Tie-2, G-CSF, and Leptin as Promising Diagnostic Biomarkers for Endometrial Cancer: A Pilot Study
Abstract
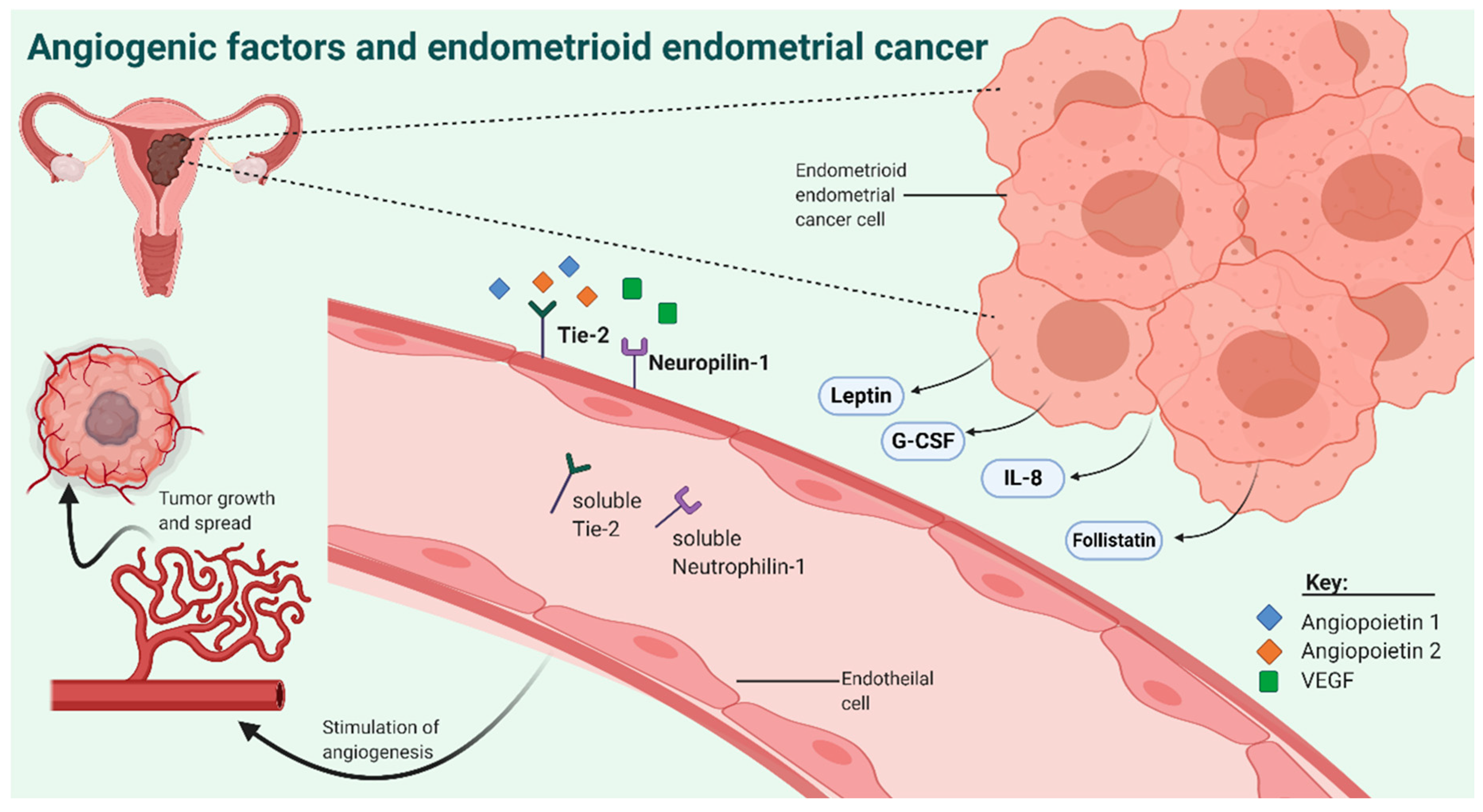
:1. Introduction
2. Experimental Section
2.1. Patient Enrolment
2.2. Measurements of AFs
2.3. Statistics
3. Results
3.1. Characteristics of the EC Patients and Control Patients
3.2. Levels of AFs in EC and Control Patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Constantine, G.D.; Kessler, G.; Graham, S.; Goldstein, S.R. Increased incidence of endometrial cancer following the women’s health initiative: An assessment of risk factors. J. Women’s Heal. 2019, 28, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Felix, A.S.; Brinton, L.A. Cancer progress and priorities: Uterine cancer. Biomarkers Prev. 2018, 27, 985–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voss, M.A.; Ganesan, R.; Ludeman, L.; McCarthy, K.; Gornall, R.; Schaller, G.; Wei, W.; Sundar, S. Should grade 3 endometrioid endometrial carcinoma be considered a type 2 cancer—A clinical and pathological evaluation. Gynecol. Oncol. 2012, 124, 15–20. [Google Scholar] [CrossRef]
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef]
- Tesfamariam Sengal, A. Editorial updates in classification and pathogenesis of endometrial cancer. Cronicon 2017, 5, 115–117. [Google Scholar]
- Murali, R.; Soslow, R.A.; Weigelt, B. Classification of endometrial carcinoma: More than two types. Lancet Oncol. 2014, 15, e268–e278. [Google Scholar] [CrossRef]
- Oberndorfer, F.; Moling, S.; Hagelkruys, L.; Grimm, C.; Polterauer, S.; Sturdza, A.; Aust, S.; Reinthaller, A.; Müllauer, L.; Schwameis, R. Risk reclassification of patients with endometrial cancer based on tumor molecular profiling: First real world data. J. Pers. Med. 2021, 11, 48. [Google Scholar] [CrossRef]
- Soslow, R.A.; Tornos, C.; Park, K.J.; Malpica, A.; Matias-Guiu, X.; Oliva, E.; Parkash, V.; Carlson, J.; McCluggage, W.G.; Gilks, C.B. Endometrial carcinoma diagnosis. Int. J. Gynecol. Pathol. 2019, 38, S64–S74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colombo, N.; Creutzberg, C.L.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; A Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: Diagnosis, treatment and follow-up. J. Gynecol. Cancer 2016, 26, 2–30. [Google Scholar] [CrossRef] [Green Version]
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial cancer. Proc. Lancet 2016, 387, 1094–1108. [Google Scholar] [CrossRef]
- Soslow, R. High-grade endometrial carcinomas - strategies for typing. Histopathology 2013, 62, 89–110. [Google Scholar] [CrossRef] [PubMed]
- Frumovitz, M.; Singh, D.K.; Meyer, L.; Smith, D.H.; Wertheim, I.; Resnik, E.; Bodurka, D.C. Predictors of final histology in patients with endometrial cancer. Gynecol. Oncol. 2004, 95, 463–468. [Google Scholar] [CrossRef]
- Larson, D.M.; Johnson, K.K.; Broste, S.K.; Krawisz, B.R.; Kresl, J.J. Comparison of D&C and office endometrial biopsy in predicting final histopathologic grade in endometrial cancer. Obstet. Gynecol. 1995, 86, 38–42. [Google Scholar] [CrossRef]
