Peripheral Nerve Regeneration Using a Cytokine Cocktail Secreted by Skeletal Muscle-Derived Stem Cells in a Mouse Model
Abstract
:1. Introduction
2. Experimental Section
2.1. Animals
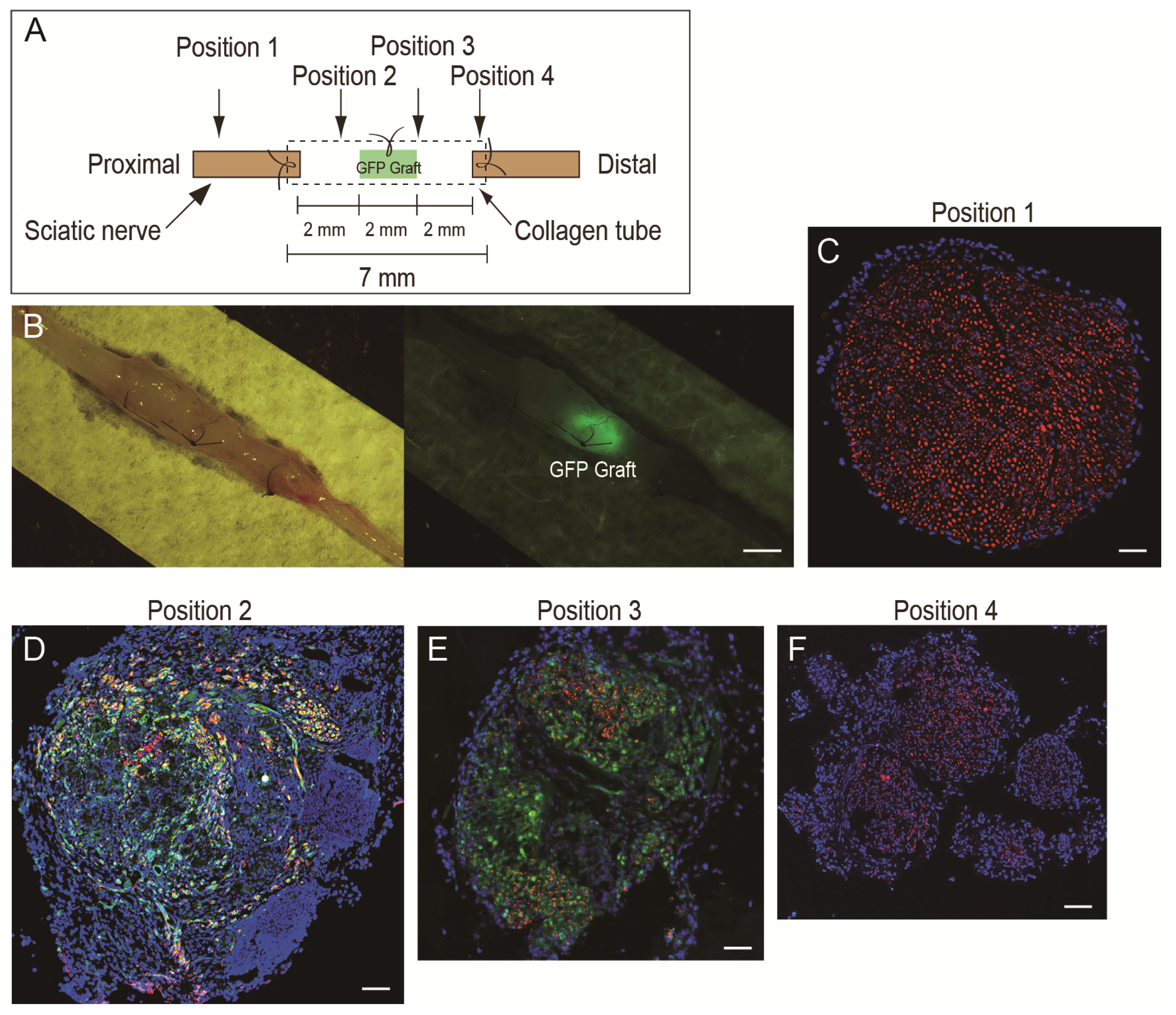
2.2. Isolation of Nerve Graft and Preparation of the Nerve Regeneration Assessment Model
2.3. Preparation and Administration of the Cytokine Cocktail
2.4. Functional Assessment of Downstream Muscles
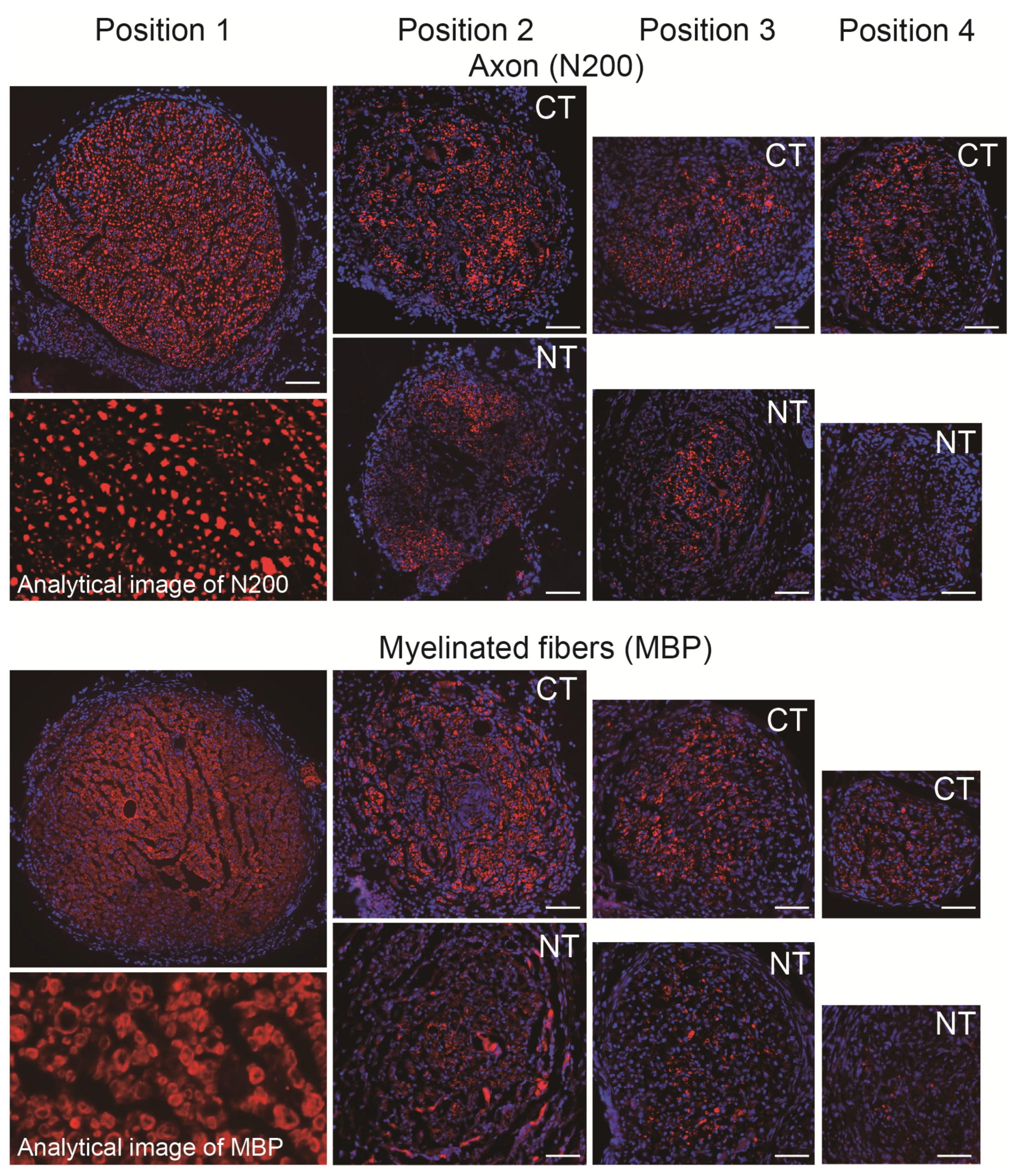
2.5. Immunohistochemistry and Numerical Analysis
2.6. Protein Analysis for the Cytokine Cocktail
2.7. Statistical Analysis
3. Results
3.1. Final Body and Muscle Mass, and Tension Output of Downstream Plantar Flexor Muscles
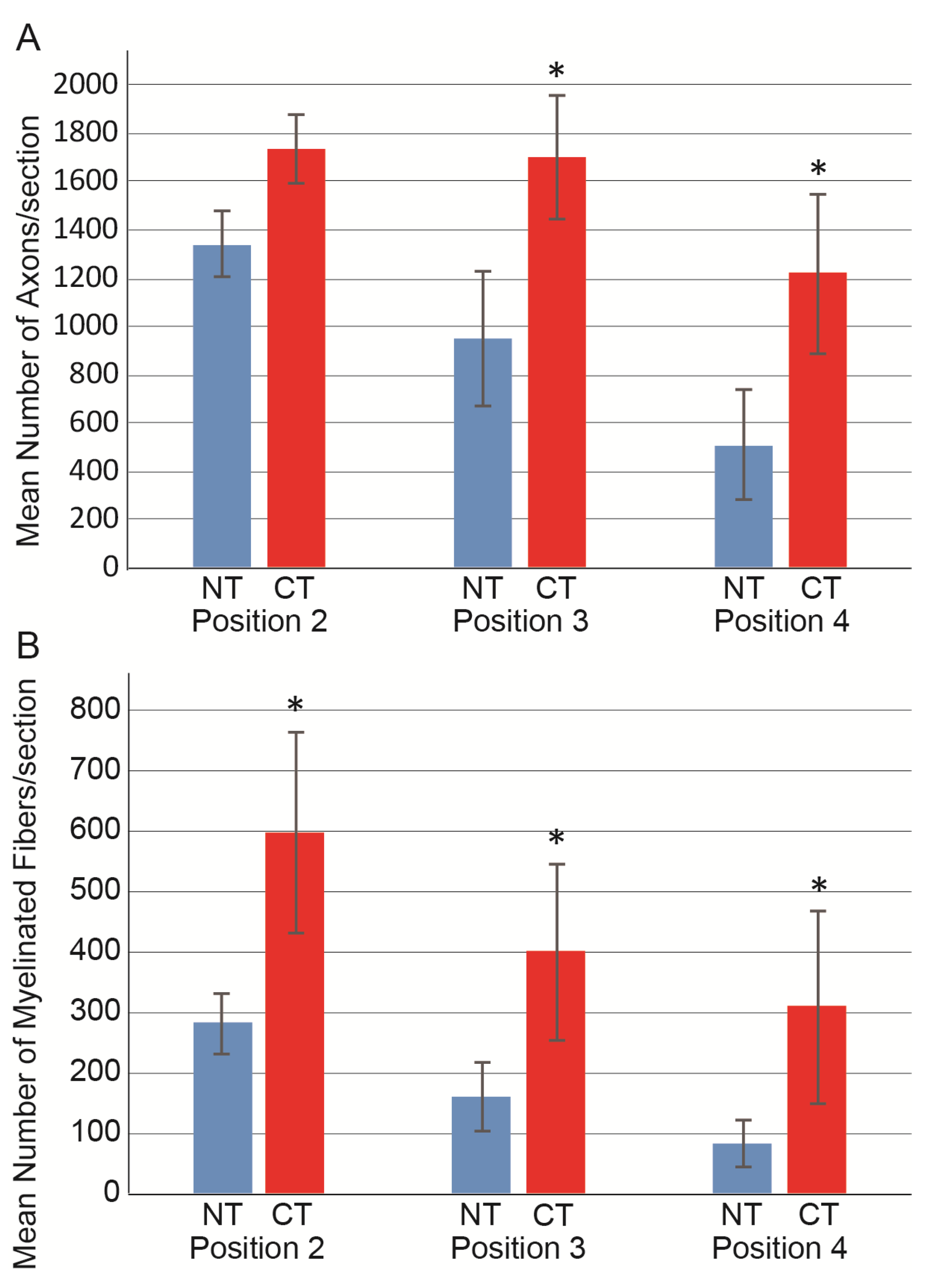
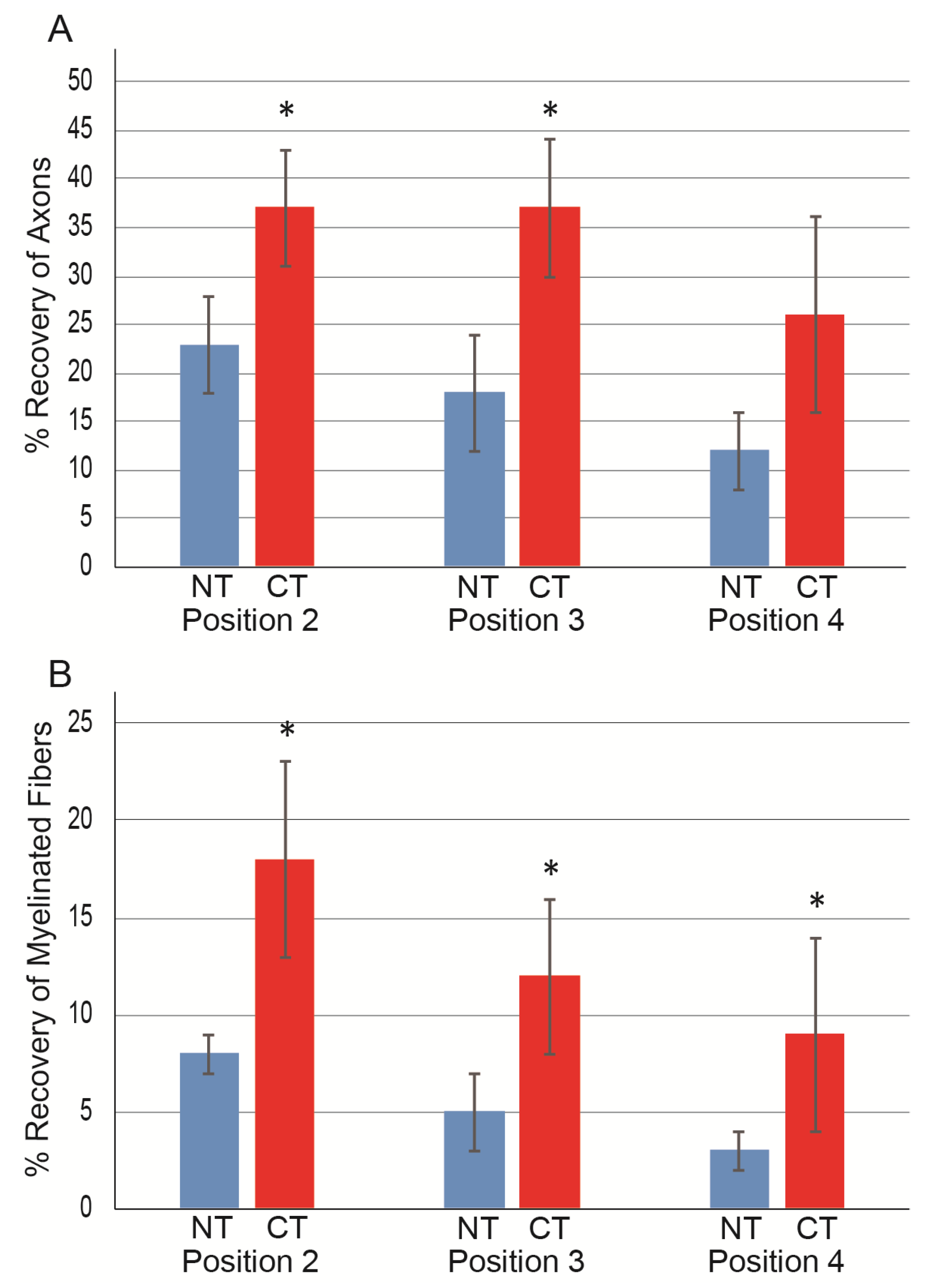
3.2. Morphological and Numerical Recoveries
3.3. Factors of the Cytokine Cocktail
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robinson, L.R. Traumatic injury to peripheral nerves. Muscle. Nerve. 2000, 23, 863–873. [Google Scholar] [CrossRef]
- Robinson, P.P.; Boissonade, F.M.; Loescher, A.R.; Smith, K.G.; Yates, J.M.; Elcock, C.; Bird, E.V.; Davies, S.L.; Smith, P.L.; Vora, A.R. Peripheral mechanisms for the initiation of pain following trigeminal nerve injury. J. Orofac. Pain 2004, 18, 287–292. [Google Scholar]
- Deumens, R.; Bozkurt, A.; Meek, M.F.; Marcus, M.A.; Joosten, E.A.; Weis, J.; Brook, G.A. Repairing injured peripheral nerves: Bridging the gap. Prog. Neurobiol. 2010, 92, 245–276. [Google Scholar] [CrossRef]
