Expression of VEGF in Peripheral Serum Is a Possible Prognostic Factor in Bone-Regeneration via Masquelet-Technique—A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Retrieval and Measurement of Serum Cytokines
2.3. Clinical and Radiological Patient Outcome
2.4. Data Analysis
2.5. Statistics
3. Results
3.1. Study Group 1: Responders
3.2. Study Group 2: Non-Responder
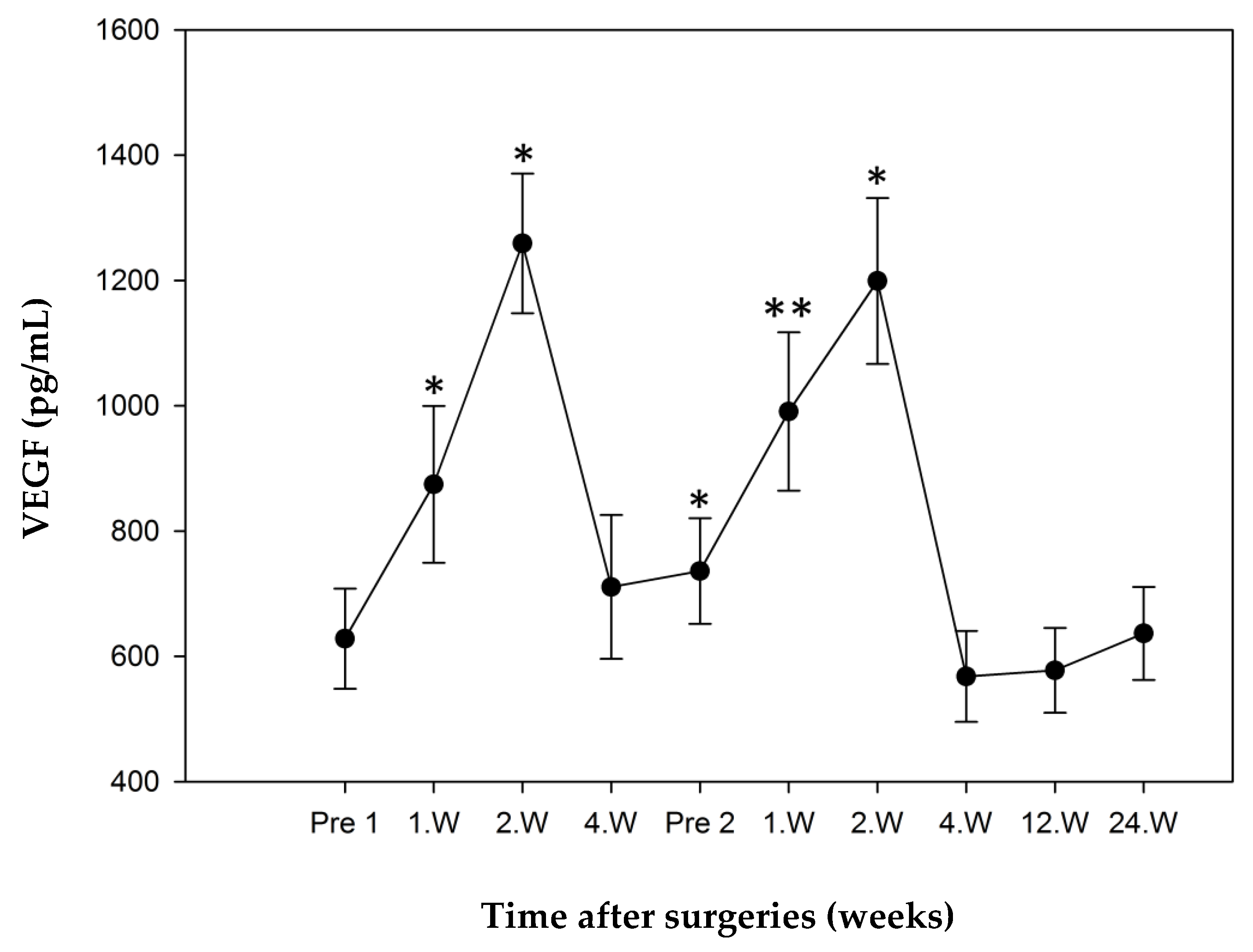
3.3. Serum Cytokine Levels
3.3.1. Study Group 1: Responders
3.3.2. Study Group 2: Non-Responder
3.3.3. Comparison of “Responders” and “Non-Responders”.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Einhorn, T.A. The Cell and Molecular Biology of Fracture Healing. Clin. Orthop. Relat. Res. 1998, 355, S7–S21. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, A.; Weiss, S.; Wölfl, C.; Schmeckenbecher, K.; Wentzensen, A.; Grützner, P.; Zimmermann, G. Cigarette smoking decreases TGF-β1 serum concentrations after long bone fracture. Injury 2010, 41, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Rothman, R.H.; Klemek, J.S.; Toton, J.J. The effect of iron deficiancy anaemia on fracture healing. Clin. Orthop. Relat. Res. 1971, 77, 276–283. [Google Scholar]
- Gaston, M.; Simpson, A.H.R.W. Inhibition of fracture healing. J. Bone Jt. Surgery. Br. Vol. 2007, 89, 1553–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, L.A.; Simpson, A.H.R. The relative incidence of fracture non-union in the Scottish population (5.17 million): A 5-year epidemiological study. BMJ Open 2013, 3. [Google Scholar] [CrossRef] [Green Version]
- Hak, D.J.; Fitzpatrick, D.C.; Bishop, J.A.; Marsh, J.L.; Tilp, S.; Schnettler, R.; Simpson, H.; Alt, V. Delayed union and nonunions: Epidemiology, clinical issues, and financial aspects. Injury 2014, 45, S3–S7. [Google Scholar] [CrossRef]
- Tay, W.-H.; De Steiger, R.; Richardson, M.; Gruen, R.; Balogh, Z.J. Health outcomes of delayed union and nonunion of femoral and tibial shaft fractures. Injury 2014, 45, 1653–1658. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Einhorn, T.A.; Marsh, D. Fracture healing: The diamond concept. Injury 2007, 38, S3–S6. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Einhorn, T.A.; Schmidmaier, G.; Marsh, D. The diamond concept-open questions. Injury 2008, 39, S5–S8. [Google Scholar] [CrossRef]