- Suh-Burgmann, E.; Hung, Y.Y.; Armstrong, M.A. Complex atypical endometrial hyperplasia: The risk of unrecognized adenocarcinoma and value of preoperative dilation and curettage. Obstet. Gynecol. 2009, 114, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Knific, T.; Vouk, K.; Smrkolj, Š.; Prehn, C.; Adamski, J.; Rižner, T.L. Models including plasma levels of sphingomyelins and phosphatidylcholines as diagnostic and prognostic biomarkers of endometrial cancer. J. Steroid Biochem. Mol. Biol. 2018, 178, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Kamel, H.F.M.; Al-Amodi, H.S.A.B. Exploitation of Gene Expression and Cancer Biomarkers in Paving the Path to Era of Personalized Medicine. Genom. Proteom. Bioinform. 2017, 15, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Sliwinska, P.; Alitalo, K.; Allen, E.; Anisimov, A.; Aplin, A.C.; Auerbach, R.; Augustin, H.G.; Bates, D.O.; van Beijnum, J.R.; Bender, R.H.F.; et al. Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis 2018, 21, 425–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nat. Cell Biol. 2000, 407, 249–257. [Google Scholar] [CrossRef]
- Hillen, F.; Griffioen, A.W. Tumour vascularization: Sprouting angiogenesis and beyond. Cancer Metastasis Rev. 2007, 26, 489–502. [Google Scholar] [CrossRef] [Green Version]
- Sherwood, L.M.; Parris, E.E.; Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef]
- Ucuzian, A.A.; Gassman, A.A.; East, A.T.; Greisler, H.P. Molecular mediators of angiogenesis. J. Burn Care Res. 2010, 31, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Tas, F.; Duranyildiz, D.; Oguz, H.; Camlica, H.; Yasasever, V.; Topuz, E. Circulating serum levels of angiogenic factors and vascular endothelial growth factor receptors 1 and 2 in melanoma patients. Melanoma Res. 2006, 16, 405–411. [Google Scholar] [CrossRef]
- Szarvas, T.; Jäger, T.; Droste, F.; Becker, M.; Kovalszky, I.; Romics, I.; Ergun, S.; Rübben, H. Serum levels of angiogenic factors and their prognostic relevance in bladder cancer. Pathol. Oncol. Res. 2008, 15, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Papa, A.; Zaccarelli, E.; Caruso, D.; Vici, P.; Panici, P.B.; Tomao, F. Targeting angiogenesis in endometrial cancer—New agents for tailored treatments. Expert Opin. Investig. Drugs 2015, 25, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Kilgore, L.C.; Partridge, E.E.; Alvarez, R.D.; Austin, J.; Shingleton, H.M.; Noojin, F.; Conner, W. Adenocarcinoma of the endometrium: Survival comparisons of patients with and without pelvic node sampling. Gynecol. Oncol. 1995, 56, 29–33. [Google Scholar] [CrossRef]
- Todo, Y.; Yamamoto, R.; Minobe, S.; Suzuki, Y.; Takeshi, U.; Nakatani, M.; Aoyagi, Y.; Ohba, Y.; Okamoto, K.; Kato, H. Risk factors for postoperative lower-extremity lymphedema in endometrial cancer survivors who had treatment including lymphadenectomy. Gynecol. Oncol. 2010, 119, 60–64. [Google Scholar] [CrossRef]
