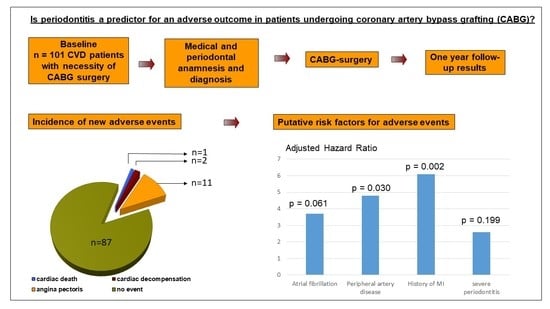
Is Periodontitis a Predictor for an Adverse Outcome in Patients Undergoing Coronary Artery Bypass Grafting? A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients with CVD
2.2. Demographic Parameters and Clinical and Cardiological Diagnostics
2.3. Dental Anamnesis and Examinations
2.4. Follow-Up
2.5. Statistics
3. Results
3.1. Incidence of New Cardiovascular Events
3.2. Cross-Section Comparisons
3.3. Multivariate Survival Analyses
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 9 December 2020).
- Deutsche Herzstiftung e.V. Deutscher Herzbericht 2018: Heart diseases in the cause of death statistics; German Heart Foundation e.V.: Frankfurt am Main, Germany, 2018; p. 27. [Google Scholar]
- Graham, M.M.; Ghali, W.A.; Faris, P.D.; Galbraith, P.D.; Norris, C.M.; Knudtson, M.L. Survival After Coronary Revascularization in the Elderly. Circulation 2002, 105, 2378–2384. [Google Scholar] [CrossRef] [PubMed]
- Toumpoulis, I.K.; Anagnostopoulos, C.E.; Balaram, S.K.; Rokkas, C.K.; Swistel, D.G.; Ashton, R.C.; Derose, J.J. Assessment of independent predictors for long-term mortality between women and men after coronary artery bypass grafting: Are women different from men? J. Thorac. Cardiovasc. Surg. 2006, 131, 343–351. [Google Scholar] [CrossRef]
- Axelsson, T.A.; Adalsteinsson, J.A.; Arnadottir, L.O.; Helgason, D.; Johannesdottir, H.; Helgadottir, S.; Orrason, A.W.; Andersen, K.; Gudbjartsson, T. Long-term outcomes after coronary artery bypass surgery in patients with diabetes. Interact. Cardiovasc. Thorac. Surg. 2020, 30, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Bonacchi, M.; Parise, O.; Matteucci, F.; Tetta, C.; Moula, A.I.; Micali, L.R.; Dokollari, A.; De Martino, M.; Sani, G.; Grasso, A.; et al. Is Peripheral Artery Disease an Independent Predictor of Isolated Coronary Artery Bypass Outcome? Hearth Lung Circ. 2020, 29, 1502–1510. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S173–S182. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Clin. Periodontol. 2018, 45, S149–S161. [Google Scholar] [CrossRef] [PubMed]
- Bahekar, A.A.; Singh, S.; Saha, S.; Molnar, J.; Arora, R. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: A meta-analysis. Am. Hearth. J. 2007, 154, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Blaizot, A.; Vergnes, J.-N.; Nuwwareh, S.; Amar, J.; Sixou, M. Periodontal diseases and cardiovascular events: Meta-analysis of observational studies. Int. Dent. J. 2009, 59, 197–209. [Google Scholar] [PubMed]
- Lockhart, P.B.; Bolger, A.F.; Papapanou, P.N.; Lockhart, P.B.; Bolger, A.F.; Papapanou, P.N.; Osinbowale, O.; Trevisan, M.; Levison, M.E.; Taubert, K.A.; et al. Periodontal disease and atherosclerotic vascular disease: Does the evidence support an independent association: A scientific statement from the American Heart Association. Circulation 2012, 125, 2520–2544. [Google Scholar] [CrossRef]
