang, C.J.; Huang, Y.C. Staphylococcus aureus ocular infection: Methicillin-resistance, clinical features, and antibiotic susceptibilities. PLoS ONE 2012, 8, e42437. [Google Scholar] [CrossRef] [PubMed]
- Elsahn, A.F.; Yildiz, E.H.; Jungkind, D.L.; Abdalla, Y.F.; Erdurmus, M.; Cremona, F.A.; Rapuano, C.J.; Hammersmith, K.M.; Cohen, E.J. In Vitro Susceptibility Patterns of Methicillin-Resistant Staphylococcus aureus and Coagulase-Negative Staphylococcus Corneal Isolates to Antibiotics. Cornea 2010, 29, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, R.P.; Kowalski, T.A.; Shanks, R.M.; Romanowski, E.G.; Karenchak, L.M.; Mah, F.S. In vitro comparison of combination and monotherapy for the empiric and optimal coverage of bacterial keratitis based on incidence of infection. Cornea 2013, 32, 830–834. [Google Scholar] [CrossRef]
- Sueke, H.; Kaye, S.B.; Neal, T.; Hall, A.; Tuft, S.; Parry, C.M. An in vitro investigation of synergy or antagonism between antimicrobial combinations against isolates from bacterial keratitis. Invest. Ophthalmol. Vis. Sci. 2010, 51, 4151–4155. [Google Scholar] [CrossRef]
- Chojnacki, M.; Philbrick, A.; Wucher, B.; Reed, J.N.; Tomaras, A.; Dunman, P.M.; Wozniak, R.A. Development of a Broad-Spectrum Antimicrobial Combination for the Treatment of Staphylococcus aureus and Pseudomonas aeruginosa Corneal Infections. Antimicrob. Agents Chemother. 2019, 63, e01929-18. [Google Scholar] [CrossRef]
- Jiang, H.; Han, S.; Guo, C.; Liu, T.; Wu, X. In vitro and in vivo effectiveness evaluation of balofloxacin in experimental Staphylococcus aureus keratitis. J. Ocul. Pharmacol. Ther. 2014, 30, 482–488. [Google Scholar] [CrossRef]
- Goktas, S.; Kurtoglu, M.G.; Sakarya, Y.; Ugurluoglu, C.; Ozcimen, M.; Sakarya, R.; Alpfidan, I.; Ivacık, I.S.; Erdogan, E.; Bukus, A. New therapy option for treatment of methicillin-resistant Staphylococcus aureus keratitis: Tigecycline. J. Ocul. Pharmacol. Ther. 2015, 31, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Romanowski, E.G.; Kowalski, T.A.; O’Connor, K.E.; Yates, K.A.; Mah, F.S.; Shanks, R.M.; Kowalski, R.P. The In Vitro Evaluation of Tigecycline and the In Vivo Evaluation of RPX-978 (0.5% Tigecycline) as an Ocular Antibiotic. J. Ocul. Pharmacol. Ther. 2016, 32, 119–126. [Google Scholar] [CrossRef]
- DelMonte, D.W.; Kim, T. Anatomy and physiology of the cornea. J. Cataract Refract. Surg. 2011, 37, 588–598. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Das, P.J.; Adhikari, P.; Marbaniang, D.; Pal, P.; Ray, S.; Mazumder, B. Novel drug delivery systems for ocular therapy: With special reference to liposomal ocular delivery. Eur. J. Ophthalmol. 2019, 29, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Ustundag-Okur, N.; Gokce, E.H.; Egrilmez, S.; Ozer, O.; Ertan, G. Novel ofloxacin-loaded microemulsion formulations for ocular delivery. J. Ocul. Pharmacol. Ther. 2014, 30, 319–332. [Google Scholar] [CrossRef]
- Balzli, C.L.; McCormick, C.C.; Caballero, A.R.; Tang, A.; O’Callaghan, R.J. The effectiveness of an improved combination therapy for experimental Staphylococcus aureus keratitis. Adv. Ther. 2010, 27, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.G.; Xin, M.; Chen, H.; Yang, L.N.; Jiang, H.R. Novel mucoadhesive polysaccharide isolated from Bletilla striata improves the intraocular penetration and efficacy of levofloxacin in the topical treatment of experimental bacterial keratitis. J. Pharm. Pharm. 2010, 62, 1152–1157. [Google Scholar] [CrossRef]