- Knific, T.; Osredkar, J.; Smrkolj, Š.; Tonin, I.; Vouk, K.; Blejec, A.; Grazio, S.F.; Rižner, T.L. Novel algorithm including CA-125, HE4 and body mass index in the diagnosis of endometrial cancer. Gynecol. Oncol. 2017, 147, 126–132. [Google Scholar] [CrossRef]
- Amant, F.; Mirza, M.R.; Koskas, M.; Creutzberg, C.L. Cancer of the corpus uteri. Int. J. Gynecol. Obstet. 2018, 143, 37–50. [Google Scholar] [CrossRef] [Green Version]
- Crean-Tate, K.K.; Reizes, O. Leptin regulation of cancer stem cells in breast and gynecologic cancer. Endocrinology 2018, 159, 3069–3080. [Google Scholar] [CrossRef] [PubMed]
- Żyła, M.M.; Kostrzewa, M.; Litwińska, E.; Szpakowski, A.; Wilczyński, J.R.; Stetkiewicz, T. The role of angiogenic factors in endometrial cancer. Menopausal Rev. 2014, 13, 122–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, K.K.; Varney, M.L.; Dave, B.J.; Bucana, C.D.; Fidler, I.J.; Singh, R.K. Expression of interleukin-8 in human metastatic endometrial carcinoma cells and its regulation by inflammatory cytokines. Int. J. Gynecol. Cancer 2001, 11, 54–60. [Google Scholar] [CrossRef]
- Myśliwiec, P.; Pawlak, K.; Kukliński, A.; Kedra, B. Combined perioperative plasma endoglin and VEGF-A assessment in colorectal cancer patients. Folia Histochem. Cytobiol. 2008, 46, 487–492. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, N. VEGF as a therapeutic target in cancer. Oncology 2005, 69, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Melincovici, C.S.; Boşca, A.B.; Şuşman, S.; Mărginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular endothelial growth factor (VEGF) – key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467. [Google Scholar] [PubMed]
- Siveen, K.S.; Prabhu, K.; Krishnankutty, R.; Kuttikrishnan, S.; Tsakou, M.; Alali, F.Q.; Dermime, S.; Mohammad, R.M.; Uddin, S. Vascular endothelial growth factor (VEGF) dignaling in tumour vascularization: Potential and challenges. Curr. Vasc. Pharmacol. 2017, 15, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Sato, Y.; Watanabe, J.; Kuramoto, H.; Kaba, S.; Fukuda, T. Angiogenic factors in normal endometrium and endometrial adenocarcinoma. Pathol. Int. 2007, 57, 140–147. [Google Scholar] [CrossRef]
- Moller, B.; Rasmussen, C.; Lindblom, B.; Olovsson, M. Expression of the angiogenic growth factors VEGF, FGF-2, EGF and their receptors in normal human endometrium during the menstrual cycle. Mol. Hum. Repord. 2001, 7, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamat, A.A.; Merritt, W.M.; Coffey, N.; Lin, Y.G.; Patel, P.R.; Broaddus, R.; Nugent, E.; Han, L.Y.; Landen, C.N.; Spannuth, W.A.; et al. Clinical and biological significance of vascular endothelial growth factor in endometrial cancer. Clin. Cancer Res. 2007, 13, 7487–7495. [Google Scholar] [CrossRef] [Green Version]
- Fujimoto, J.; Ichigo, S.; Hirose, R.; Sakaguchi, H.; Tamaya, T. Expressions of vascular endothelial growth factor (VEGF) and its mRNA in uterine endometrial cancers. Cancer Lett. 1998, 134, 15–22. [Google Scholar] [CrossRef]