- Calori, G.M.; Giannoudis, P.V. Enhancement of fracture healing with the diamond concept: The role of the biological chamber. Injury 2011, 42, 1191–1193. [Google Scholar] [CrossRef]
- Chong, K.W.; Woon, C.Y.L.; Wong, M.K. Induced Membranes—A Staged Technique of Bone-Grafting for Segmental Bone Loss: Surgical Technique. JBJS Essent. Surg. Tech. 2011, 93, 85–91. [Google Scholar] [CrossRef]
- Aurégan, J.-C.; Bégué, T. Induced membrane for treatment of critical sized bone defect: A review of experimental and clinical experiences. Int. Orthop. 2014, 38, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, N.T.; Kates, S.L. Advances on the Masquelet technique using a cage and nail construct. Arch. Orthop. Trauma Surg. 2011, 132, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Masquelet, A.-C.; Fitoussi, F.; Begue, T.; Muller, G.P. Reconstruction of the long bones by the induced membrane and spongy autograft. Ann. Chir. Plast. Esthet. 2000, 45, 346–353. [Google Scholar] [PubMed]
- Pelissier, P.H.; Masquelet, A.C.; Bareille, R.; Pelissier, S.M.; Amedee, J. Induced membranes secrete growth factors including vascular and osteoinductive factors and could stimulate bone regeneration. J. Orthop. Res. 2004, 22, 73–79. [Google Scholar] [CrossRef]
- Viateau, V.; Guillemin, G.; Calando, Y.; Logeart, D.; Oudina, K.; Sedel, L.; Hannouche, D.; Bousson, V.; Petite, H. Induction of a Barrier Membrane to Facilitate Reconstruction of Massive Segmental Diaphyseal Bone Defects: An Ovine Model. Vet. Surg. 2006, 35, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Pélissier, P.; Lefevre, Y.; Delmond, S.; Villars, F.; Vilamitjana-Amedee, J. Influence des membranes induites sur l’ostéogenèse hétérotopique au sein d’un complexe ostéo-inducteur. Étude expérimentale chez le lapin. Ann. Chir. Plast. Esthét. 