- Pfister, B.J.; Gordon, T.; LoVerde, J.R.; Kochar, A.S.; MacKinnon, S.E.; Cullen, D.K. Biomedical Engineering Strategies for Peripheral Nerve Repair: Surgical Applications, State of the Art, and Future Challenges. Crit. Rev. Biomed. Eng. 2011, 39, 81–124. [Google Scholar] [CrossRef]
- Dezawa, M.; Ishikawa, H.; Hoshino, M.; Itokazu, Y.; Nabeshima, Y.-I. Potential of Bone Marrow Stromal Cells in Applications for Neuro-Degenerative, Neuro-Traumatic and Muscle Degenerative Diseases. Curr. Neuropharmacol. 2005, 3, 257–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radtke, C.; Wewetzer, K.; Reimers, K.; Vogt, P.M. Transplantation of Olfactory Ensheathing Cells as Adjunct Cell Therapy for Peripheral Nerve Injury. Cell Transplant. 2011, 20, 145–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kingham, P.J.; Kalbermatten, D.F.; Mahay, D.; Armstrong, S.J.; Wiberg, M.; Terenghi, G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp. Neurol. 2007, 207, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, T.; Hirata, M.; Soeda, S.; Nakajima, N.; Saito, K.; Nakazato, K.; Okada, Y.; Hashimoto, H.; Uchiyama, Y.; Mochida, J. Preferential and Comprehensive Reconstitution of Severely Damaged Sciatic Nerve Using Murine Skeletal Muscle-Derived Multipotent Stem Cells. PLoS ONE 2014, 9, e91257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamaki, T. Paracrine effect of skeletal muscle-derived stem cell transplantation: The case of peripheral nerve long-gap injury therapy. Stem. Cell Res. Th. 2016, 1, 1–5. [Google Scholar]