- Shaarawy, M.; El-Sharkawy, S.A. Biomarkers of intrinsic angiogenic and anti-angiogenic activity in patients with endometrial hyperplasia and endometrial cancer. Acta Oncol. 2001, 40, 513–518. [Google Scholar] [CrossRef]
- Okon, I.S.; Ding, Y.; Coughlan, K.A.; Wang, Q.; Song, P.; Benbrook, D.M.; Zou, M.H. Aberrant NRP-1 expression serves as predicator of metastatic endometrial and lung cancers. Oncotarget 2015, 7, 7970–7978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oplawski, M.; Dziobek, K.; Grabarek, B.; Zmarzły, N.; Dabrus, D.; Januszyk, P.; Brus, R.; Tomala, B.; Boron, D. Expression of NRP-1 and NRP-2 in endometrial cancer. Curr. Pharm. Biotechnol. 2019, 20, 254–260. [Google Scholar] [CrossRef]
- Ren, P.; Chen, F.-F.; Liu, H.-Y.; Cui, X.-L.; Sun, Y.; Guan, J.-L.; Liu, Z.-H.; Liu, J.-G.; Wang, Y.-N. High serum levels of follistatin in patients with ovarian cancer. J. Int. Med. Res. 2012, 40, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Ciarmela, P.; Florio, P.; Sigurdardottir, M.; Toti, P.; Maguer-Satta, V.; Rimokh, R.; Altomare, A.; Tosi, P.; Petraglia, F. Follistatin-related gene expression, but not follistatin expression, is decreased in human endometrial adenocarcinoma. Eur. J. Endocrinol. 2004, 151, 251–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhlén, M.; Björling, E.; Agaton, C.; Szigyarto, C.A.-K.; Amini, B.; Andersen, E.; Andersson, A.-C.; Angelidou, P.; Asplund, A.; Asplund, C.; et al. A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol. Cell. Proteom. 2005, 4, 1920–1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fagiani, E.; Christofori, G. Angiopoietins in angiogenesis. Cancer Lett. 2013, 328, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Augustin, H.G. The role of the angiopoietins in vascular morphogenesis. Angiogenesis 2009, 12, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Liu, D.; Fueyo, J.; Gomez-Manzano, C. Tie2: A journey from normal angiogenesis to cancer and beyond. Histol. Histopathol. 2008, 23, 773–780. [Google Scholar] [PubMed]
- Kelesidis, T.; Kelesidis, I.; Chou, S.; Mantzoros, C.S. Narrative review: The role of leptin in human physiology: Emerging clinical applications. Ann. Intern. Med. 2010, 152, 93. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-Y.; Kwon, H.M.; Lim, H.J.; Hong, B.K.; Lee, J.Y.; Park, B.E.; Jang, Y.S.; Cho, S.Y.; Kim, H.-S. Potential role of leptin in angiogenesis: Leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro. Exp. Mol. Med. 2001, 33, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Mabuchi, S.; Yamasaki, M.; Yoshimura, M.; Murata, Y. Grave outcome of granulocyte colony-stimulating factor-producing endometrial cancer: A case report and literature review. J. Obstet. Gynaecol. Res. 2012, 39, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Mikami, M.; Tanaka, K.; Komiyama, S.; Ishikawa, M.; Hirose, T. Primary serous carcinoma of the peritoneum producing granulocyte colony-stimulating factor. Acta Obstet. Gynecol. Scand. 2005, 84, 820–822. [Google Scholar] [CrossRef] [PubMed]