2009, 54, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Klaue, K.; Knothe, U.; Anton, C.; Pfluger, D.H.; Stoddart, M.; Masquelet, A.C.; Perren, S.M. Bone regeneration in long-bone defects: Tissue compartmentalisation? In vivo study on bone defects in sheep. Injury 2009, 40, S95–S102. [Google Scholar] [CrossRef]
- Pelissier, P.; Bollecker, V.; Martin, D.; Baudet, J. Reconstruction du pied per la technique “bi-Masquelet”. Ann. Chir. Plast. Esthét. 2002, 47, 304–307. [Google Scholar] [CrossRef]
- Powerski, M.; Maier, B.; Frank, J.; Marzi, I. Treatment of severe osteitis after elastic intramedullary nailing of a radial bone shaft fracture by using cancellous bone graft in Masquelet technique in a 13-year-old adolescent girl. J. Pediatr. Surg. 2009, 44, e17–e19. [Google Scholar] [CrossRef]
- Largey, A.; Faline, A.; Hebrard, W.; Hamoui, M.; Canovas, F. Management of massive traumatic compound defects of the foot. Orthop. Traumatol. Surg. Res. 2009, 95, 301–304. [Google Scholar] [CrossRef] [Green Version]
- Biau, D.J.; Pannier, S.; Masquelet, A.C.; Glorion, C. Case Report: Reconstruction of a 16-cm Diaphyseal Defect after Ewing’s Resection in a Child. Clin. Orthop. Relat. Res. 2009, 467, 572–577. [Google Scholar] [CrossRef] [Green Version]
- Makridis, K.G.; Theocharakis, S.; Fragkakis, E.M.; Giannoudis, P.V. Reconstruction of an extensive soft tissue and bone defect of the first metatarsal with the use of Masquelet technique: A case report. Foot Ankle Surg. 2014, 20, e19–e22. [Google Scholar] [CrossRef]
- Pelissier, P.; Boireau, P.; Martin, D.; Baudet, J. Bone Reconstruction of the Lower Extremity: Complications and Outcomes. Plast. Reconstr. Surg. 2003, 111, 2223–2229. [Google Scholar] [CrossRef]
- Schöttle, P.B.; Werner, C.M.L.; Dumont, C.E. Two-stage reconstruction with free vascularized soft tissue transfer and conventional bone graft for infected nonunions of the tibia: 6 patients followed for 1.5 to 5 years. Acta Orthop. 2005, 76, 878–883. [Google Scholar] [CrossRef]