- Tamaki, T. Therapeutic capacities of human and mouse skeletal muscle-derived stem cells for a long gap peripheral nerve injury. Neural Regen. Res. 2017, 12, 1811. [Google Scholar] [CrossRef]
- Tamaki, T.; Hirata, M.; Nakajima, N.; Saito, K.; Hashimoto, H.; Soeda, S.; Uchiyama, Y.; Watanabe, M. A Long-Gap Peripheral Nerve Injury Therapy Using Human Skeletal Muscle-Derived Stem Cells (Sk-SCs): An Achievement of Significant Morphological, Numerical and Functional Recovery. PLoS ONE 2016, 11, e0166639. [Google Scholar] [CrossRef]
- Hesseler, M.J.; Shyam, N. Platelet-rich plasma and its utility in medical dermatology: A systematic review. J. Am. Acad. Dermatol. 2019, 81, 834–846. [Google Scholar] [CrossRef] [Green Version]
- Zotti, F.; Albanese, M.; Rodella, L.F.; Nocini, P.F. Platelet-Rich Plasma in Treatment of Temporomandibular Joint Dysfunctions: Narrative Review. Int. J. Mol. Sci. 2019, 20, 277. [Google Scholar] [CrossRef] [Green Version]
- Okabe, M.; Ikawa, M.; Kominami, K.; Nakanishi, T.; Nishimune, Y. ’Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997, 407, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Tamaki, T.; Akatsuka, A.; Ando, K.; Nakamura, Y.; Matsuzawa, H.; Hotta, T.; Roy, R.R.; Edgerton, V.R. Identification of myogenic-endothelial progenitor cells in the interstitial spaces of skeletal muscle. J. Cell Biol. 2002, 157, 571–577. [Google Scholar] [CrossRef] [Green Version]
- Tamaki, T.; Akatsuka, A.; Okada, Y.; Matsuzaki, Y.; Okano, H.; Kimura, M. Growth and differentiation potential of main- and side-population cells derived from murine skeletal muscle. Exp. Cell Res. 2003, 291, 83–90. [Google Scholar] [CrossRef]