- Ustundag-Okur, N.; Gokce, E.H.; Bozbiyik, D.I.; Egrilmez, S.; Ertan, G.; Ozer, O. Novel nanostructured lipid carrier-based inserts for controlled ocular drug delivery: Evaluation of corneal bioavailability and treatment efficacy in bacterial keratitis. Exp. Opin. Drug Deliv. 2015, 12, 1791–1807. [Google Scholar] [CrossRef]
- Abd El-bary, A.; Kamal Ibrahim, H.; Saeed Hazaa, B. Topical Drug Delivery for Effective Treatment of Bacterial Infections of the Anterior Segment of the Eye. Asian J. Pharm. Clin. Res. 2018, 11, 13–17. [Google Scholar] [CrossRef][Green Version]
- Mishra, G.P.; Bagui, M.; Tamboli, V.; Mitra, A.K. Recent applications of liposomes in ophthalmic drug delivery. J. Drug Deliv. 2011, 2011, 863734. [Google Scholar] [CrossRef]
- Kramer, A.; Eberlein, T.; Müller, G.; Dissemond, J.; Assadian, O. Re-evaluation of polihexanide use in wound antisepsis in order to clarify ambiguities of two animal studies. J. Wound Care 2019, 28, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Chrysouli, M.P.; Banti, C.N.; Milionis, I.; Koumasi, D.; Raptopoulou, C.P.; Psycharis, V.; Sainis, I.; Hadjikakou, S.K. A water-soluble silver(I) formulation as an effective disinfectant of contact lenses cases. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 93, 902–910. [Google Scholar] [CrossRef]
- Aveyard, J.; Deller, R.C.; Lace, R.; Williams, R.L.; Kaye, S.B.; Kolegraff, K.N.; Curran, J.M.; D’Sa, R.A. Antimicrobial Nitric Oxide Releasing Contact Lens Gels for the Treatment of Microbial Keratitis. ACS Appl. Mater. Interfaces 2019, 11, 37491–37501. [Google Scholar] [CrossRef] [PubMed]
- Hui, A.; Willcox, M.; Jones, L. In vitro and in vivo evaluation of novel ciprofloxacin-releasing silicone hydrogel contact lenses. Invest. Ophthalmol. Vis Sci. 2014, 55, 4896–4904. [Google Scholar] [CrossRef] [PubMed]
- Ubani-Ukoma, U.; Gibson, D.; Schultz, G.; Silva, B.O.; Chauhan, A. Evaluating the potential of drug eluting contact lenses for treatment of bacterial keratitis using an ex vivo corneal model. Int. J. Pharm. 2019, 565, 499–508. [Google Scholar] [CrossRef]
- Luo, L.J.; Lin, T.Y.; Yao, C.H.; Kuo, P.Y.; Matsusaki, M.; Harroun, S.G.; Huang, C.C.; Lai, J.Y. Dual-functional gelatin-capped silver nanoparticles for antibacterial and antiangiogenic treatment of bacterial keratitis. J. Colloid Interface Sci. 2019, 536, 112–126. [Google Scholar] [CrossRef]
- Upadhyay, S.U.; Chavan, S.K.; Gajjar, D.U.; Upadhyay, U.M.; Patel, J.K. Nanoparticles laden In situ gel for sustained drug release after topical ocular administration. J. Drug Deliv. Sci. Technol. 2020, 57, 101736. [Google Scholar]
- Long, Y.; Li, Z.; Bi, Q.; Deng, C.; Chen, Z.; Bhattachayya, S.; Li, C. Novel polymeric nanoparticles targeting the lipopolysaccharides of Pseudomonas aeruginosa. Int. J. Pharm. 2016, 502, 232–241. [Google Scholar] [CrossRef]
- Silva, N.C.; Sarmento, B.; Pintado, M. The importance of antimicrobial peptides and their potential for therapeutic use in ophthalmology. Int. J. Antimicrob. Agents. 2013, 41, 5–10. [Google Scholar] [CrossRef]
- Parmar, A.; Lakshminarayanan, R.; Iyer, A.; Mayandi, V.; Leng Goh, E.T.; Lloyd, D.G.; Chalasani, M.L.; Verma, N.K.; Prior, S.H.; Beuerman, R.W.; et al. Design and Syntheses of Highly Potent Teixobactin Analogues against Staphylococcus aureus, Methicillin-Resistant Staphylococcus aureus (MRSA), and Vancomycin-Resistant Enterococci (VRE) in Vitro and in Vivo. J. Med. Chem. 2018, 61, 2009–2017. [Google Scholar] [CrossRef]