- Janket, S.-J.; Baird, A.E.; Chuang, S.-K.; Jones, J.A. Meta-analysis of periodontal disease and risk of coronary heart disease and stroke. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2003, 95, 559–569. [Google Scholar] [CrossRef]
- Humphrey, L.L.; Fu, R.; Buckley, D.I.; Freeman, M.; Helfand, M. Periodontal disease and coronary heart disease incidence: A sys-tematic review and meta-analysis. J. Gen. Intern. Med. 2008, 23, 2079–2086. [Google Scholar] [CrossRef]
- Dietrich, T.; Sharma, P.; Walter, C.; Weston, P.; Beck, J. The epidemiological evidence behind the association between periodontitis and incident atherosclerotic cardiovascular disease. J. Periodontol. 2013, 84, S70–S84. [Google Scholar] [CrossRef] [PubMed]
- Yun, P.L.W.; Decarlo, A.A.; Chapple, C.C.; Hunter, N. Functional Implication of the Hydrolysis of Platelet Endothelial Cell Adhesion Molecule 1 (CD31) by Gingipains of Porphyromonas gingivalis for the Pathology of Periodontal Disease. Infect. Immun. 2005, 73, 1386–1398. [Google Scholar] [CrossRef]
- Zhang, W.; Daly, C.G.; Mitchell, D.; Curtis, B.H. Incidence and magnitude of bacteraemia caused by flossing and by scaling and root planing. J. Clin. Periodontol. 2013, 40, 41–52. [Google Scholar] [CrossRef]
- Hirschfeld, J.; Kawai, T. Oral inflammation and bacteremia: Implications for chronic and acute systemic diseases involving major organs. Cardiovasc. Hematol. Disord. Targets 2015, 15, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Geerts, S.O.; Nys, M.; De Mol, P.; Charpentier, J.; Albert, A.; Legrand, V.; Rompen, E.H. Systemic Release of Endotoxins Induced by Gentle Mastication: Association With Periodontitis Severity. J. Periodontol. 2002, 73, 73–78. [Google Scholar] [CrossRef]
- Haraszthy, V.I.; Zambon, J.J.; Trevisan, M.; Zeid, M.; Genco, R.J. Identification of periodontal pathogens in atheromatous plaques. J. Periodontol. 2000, 71, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Atarbashi-Moghadam, F.; Havaei, S.R.; Havaei, S.A.; Hosseini, N.S.; Behdadmehr, G.; Atarbashi-Moghadam, S. Periopathogens in atherosclerotic plaques of patients with both cardiovascular disease and chronic periodontitis. ARYA Atheroscler. 2018, 14, 53–57. [Google Scholar]
- Ziebolz, D.; Rost, C.; Schmidt, J.; Waldmann-Beushausen, R.; Schöndube, F.A.; Mausberg, R.F.; Danner, B.C. Periodontal Bacterial DNA and Their Link to Human Cardiac Tissue: Findings of a Pilot Study. Thorac. Cardiovasc. Surg. 2015, 66, 83–90. [Google Scholar] [CrossRef]