- Granger, J.M.; Kontoyiannis, D.P. Etiology and outcome of extreme leukocytosis in 758 nonhematologic cancer patients. Cancer 2009, 115, 3919–3923. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.H.; Moll, S.; Houghton, D.; O’Connor, S.; Soper, J.T. Leukocytosis due to markedly elevated granulocyte-colony stimulating factor levels in a patient with endometrial cancer: Case report and literature review. Gynecol. Oncol. Rep. 2017, 20, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Huang, L.; Zhang, S. Preoperative serum CA125: A useful marker for surgical management of endometrial cancer. BMC Cancer 2015, 15, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modarres-Gilani, M.; Vaezi, M.; Shariat, M.; Zamani, N.; Nourizadeh, R. The prognostic role of preoperative serum CA125 levels in patients with advanced endometrial carcinoma. Cancer Biomarkers 2017, 20, 135–141. [Google Scholar] [CrossRef]
- Romera, A.E.; Guardiola, T.C.; Vielba, M.B.; Torralba, C.D.B.; Martín, P.J.C.; Mainar, L.B. HE 4 tumor marker as a predictive factor for lymphatic metastasis in endometrial cancer. Int. J. Gynecol. Obstet. 2020, 149, 265–268. [Google Scholar] [CrossRef]


| Control Patients n = 38 (100%) | EC Patients n = 38 (100%) | pa Values | ||
|---|---|---|---|---|
| Age category | 50–59.9 years | 10 (26.3) | 11 (28.9) | ns |
| 60–69.9 years | 12 (31.6) | 14 (36.8) | ||
| 70–79.9 years | 15 (39.5) | 12 (31.6) | ||
| >80 years | 1 (2.6) | 1 (2.6) | ||
| Body mass index (kg/m2) | 18.6–24.9 (normal weight) | 10 (26.3) | 4 (10.5) | 0.001 |
| 25–29.9 (overweight) | 16 (42.1) | 15 (39.5) | ||
| 30–34.9 (class I obesity) | 6 (15.8) | 9 (23.7) | ||
| 35–39.9 (class II obesity) | 1 (2.6) | 6 (15.8) | ||
| 40–49.9 (class III obesity) | 0 (0) | 4 (10.5) | ||
| Missing data | 5 (13.2) | 0 (0) | ||
| Smoking status | Nonsmoker | 29 (76.3) | 30 (78.9) | ns |
| Smoker | 2 (5.3) | 3 (7.9) | ||
| Occasional smoker | 2 (5.3) | 2 (5.3) | ||
| Former smoker | 3 (7.9) | 3 (7.9) | ||
| Missing data | 2 (5.3) | 0 (0) | ||
| Hormonal therapy in the past | No | 21 (55.3) | 25 (65.8) | ns |
| Yes | 3 (7.9) | 1 (2.6) | ||
| Missing | 14 (36.8) | 12 (31.6) | ||
| Peroral contraception in the past | No | 18 (47.4) | 16 (42.1) | ns |
| Yes | 7 (18.4) | 7 (18.4) | ||
| Missing | 13 (34.2) | 15 (39.5) | ||
| Medication intake in last 7 days | No | 0 (0) | 1 (2.6) | ns |
| Yes | 33 (86.8) | 28 (73.7) | ||
| Missing data | 5 (13.2) | 9 (23.7) |
| Control Patients n = 38 | EC Patients n = 38 | pa Values (<0.05) | |||
|---|---|---|---|---|---|
| Median | Range | Median | Range | ||
| angiostatin | 68,762.7 | 26,019.6–131,674.6 | 68,968.4 | 10,337.8–133,835.1 | |
| sAXL | 1352.0 | 447.3–2214.6 | 1400.2 | 487.3–1932.0 | |
| sc-Kit/SCFR | 25,473.2 | 6497.9–44,838.5 | 27,755.8 | 6382.0–47,182.2 | |