- Seta, H.; Maki, D.; Kazuno, A.; Yamato, I.; Nakajima, N.; Soeda, S.; Uchiyama, Y.; Tamaki, T. Voluntary Exercise Positively Affects the Recovery of Long-Nerve Gap Injury Following Tube-Bridging with Human Skeletal Muscle-Derived Stem Cell Transplantation. J. Clin. Med. 2018, 7, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamaki, T.; Uchiyama, Y.; Okada, Y.; Ishikawa, T.; Sato, M.; Akatsuka, A.; Asahara, T. Functional Recovery of Damaged Skeletal Muscle Through Synchronized Vasculogenesis, Myogenesis, and Neurogenesis by Muscle-Derived Stem Cells. Circulation 2005, 112, 2857–2866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamaki, T.; Uchiyama, Y.; Hirata, M.; Hashimoto, H.; Nakajima, N.; Saito, K.; Terachi, T.; Mochida, J. Therapeutic isolation and expansion of human skeletal muscle-derived stem cells for the use of muscle-nerve-blood vessel reconstitution. Front. Physiol. 2015, 6, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, M.; Hughart, R.; Champlain, A.; Geisler, A.; Paghdal, K.; Whiting, D.; Hammel, J.A.; Maisel, A.; Rapcan, M.J.; West, D.P.; et al. Effect of Platelet-Rich Plasma Injection for Rejuvenation of Photoaged Facial Skin: A Randomized Clinical Trial. JAMA Dermatol. 2018, 154, 1447–1452. [Google Scholar] [CrossRef]
- Gato-Calvo, L.; Magalhaes, J.; Ruiz-Romero, C.; Blanco, F.J.; Burguera, E.F. Platelet-rich plasma in osteoarthritis treatment: Review of current evidence. Ther. Adv. Chronic Dis. 2019, 10, 5567. [Google Scholar] [CrossRef] [Green Version]