- Padilla, C.; Lobos, O.; Hubert, E.; Gonzalez, C.; Matus, S.; Pereira, M.; Hasbun, S.; Descouvieres, C. Periodontal pathogens in atheromatous plaques isolated from patients with chronic periodontitis. J. Periodontal Res. 2006, 41, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Shiheido, Y.; Maejima, Y.; Suzuki, J.I. Porphyromonas gingivalis, a periodontal pathogen, enhances myocardial vulnera-bility, thereby promoting post-infarct cardiac rupture. J. Mol. Cell. Cardiol. 2016, 99, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Schulz, S.; Reichert, S.; Grollmitz, J.; Friebe, L.; Kohnert, M.; Hofmann, B.; Schaller, H.G.; Kllawonn, F.; Shi, R. The role of Sacchari-bacteria (TM7) in the oral microbiome as a predictor for secondary cardiovascular events. Int. J. Cardiol. 2020. accept. [Google Scholar] [CrossRef]
- Adler, Y.; Charron, P.; Imazio, M.; Badano, L.; Barón-Esquivias, G.; Bogaert, J. ESC Scientific Document Group. Eur. Heart J. 2015, 36, 2921–2964. [Google Scholar] [CrossRef]
- Löe, H.; Silness, J. Periodontal Disease in Pregnancy I. Prevalence and Severity. Acta Odontol. Scand. 1963, 21, 533–551. [Google Scholar] [CrossRef]
- Ainamo, J.; Bay, I. Problems and proposals for recording gingivitis and plaque. Int. Dent. J. 1975, 25, 229–235. [Google Scholar]
- Grouven, U.; Bender, R.; Ziegler, A.; Lange, S. Vergleich von Messmethoden. DMW Dtsch. Med. Wochenschr. 2007, 132, e69–e73. [Google Scholar] [CrossRef]
- Page, R.C.; Eke, P.I. Case Definitions for Use in Population-Based Surveillance of Periodontitis. J. Periodontol. 2007, 78, 1387–1399. [Google Scholar] [CrossRef]
- Nesse, W.; Abbas, F.; Van Der Ploeg, I.; Spijkervet, F.K.L.; Dijkstra, P.U.; Vissink, A. Periodontal inflamed surface area: Quantifying inflammatory burden. J. Clin. Periodontol. 2008, 35, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global Prevalence of Periodontal Disease and Lack of Its Surveillance. Sci. World J. 2020, 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jordan, R.A.; Micheelis, W. Fünfte Deutsche Mundgesundheitsstudie (DMS V), 1st ed.; Deutscher Zahnärzte Verlag DÄV: Köln, Germany, 2016; pp. 396–413. [Google Scholar]
- Dorn, J.M.; Genco, R.J.; Grossi, S.G.; Falkner, K.L.; Hovey, K.M.; Iacoviello, L.; Trevisan, M. Periodontal Disease and Recurrent Cardiovascular Events in Survivors of Myocardial Infarction (MI): The Western New York Acute MI Study. J. Periodontol. 2010, 81, 502–511. [Google Scholar] [CrossRef]
- Renvert, S.; Ohlsson, O.; Pettersson, T.; Persson, G.R. Periodontitis: A Future Risk of Acute Coronary Syndrome? A Follow-Up Study Over 3 Years. J. Periodontol. 2010, 81, 992–1000. [Google Scholar] [CrossRef]
- Chu, D.; Bakaeen, F.G.; Wang, X.L.; Dao, T.K.; Lemaire, S.A.; Coselli, J.S.; Huh, J. The Impact of Peripheral Vascular Disease on Long-Term Survival After Coronary Artery Bypass Graft Surgery. Ann. Thorac. Surg. 2008, 86, 1175–1180. [Google Scholar] [CrossRef]