- Clemens, L.E.; Jaynes, J.; Lim, E.; Kolar, S.S.; Reins, R.Y.; Baidouri, H.; Hanlon, S.; McDermott, A.M.; Woodburn, K.M. Designed Host Defense Peptides for the Treatment of Bacterial Keratitis. Invest. Ophthalmol. Vis Sci. 2017, 58, 6273–6281. [Google Scholar] [CrossRef]
- Venkatesh, M.; Barathi, V.A.; Goh, E.T.L.; Anggara, R.; Fazil, M.; Ng, A.J.Y.; Harini, S.; Aung, T.T.; Fox, S.J.; Liu, S.; et al. Antimicrobial Activity and Cell Selectivity of Synthetic and Biosynthetic Cationic Polymers. Antimicrob. Agents Chemother. 2017, 61, e00469-17. [Google Scholar] [CrossRef]
- Kowalski, R.P.; Romanowski, E.G.; Yates, K.A.; Mah, F.S. An Independent Evaluation of a Novel Peptide Mimetic, Brilacidin (PMX30063), for Ocular Anti-infective. J. Ocul. Pharmacol. Ther. 2016, 32, 23–27. [Google Scholar] [CrossRef] [PubMed]
- O’Brart, D.P. Corneal collagen cross-linking: A review. J. Optom. 2014, 7, 113–124. [Google Scholar] [CrossRef]
- Tayapad, J.B.; Viguilla, A.Q.; Reyes, J.M. Collagen cross-linking and corneal infections. Curr. Opin. Ophthalmol. 2013, 24, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Richoz, O.; Kling, S.; Hoogewoud, F.; Hammer, A.; Tabibian, D.; Francois, P.; Schrenzel, J.; Hafezi, F. Antibacterial efficacy of accelerated photoactivated chromophore for keratitis-corneal collagen cross-linking (PACK-CXL). J. Refract. Surg. 2014, 30, 850–854. [Google Scholar] [CrossRef] [PubMed]
- Halili, F.; Arboleda, A.; Durkee, H.; Taneja, M.; Miller, D.; Alawa, K.A.; Aguilar, M.C.; Amescua, G.; Flynn, H.W., Jr.; Parel, J.M. Rose Bengal- and Riboflavin-Mediated Photodynamic Therapy to Inhibit Methicillin-Resistant Staphylococcus aureus Keratitis Isolates. Am. J. Ophthalmol. 2016, 166, 194–202. [Google Scholar] [CrossRef]
- Su, G.; Wei, Z.; Wang, L.; Shen, J.; Baudouin, C.; Labbé, A.; Liang, Q. Evaluation of Toluidine Blue-Mediated Photodynamic Therapy for Experimental Bacterial Keratitis in Rabbits. Transl. Vis. Sci. Technol. 2020, 9, 13. [Google Scholar] [CrossRef]
- Kilic, B.B.; Altiors, D.D.; Demirbilek, M.; Ogus, E. Comparison between corneal cross-linking, topical antibiotic and combined therapy in experimental bacterial keratitis model. Saudi J. Ophthalmol. 2018, 32, 97–104. [Google Scholar] [CrossRef]
- Rapuano, P.B.; Scanameo, A.H.; Amponin, D.E.; Paulose, S.A.; Zyablitskaya, M.; Takaoka, A.; Suh, L.H.; Nagasaki, T.; Trokel, S.L.; Paik, D.C. Antimicrobial Studies Using the Therapeutic Tissue Cross-Linking Agent, Sodium Hydroxymethylglycinate: Implication for Treating Infectious Keratitis. Invest. Ophthalmol. Vis Sci. 2018, 59, 332–337. [Google Scholar] [CrossRef]
- Iseli, H.P.; Thiel, M.A.; Hafezi, F.; Kampmeier, J.; Seiler, T. Ultraviolet A/Riboflavin Corneal Cross-linking for Infectious Keratitis Associated With Corneal Melts. Cornea 2008, 27, 590–594. [Google Scholar] [CrossRef]
- Makdoumi, K.; Mortensen, J.; Crafoord, S. Infectious keratitis treated with corneal crosslinking. Cornea 2010, 29, 1353–1358. [Google Scholar] [CrossRef]
- Morén, H.; Malmsjö, M.; Mortensen, J.; Ohrström, A. Riboflavin and ultraviolet a collagen crosslinking of the cornea for the treatment of keratitis. Cornea 2010, 29, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.A.; Bovelle, R.; Han, G.; Kwagyan, J. Corneal collagen cross-linking for bacterial infectious keratitis. Cochrane Database Syst. Rev. 2020, 6, CD013001. [Google Scholar] [PubMed]