- Zwetyenga, N.; Catros, S.; Emparanza, A.; Deminiere, C.; Siberchicot, F.; Fricain, J.C. Mandibular reconstruction using induced membranes with autologous cancellous bone graft and HA-βTCP: Animal model study and preliminary results in patients. Int. J. Oral Maxillofac. Surg. 2009, 38, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Yi-Loong Woon, C.; Chong, K.W.; Wong, M.K. Induced Membranes—A Staged Technique of Bone-Grafting for Segmental Bone Loss: A Report of Two Cases and a Literature Review. JBJS Case Connect. 2010, 92, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Stafford, P.R.; Norris, B.L. Reamer-irrigator-aspirator bone graft and bi Masquelet technique for segmental bone defect nonunions: A review of 25 cases. Injury 2010, 41, S72–S77. [Google Scholar] [CrossRef]
- Flamans, B.; Pauchot, J.; Petite, H.; Blanchet, N.; Rochet, S.; Garbuio, P.; Tropet, Y.; Obert, L. Pertes de substance osseuse à la main et au poignet traitées en urgence par technique de la membrane induite (technique de Masquelet). Chir. Main 2010, 29, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Apard, T.; Bigorre, N.; Cronier, P.; Duteille, F.; Bizot, P.; Massin, P. Two-stage reconstruction of post-traumatic segmental tibia bone loss with nailing. Orthop. Traumatol. Surg. Res. 2010, 96, 549–553. [Google Scholar] [CrossRef] [Green Version]
- Donegan, D.J.; Scolaro, J.; Matuszewski, P.E.; Mehta, S. Staged Bone Grafting Following Placement of an Antibiotic Spacer Block for the Management of Segmental Long Bone Defects. Orthopedics 2011, 34, e730–e735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karger, C.; Kishi, T.; Schneider, L.; Fitoussi, F.; Masquelet, A.-C. Treatment of posttraumatic bone defects by the induced membrane technique. Orthop. Traumatol. Surg. Res. 2012, 98, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Villemagne, T.; Bonnard, C.; Accadbled, F.; L’Kaissi, M.; De Billy, B.; Sales de Gauzy, J. Intercalary Segmental Reconstruction of Long Bones after Malignant Tumor Resection Using Primary Methyl Methacrylat Cement Spacer Interposition and Secondary Bone Grafting: The Induced Membrane Technique. Pediatric Orthop. 