- Van Straten, A.H.M.; Firanescu, C.; Soliman Hamad, M.A. Peripheral vascular disease as a predictor of survival after coronary artery bypass grafting: Comparison with a matched general population. Ann. Thorac. Surg. 2010, 89, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Herlitz, J.; Brandrup, G.; Haglid, M.; Karlson, B.; Albertsson, P.; Lurje, L.; Westberg, S.; Karlsson, T. Death, Mode of Death, Morbidity, and Rehospitalization after Coronary Artery Bypass Grafting in Relation to Occurrence of and Time Since a Previous Myocardial Infarction. Thorac. Cardiovasc. Surg. 1997, 45, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Batra, G.; Ahlsson, A.; Lindahl, B.; Lindhagen, L.; Wickbom, A.; Oldgren, J. Atrial fibrillation in patients undergoing coronary artery surgery is associated with adverse outcome. Upsala J. Med Sci. 2019, 124, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Fengsrud, E.; Englund, A.; Ahlsson, A. Pre- and postoperative atrial fibrillation in CABG patients have similar prognostic impact. Scand. Cardiovasc. J. 2016, 51, 21–27. [Google Scholar] [CrossRef]
- Ngaage, D.L.; Schaff, H.V.; Mullany, C.J.; Sundt, T.M.; Dearani, J.A.; Barnes, S.; Daly, R.C.; Orszulak, T.A. Does preoperative atrial fibrillation influence early and late outcomes of coronary artery bypass grafting? J. Thorac. Cardiovasc. Surg. 2007, 133, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, A.S.; Richter, G.M.; Groessner-Schreiber, B.; Noack, B.; Nothnagel, M.; El Mokhtari, N.-E.; Loos, B.G.; Jepsen, S.; Schreiber, S. Identification of a Shared Genetic Susceptibility Locus for Coronary Heart Disease and Periodontitis. PLoS Genet. 2009, 5, e1000378. [Google Scholar] [CrossRef] [PubMed]
- Reichert, S.; Schulz, S.; Benten, A.C.; Lutze, A.; Seifert, T.; Schlitt, M.; Werdan, K.; Hofmann, B.; Wienke, A.; Schaller, H.G. Per-iodontal conditions and incidence of new cardiovascular events among patients with coronary vascular disease. J. Clin. Periodontol. 2016, 43, 918–925. [Google Scholar] [CrossRef]

| Variable | Entire Study Cohort n = 101, Median (25th/75th Percentile) or n (%) | Event n = 14, Median (25th/75th Percentile) or n (%) | No event n = 87, Median (25th/75th Percentile) or n (%) | p-Values |
|---|---|---|---|---|
| age (years) | 69.0 (60.0/75.0) | 71.0 (60.8/75.3) | 69.0 (60.0/74.0) | 0.646 * |
| females | 12 (11.9) | 2 (14.3) | 10 (11.5) | |
| males | 89 (88.1) | 12 (85.7) | 77 (88.5) | 0.671 *** |
| BMI (kg/m2) | 28.7 (25.6/31.0) | 29.7 (25.4/31.8) | 28.7 (25.6/30.5) | 0.476 * |
| smoking | ||||
| current | 22 (21.8) | 3 (21.4) | 19 (21.8) | |
| past | 42 (41.6) | 6 (42.9) | 36 (41.4) | |
| never | 37 (36.6) | 5 (35.7) | 32 (36.8) | 0.995 ** |
| pack years | 7.5 (0/22.5) | 3.0 (0/21.3) | 8.0 (0/22.5) | 0.686 * |
| Variable | Entire Study Cohort n = 101, Median (25th/75th Percentile) or n (%) | Event n = 14, Median (25th/75th Percentile) or n (%) | No event n = 87, Median (25th/75th Percentile) or n (%) | p-Values |
|---|---|---|---|---|
| Affected coronaries | ||||
| One-vessel disease | 5 (5.0) | 2 (14.3) | 3 (3.4) | |
| Two-vessel disease | 21 (20.8) | 3 (21.4) | 18 (20.7) | |
| Three-vessel disease | 75 (74.3) | 9 (64.3) | 66 (75.9) | 0.214 ** |