- Middleton, K.K.; Barro, V.; Muller, B.; Terada, S.; Fu, F.H. Evaluation of the Effects of Platelet-Rich Plasma (PRP) Therapy Involved in the Healing of Sports-Related Soft Tissue Injuries. IOWA Orthop. J. 2012, 32, 150–163. [Google Scholar]
- Samadi, P.; Sheykhhasan, M.; Khoshinani, H.M. The Use of Platelet-Rich Plasma in Aesthetic and Regenerative Medicine: A Comprehensive Review. Aesthetic Plast. Surg. 2019, 43, 803–814. [Google Scholar] [CrossRef]
- Reddy, S.H.R.; Reddy, R.; Babu, N.C.; Ashok, G.N. Stem-cell therapy and platelet-rich plasma in regenerative medicines: A review on pros and cons of the technologies. J. Oral Maxillofac. Pathol. 2018, 22, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Schuckert, K.-H.; Jopp, S.; Osadnik, M. The Use of Platelet Rich Plasma, Bone Morphogenetic Protein-2 and Different Scaffolds in Oral and Maxillofacial Surgery—Literature Review in Comparison with Own Clinical Experience. J. Oral Maxillofac. Res. 2011, 2, e2. [Google Scholar] [CrossRef] [Green Version]
- Hesseler, M.J.; Shyam, N. Platelet-rich plasma and its utility in the treatment of acne scars: A systematic review. J. Am. Acad. Dermatol. 2019, 80, 1730–1745. [Google Scholar] [CrossRef] [PubMed]
- Forogh, B.; Mianehsaz, E.; Shoaee, S.; Ahadi, T.; Raissi, G.R.; Sajadi, S. Effect of single injection of Platelet-Rich Plasma in comparison with corticosteroid on knee osteoarthritis: A double-blind randomized clinical trial. J. Sports Med. Phys. Fit. 2015, 56, 901–908. [Google Scholar]