2011, 31, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.M.; Lau, T.W.; Li, X.; Fang, C.; Yeung, K.W.; Leung, F. Masquelet Technique for Treatment of Posttraumatic Bone Defects. Sci. World J. 2014, 2014, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keramaris, N.; Calori, G.; Nikolaou, V.; Schemitsch, E.; Giannoudis, P. Fracture vascularity and bone healing: A systematic review of the role of VEGF. Injury 2008, 39, S45–S57. [Google Scholar] [CrossRef]
- Saran, U.; Piperni, S.G.; Chatterjee, S. Role of angiogenesis in bone repair. Arch. Biochem. Biophys. 2014, 561, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Devescovi, V.; Leonardi, E.; Ciapetti, G.; Cenni, E. Growth factors in bone repair. Chir. Organi Mov. 2008, 92, 161–168. [Google Scholar] [CrossRef]
- Hankenson, K.D.; Dishowitz, M.; Gray, C.; Schenker, M. Angiogenesis in bone regeneration. Injury 2011, 42, 556–561. [Google Scholar] [CrossRef] [Green Version]
- Kobbe, P.; Tarkin, I.S.; Pape, H.C. Use of the ‘reamer irrigator aspirator’ system for non-infected tibial non-union after failed iliac crest grafting. Injury 2008, 39, 796–800. [Google Scholar] [CrossRef]
- Pape, H.-C.; Tarkin, I.S. Reamer Irrigator Aspirator: A New Technique for Bone Graft Harvesting from the Intramedullary Canal. Oper. Tech. Orthop. 2008, 18, 108–113. [Google Scholar] [CrossRef]
- Moghaddam, A.; Müller, U.; Roth, H.; Wentzensen, A.; Grützner, P.; Zimmermann, G. TRACP 5b and CTX as osteological markers of delayed fracture healing. Injury 2011, 42, 758–764. [Google Scholar] [CrossRef]
- Zimmerman, G.; Henle, P.; Kusswetter, M.; Moghaddam, A.; Wentzensen, A.; Richter, W.; Weiss, S.; Shyamsundar, S. TGF-β1 as a marker of delayed fracture healing. Bone 2006, 38, 456–457. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L. Cotinine as a Biomarker of Environmental Tobacco Smoke Exposure. Epidemiol. Rev. 1996, 18, 188–204. [Google Scholar] [CrossRef] [Green Version]