| Angina pectoris grade | ||||
| CCS 0 | 32 (31.7) | 7 (50) | 25 (28.7) | |
| CCS I | 13 (12.9) | 1 (7.7) | 12 (13.8) | |
| CCS II | 25 (24.8) | 2 (14.3) | 23 (26.4) | |
| CCS III | 18 (17.8) | 2 (14.3) | 16 (18.4) | |
| CCS IV | 13 (12.9) | 2 (14.3) | 11 (12.6) | 0.599 ** |
| History of | ||||
| Diabetes mellitus | 40 (39.6) | 7 (50) | 33 (37.9) | 0.397 *** |
| Hypertension | 88 (87.1) | 14 (100) | 74 (85.1) | 0.205 *** |
| Dyslipoproteinemia | 81 (80.2) | 13 (92.9) | 68 (78.2) | 0.291 *** |
| PAD | 16 (15.8) | 5 (35.7) ↑ | 11 (12.6) | 0.044 *** |
| CVD | 39 (38.6) | 5 (35.7) | 34 (39.1) | 1.00 *** |
| MI | 28 (27,7) | 8 (57.1) ↑ | 20 (23.0) | 0.020 *** |
| stroke/TIA | 9 (8.9) | 0 (0) | 9 (10.3) | 0.354 *** |
| Angina pectoris | 75 (74.3) | 10 (71.4) | 65 (74.7) | 0.752 *** |
| PTCA/stent | 15 (14.9) | 3 (21.4) | 12 (13.8) | 0.433 *** |
| Atrial fibrillation | 14 (13.9) | 4 (28.6) | 10 (11.5) | 0.102 *** |
| Blood values | ||||
| INR | 1.04 (0.99/1.11) | 1.04 (0.95/1,12) | 1.04 (0.99/1.10) | 0.984 * |
| Hb (mmol/L) | 8.8 (8.3/9.4) | 8.4 (8.1/9.4) | 8.8 (8.3/9.4) | 0.437 * |
| Hematocrit 1/L | 0.41 (0.39/0.43) | 0.4 (0.38/0.43) | 0.41 (0.39/0.43) | 0.778 * |
| Creatinine (µmol/L) | 85 (75.5/100) | 86.5 (79.5/100.3) | 85.0 (74.0/99.0) | 0.440 * |
| Urea (mmol/L) | 5.9 (4.5/7.2) | 6.8 (5.4/7.6) | 5.6 (4.3/6.8) | 0.065 * |
| HbA1C (mmol/mol) | 37.6 (31.2/44.4) | 39.0 (35.1/52.6) | 40.1 (35.9/48.8) | 0.769 * |
| CRP (mg/L) | 2.6 (1.2/6.6) | 1.4 (0.7/3.9) | 2.8 (1.4/6.9) | 0.052 * |
| Leukocytes (Gpt/L) | 6.5 (7.6/9.1) | 7.2 (5.5/8.5) | 7.6 (6.6/9.5) | 0.210 * |
| Platelet (Gpt/L) | 238.0 (193.0/269.5) | 225.0 (184.0/259.8) | 239.0 (193.0/280.0) | 0.401 * |
| Drugs | ||||
| Lipid lowering drugs | 90 (89.1) | 14 (100.0) | 76 (87.4) | 0.354 *** |
| Oral anticoagulants | 11 (10.9) | 4 (28.6) ↑ | 7 (8.0) | 0.044 *** |
| Antiarrhythmics | 2 (2.0) | 2 (14.3) ↑ | 0 (0.0) | 0.018 *** |
| Variable | Entire Study Cohort n = 101, Median (25th/75th Percentile) or n (%) | Event n = 14, Median (25th/75th Percentile) or n (%) | No event n = 87, Median (25th/75th Percentile) or n (%) | p-Values |
|---|---|---|---|---|
| Dental anamnesis | ||||
| tooth brushing/d | ||||
| 1 | 15 (14.9) | 1 (7.1) | 14 (16.1) | |
| 2 | 80 (79.2) | 12 (85.7) | 68 (78.2) | |
| 3 | 6 (5.9) | 1 (7.1) | 5 (85.7) | 0.678 ** |
| Use of floss/interdental | ||||
| brushes | ||||
| 29 (28.7) | 5 (35.7) | 24 (27.6) | 0.563 *** | |
| Previous SRP | ||||
| 12 (11.9) | 3 (21.4) | 9 (10.3) | 0.366 *** | |
| Early tooth loss among first-degree relatives | ||||
| Yes | ||||
| No | ||||
| Unknown | 26 (25.7) | 8 (51.7) ↑ | 18 (20.7) | |
| Periodontitis (CDC) | ||||
| No or mild | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Moderate | 29 (28.7) | 3 (21.4) | 26 (29.9) | |
| Severe | 72 (71.3) | 11 (78.6) | 61 (70.1) | 0.516 ** |
| Plaque index (%) | 1.3 (0.9/1.7) | 1.2(0.8/1.8) | 1.3 (1.0/1.7) | 0.293 * |