- Prajna, N.V.; Radhakrishnan, N.; Lalitha, P.; Rajaraman, R.; Narayana, S.; Austin, A.F.; Porco, T.C.; Lietman, T.M.; Rose-Nussbaumer, J. Cross-Linking Assisted Infection Reduction (CLAIR): A Randomized Clinical Trial Evaluating the Effect of Adjuvant Cross-Linking on Bacterial Keratitis. Cornea 2020, 00, 1–5. [Google Scholar]
- Reitberger, H.H.; Czugala, M.; Chow, C.; Mohr, A.; Burkovski, A.; Gruenert, A.K.; Schoenebeck, R.; Fuchsluger, T..A. Argon Cold Plasma-A Novel Tool to Treat Therapy-resistant Corneal Infections. Am. J. Ophthalmol. 2018, 190, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Ishida, W.; Uchiyama, J.; Rashel, M.; Kato, S.; Morita, T.; Muraoka, A.; Sumi, T.; Matsuzaki, S.; Daibata, M.; et al. Pseudomonas aeruginosa keratitis in mice: Effects of topical bacteriophage KPP12 administration. PLoS ONE 2012, 7, e47742. [Google Scholar] [CrossRef] [PubMed]
- Fadlallah, A.; Chelala, E.; Legeais, J. Corneal Infection Therapy with Topical Bacteriophage Administration. Open Ophthalmol. J. 2015, 9, 167–168. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Bukhari, S.; Andleeb, S.; Ali, M.; Raza, S.; Nawaz, M.A.; Hussain, T.; Rahman, S.U.; Shah, S.S. Bacteriophages: An overview of the control strategies against multiple bacterial infections in different fields. J. Basic Microbiol. 2019, 59, 123–133. [Google Scholar] [CrossRef] [PubMed]


| Peptide/Protein Name | Classification | Mechanism of Action | Study |
|---|---|---|---|
| LyeTxI-b | Synthetic peptide derived from Lycosa erythrognatha spider venom | Alters permeabilisation and forms pores within bacterial membrane | Silva et al. (2019) [7] |
| D-Arg4-Leu10-Teixobactin | Analogue of teixobactin, a cyclic depsipeptide | Binds to pyrophosphate motifs of bacterial cell-wall substrates, such as lipid II (precursor of peptidoglycan), and lipid III (precursor of teichoic acid) | Parmar et al. (2018) [80] |
| RP442, RP443, RP444 | Designed host-defence peptide | Electrostatic interactions with bacterial membrane | Clemens et al. (2017) [81] |
| poly-ε-lysine | Biosynthetic polymer | Induces a loss in membrane potential | Venkatesh et al. (2017) [82] |
| Brilacidin (PMX30063) | Defensin mimetic | Membrane depolarisation | Kowalski et al. (2016) [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.W.; Somerville, T.; Kaye, S.B.; Romano, V. Staphylococcus aureus Keratitis: Incidence, Pathophysiology, Risk Factors and Novel Strategies for Treatment. J. Clin. Med. 2021, 10, 758. https://doi.org/10.3390/jcm10040758
Lee JW, Somerville T, Kaye SB, Romano V. Staphylococcus aureus Keratitis: Incidence, Pathophysiology, Risk Factors and Novel Strategies for Treatment. Journal of Clinical Medicine. 2021; 10(4):758. https://doi.org/10.3390/jcm10040758
Chicago/Turabian StyleLee, Jason W., Tobi Somerville, Stephen B. Kaye, and Vito Romano. 2021. "Staphylococcus aureus Keratitis: Incidence, Pathophysiology, Risk Factors and Novel Strategies for Treatment" Journal of Clinical Medicine 10, no. 4: 758. https://doi.org/10.3390/jcm10040758
APA StyleLee, J. W., Somerville, T., Kaye, S. B., & Romano, V. (2021). Staphylococcus aureus Keratitis: Incidence, Pathophysiology, Risk Factors and Novel Strategies for Treatment. Journal of Clinical Medicine, 10(4), 758. https://doi.org/10.3390/jcm10040758