- Weiss, S.; Henle, P.; Bidlingmaier, M.; Moghaddam, A.; Kasten, P.; Zimmermann, G. Systemic response of the GH/IGF-I axis in timely versus delayed fracture healing. Growth Horm. IGF Res. 2008, 18, 205–212. [Google Scholar] [CrossRef]
- Sarahrudi, K.; Thomas, A.; Braunsteiner, T.; Wolf, H.; Vécsei, V.; Aharinejad, S. VEGF serum concentrations in patients with long bone fractures: A comparison between impaired and normal fracture healing. J. Orthop. Res. 2009, 27, 1293–1297. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Zimmermann, G.; Pufe, T.; Varoga, D.; Henle, P. The systemic angiogenic response during bone healing. Arch. Orthop. Trauma Surg. 2008, 129, 989–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, S.; Zimmermann, G.; Baumgart, R.; Kasten, P.; Bidlingmaier, M.; Henle, P. Systemic regulation of angiogenesis and matrix degradation in bone regeneration—Distraction osteogenesis compared to rigid fracture healing. Bone 2005, 37, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Beamer, B.; Hettrich, C.; Lane, J. Vascular Endothelial Growth Factor: An Essential Component of Angiogenesis and Fracture Healing. HSS J. 2010, 6, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Aho, O.-M.; Lehenkari, P.; Ristiniemi, J.; Lehtonen, S.; Risteli, J.; Leskelä, H.-V. The Mechanism of Action of Induced Membranes in Bone Repair. J. Bone Jt. Surg. Am. Vol. 2013, 95, 597–604. [Google Scholar] [CrossRef]
- Henrich, D.; Seebach, C.; Nau, C.; Basan, S.; Relja, B.; Wilhelm, K.; Schaible, A.; Frank, J.; Barker, J.; Marzi, I. Establishment and characterization of the Masquelet induced membrane technique in a rat femur critical-sized defect model. J. Tissue Eng. Regen. Med. 2016, 10, E382–E396. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Faour, O.; Goff, T.; Kanakaris, N.; Dimitriou, R. Masquelet technique for the treatment of bone defects: Tips-tricks and future directions. Injury 2011, 42, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, M.; Guyatt, G.H.; Swiontkowski, M.F.; Tornetta, P., III; Sprague, S.; Schemitsch, E.H. A lack of consensus in the assessment of fracture healing among orthopaedic surgeons. J. Orthop. Trauma 2002, 16, 562–566. [Google Scholar] [CrossRef] [PubMed]