| sHer2 | 4686.2 | 2750.8–6958.6 | 4589.5 | 2247.6–7616.4 | |
| sHer3 | 5310.9 | 735.3–7160.7 | 4921.2 | 2174.1–8658.4 | |
| sE-Selectin | 88,127.2 | 42,351.2–164,728.6 | 84,245.3 | 35,178.1–132,759.8 | |
| sHGFR/c-Met | 39,388.4 | 12,640.9–86,504.3 | 38,925.6 | 22,210.5–74,766.1 | |
| Tenascin C | 11,069.2 | 1242.1–22,237.3 | 10,313.6 | 1179.9–18,276.6 | |
| PDGF-AB/BB | 921.7 | 266.2–2355.9 | 914.4 | 338.5–9049.4 | |
| sIL-6Ralpha | 26,213.5 | 8505.9–40,310.4 | 27,467.1 | 5581.6–42,348.2 | |
| sTie-2 | 9862.9 | 2465.8–16,502.5 | 8191.4 | 5314.6–13,762.4 | 0.0218 |
| Thrombospondin-2 | 8696.5 | 1347.0–26,885.0 | 7591.7 | 910.6–18,021.5 | |
| sNeuropilin-1 | 387,531.5 | 55,935.1–916,924.7 | 459,548.5 | 81,804.2–775,122.2 | |
| sEGFR | 1157.7 | 321.9–1949.8 | 1212.5 | 386.9–2136.5 | |
| suPAR | 9886.1 | 3055.3–18,468.5 | 10,253.5 | 4365.7–16,059.8 | |
| sVEGFR1 | 1049.8 | 114.8–2381.0 | 979.1 | 142.4–2116.0 | |
| sVEGFR3 | 14,534.9 | 1908.7–28,017.4 | 12,534.2 | 546.7–26,452.6 | |
| sPECAM-1 | 5301.3 | 1907.4–7014.2 | 4814.0 | 1364.5–7573.7 | |
| Osteopontin | 4620.1 | 1745.7–9509.6 | 4037.6 | 1721.5–9583.0 | |
| sVEGFR2 | 12,044.6 | 6781.0–17,613.6 | 12,520.4 | 6115.8–18,684.0 | |
| Angiopoietin-2 | 2442.0 | 594.3–7937.8 | 2209.4 | 733.3–5191.5 | |
| BMP-9 | 103.2 | 7.9–244.7 | 110.1 | 19.2–847.6 | |
| Endoglin | 1741.2 | 589.4–3370.5 | 1730.9 | 364.4–2808.5 | |
| Follistatin | 1054.8 | 190.3–1901.1 | 1032.3 | 153.4–2692.5 | |
| G-CSF | 214.8 c | 29.0–578.6 | 158.1 c | 16.8–384.3 | 0.0175 |
| HB-EGF | 40.9 | 2.8–169.3 | 32.4 | 8.0–153.0 | |
| HGF | 221.1 | 127.8–458.6 | 277.2 | 93.4–589.1 | |
| Leptin | 40,208.9 | 12,646.1–12,7802.3 | 50,185.7 | 10,869.2–240,758.2 | 0.0451 |
| VEGF-C | 911.7 | 350.6–3110.3 | 881.6 | 169.4–2508.0 | |
| VEGF-D | 151.9 | 3.7–481.9 | 150.1 | 27.4–469.8 | |
| EGF | 21.8 d | 1.5–119.1 | 21.8 d | 1.1–70.4 | |
| Endothelin-1 b | - | - | - | - | |
| FGF-1 b | - | - | - | - | |
| FGF-2 | 120.4 | 65.8–240.7 | 120.4 | 65.8–240.7 | |
| IL-8 | 4.2 | 0.6–21.2 | 4.4 | 1.7–14.3 | |
| PLGF | 8.6 | 1.8–36.9 | 7.1 | 1.8–265.8 | |
| VEGF-A | 67.4 | 6.8–771.8 | 77.9 | 6.8–225.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roškar, L.; Klančič, T.; Knific, T.; Rižner, T.L.; Smrkolj, Š. Tie-2, G-CSF, and Leptin as Promising Diagnostic Biomarkers for Endometrial Cancer: A Pilot Study. J. Clin. Med. 2021, 10, 765. https://doi.org/10.3390/jcm10040765
Roškar L, Klančič T, Knific T, Rižner TL, Smrkolj Š. Tie-2, G-CSF, and Leptin as Promising Diagnostic Biomarkers for Endometrial Cancer: A Pilot Study. Journal of Clinical Medicine. 2021; 10(4):765. https://doi.org/10.3390/jcm10040765
Chicago/Turabian StyleRoškar, Luka, Teja Klančič, Tamara Knific, Tea Lanišnik Rižner, and Špela Smrkolj. 2021. "Tie-2, G-CSF, and Leptin as Promising Diagnostic Biomarkers for Endometrial Cancer: A Pilot Study" Journal of Clinical Medicine 10, no. 4: 765. https://doi.org/10.3390/jcm10040765