| Bleeding index (%) | 18.0 (10.1/33.3) | 19.0 (13.8/37.0) | 17.5 (9.6/33.3) | 0.220 * |
| Pocket depth (mm) | 3.0 (2.6/3.6) | 2.8 (2.6/3.5) | 3.0 (2.6/3.6) | 0.738 * |
| % sites with PD | ||||
| <3 mm | 34.4 (23.3/52.7) | 34.8 (23.2/59.1) | 34.4 (23.3/52.6) | 0.976 * |
| 3–5 mm | 56.7 (45.0/67.9) | 64.2 (40.7/70.5) | 56.3 (45.2/66.7) | 0.353 * |
| >5 mm | 1.7 (0/8.3) | 1.2 (0/5.9) | 1.7 (0/9.1) | 0.373 * |
| Attachment loss (mm) | 3.9 (3.1/4.9) | 3.9 (3.1/5.0) | 3.9 (3.2/5.0) | 0.705 * |
| % sites with CAL | ||||
| <3 mm | 16.7 (3.9/32.7) | 13.5 (3.5/44.3) | 19.3 (4.2/32.1) | 0.871 * |
| 3–5 mm | 59.4 (47.4/68.5) | 60.0 (44.3/76.3) | 59.4 (48.3/68.2) | 0.596 * |
| >5 mm | 12.7 (3.2/33.3) | 11.8 (1.8/35.0) | 12.7 (3.2/33.3) | 0.735 * |
| PESA (mm2) | 1187.8 (831.4/1617.3) | 1393.7 (966.2/1778.6) | 1165.2 (812.3/1577.9) | 0.453 * |
| PISA (mm2) | 194.6 (107.6/405.9) | 289.7 (164.8/407.9) | 191.4 (103.1/419.9) | 0.515 * |
| DMF/T | 18 (14.0/22.0) | 16.0 (12.0/21.3) | 19.0 (14.0/23.0) | 0.266 * |
| Missing teeth | 7 (3.0/15.0) | 6.0 (2.0/9.8) | 7.0 (3.0/17.0) | 0.695 * |
| Teeth with open furcations | 0 (0/2.0) | 1.0 (0.0/2.0) | 0.0 (0.0/2.0) | 0.517 * |
| Confounding Variables | Hazard Ratio | 95% Lower | CI Upper | p-Values |
|---|---|---|---|---|
| Age | 0.986 | 0.912 | 1.067 | 0.725 |
| Gender (female) | 1.601 | 0.321 | 7.976 | 0.566 |
| Pack years | 0.960 | 0.916 | 1.005 | 0.082 |
| Severe periodontitis | 2.559 | 0.610 | 10.743 | 0.199 |
| Atrial fibrillation | 3.701 | 0.941 | 14.562 | 0.061 |
| PAD | 4.836 | 1.162 | 20.126 | 0.030 |
| Previous MI | 6.056 | 1.892 | 19.379 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reichert, S.; Schulz, S.; Friebe, L.; Kohnert, M.; Grollmitz, J.; Schaller, H.-G.; Hofmann, B. Is Periodontitis a Predictor for an Adverse Outcome in Patients Undergoing Coronary Artery Bypass Grafting? A Pilot Study. J. Clin. Med. 2021, 10, 818. https://doi.org/10.3390/jcm10040818
Reichert S, Schulz S, Friebe L, Kohnert M, Grollmitz J, Schaller H-G, Hofmann B. Is Periodontitis a Predictor for an Adverse Outcome in Patients Undergoing Coronary Artery Bypass Grafting? A Pilot Study. Journal of Clinical Medicine. 2021; 10(4):818. https://doi.org/10.3390/jcm10040818
Chicago/Turabian StyleReichert, Stefan, Susanne Schulz, Lisa Friebe, Michael Kohnert, Julia Grollmitz, Hans-Günter Schaller, and Britt Hofmann. 2021. "Is Periodontitis a Predictor for an Adverse Outcome in Patients Undergoing Coronary Artery Bypass Grafting? A Pilot Study" Journal of Clinical Medicine 10, no. 4: 818. https://doi.org/10.3390/jcm10040818
APA StyleReichert, S., Schulz, S., Friebe, L., Kohnert, M., Grollmitz, J., Schaller, H.-G., & Hofmann, B. (2021). Is Periodontitis a Predictor for an Adverse Outcome in Patients Undergoing Coronary Artery Bypass Grafting? A Pilot Study. Journal of Clinical Medicine, 10(4), 818. https://doi.org/10.3390/jcm10040818