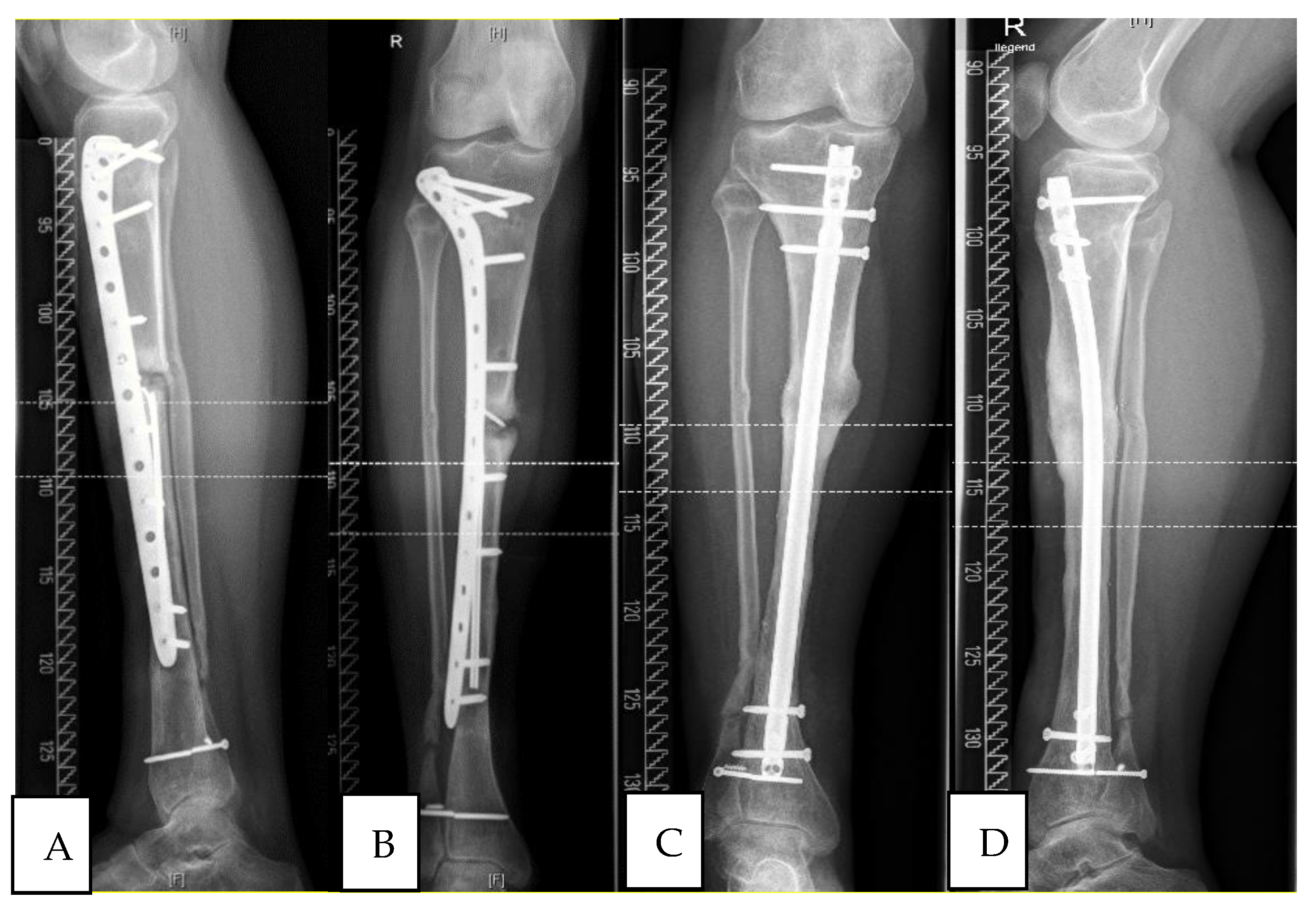
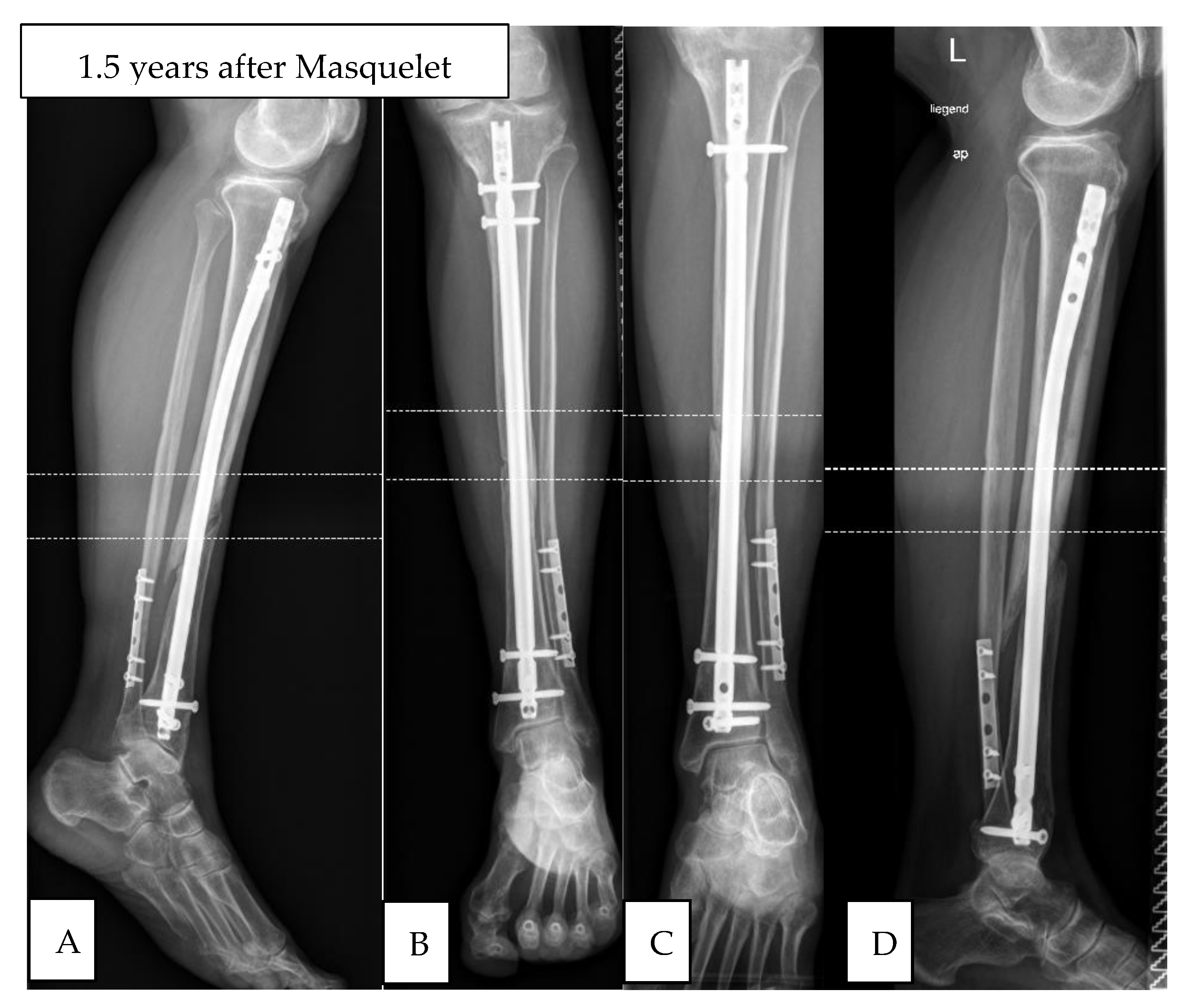


| No. | Gender | Age | BMI | Localisation | Side | Pre-OPs | Previous Infection | Fixation | Bone Graft | Diabetes | Smoker | Cotinin |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F001 | M | 72 | 22.8 | Tibia | left | >10 | yes | Nail | RIA, free fibula | no | no | positive |
| F006 | M | 45 | 19.8 | Tibia | left | 4 | yes | Plate | RIA | no | yes | positive |
| F009 | F | 50 | 32.5 | Femur | left | 2 | no | Nail | RIA | no | no | negative |
| F010 | M | 64 | 25.1 | Tibia | right | 3 | yes | Nail | Iliac crest | yes | yes | negative |
| F012 | M | 30 | 26.7 | Tibia | right | >10 | yes | Nail | RIA | no | no | positive |
| F013 | M | 51 | 29.9 | Tibia | right | 4 | yes | Plate | RIA, iliac crest | no | no | negative |
| F021 * | M | 59 | 22.8 | Femur | left | 4 | no | Plate | Iliac crest | no | yes | positive |
| F025 | M | 53 | 29.7 | Tibia | right | 16 | yes | Nail | RIA, iliac crest | yes | no | positive |
| F026 | M | 50 | 27.5 | Femur | left | 4 | no | Nail | RIA | no | no | positive |
| F027 | M | 45 | 44.8 | Femur | right | 2 | no | Plate | RIA | yes | no | negative |
| F028 | F | 38 | 23.1 | Tibia | right | 4 | no | Plate | RIA, iliac crest | no | no | negative |
| F029 | M | 55 | 21.9 | Tibia | left | 3 | no | Plate | RIA, iliac crest | no | yes | positive |
| F030 | M | 60 | 25.9 | Tibia | right | 1 | no | Plate | RIA, iliac crest | no | no | negative |
| F031 | M | 43 | 26.3 | Tibia | left | 10 | yes | Plate | RIA | no | no | negative |
| No. | Gender | Age | BMI | Localisation | Side | Pre-OPs | Previous Infection | Fixation | Bone Graft | Diabetes | Smoker | Cotinin |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F002 | F | 51 | 30.4 | Femur | left | >10 | yes | Plate | RIA, iliac crest | no | yes | positive |
| F003 | F | 57 | 22.2 | Femur | left | 17 | yes | Nail | RIA | no | yes | positive |
| F004 * | F | 60 | 39.2 | Femur | right | 3 | No | Plate | RIA | no | no | negative |
| F005 | F | 73 | 37.5 | Humerus | left | 1 | No | Plate | iliac crest | yes | no | negative |
| F007 * | M | 44 | 25.2 | Tibia | left | 12 | yes | Nail | RIA | no | no | negative |
| F008 | F | 71 | 29.7 | Femur | right | 14 | yes | Nail | RIA, iliac crest | no | no | negative |
| F018 | M | 71 | 26.3 | Tibia | right | 4 | yes | Nail | RIA | yes | no | negative |
| F023 | F | 41 | 34.6 | Femur | right | 2 | No | Plate | RIA | no | yes | negative |
| F035 | F | 78 | 23.0 | Femur | right | 2 | No | Plate | iliac crest | no | no | negative |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanner, M.C.; Boxriker, S.; Haubruck, P.; Child, C.; Westhauser, F.; Fischer, C.; Schmidmaier, G.; Moghaddam, A. Expression of VEGF in Peripheral Serum Is a Possible Prognostic Factor in Bone-Regeneration via Masquelet-Technique—A Pilot Study. J. Clin. Med. 2021, 10, 776. https://doi.org/10.3390/jcm10040776
Tanner MC, Boxriker S, Haubruck P, Child C, Westhauser F, Fischer C, Schmidmaier G, Moghaddam A. Expression of VEGF in Peripheral Serum Is a Possible Prognostic Factor in Bone-Regeneration via Masquelet-Technique—A Pilot Study. Journal of Clinical Medicine. 2021; 10(4):776. https://doi.org/10.3390/jcm10040776
Chicago/Turabian StyleTanner, Michael C., Sonja Boxriker, Patrick Haubruck, Christopher Child, Fabian Westhauser, Christian Fischer, Gerhard Schmidmaier, and Arash Moghaddam. 2021. "Expression of VEGF in Peripheral Serum Is a Possible Prognostic Factor in Bone-Regeneration via Masquelet-Technique—A Pilot Study" Journal of Clinical Medicine 10, no. 4: 776. https://doi.org/10.3390/jcm10040776
APA StyleTanner, M. C., Boxriker, S., Haubruck, P., Child, C., Westhauser, F., Fischer, C., Schmidmaier, G., & Moghaddam, A. (2021). Expression of VEGF in Peripheral Serum Is a Possible Prognostic Factor in Bone-Regeneration via Masquelet-Technique—A Pilot Study. Journal of Clinical Medicine, 10(4), 776. https://doi.org/10.3390/jcm10040776