- Keene, D.J.; Alsousou, J.; Harrison, P.; Hulley, P.; Wagland, S.; Parsons, S.R.; Thompson, J.Y.; O’Connor, H.M.; Schlüssel, M.M.; Dutton, S.J.; et al. Platelet rich plasma injection for acute Achilles tendon rupture: PATH-2 randomised, placebo controlled, superiority trial. BMJ 2019, 367, l6132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, S.; Loibl, M.; Herrmann, M. Platelet-Rich Plasma in Tissue Engineering: Hype and Hope. Eur. Surg. Res. 2018, 59, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Le, A.D.K.; Enweze, L.; DeBaun, M.R.; Dragoo, J.L. Platelet-Rich Plasma. Clin. Sports Med. 2019, 38, 17–44. [Google Scholar] [CrossRef]
- Wang, D.; Rodeo, S.A. Platelet-Rich Plasma in Orthopaedic Surgery. JBJS Rev. 2017, 5, e7. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, T.; Okada, Y.; Uchiyama, Y.; Tono, K.; Masuda, M.; Wada, M.; Hoshi, A.; Akatsuka, A. Synchronized reconstitution of muscle fibers, peripheral nerves and blood vessels by murine skeletal muscle-derived CD34−/45− cells. Histochem. Cell Biol. 2007, 128, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, T.; Soeda, S.; Hashimoto, H.; Saito, K.; Sakai, A.; Nakajima, N.; Masuda, M.; Fukunishi, N.; Uchiyama, Y.; Terachi, T.; et al. 3D reconstitution of nerve–blood vessel networks using skeletal muscle-derived multipotent stem cell sheet pellets. Regen. Med. 2013, 8, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Tamaki, T.; Hirata, M.; Uchiyama, Y.; Sato, M.; Mochida, J. Reconstitution of the complete rupture in musculotendinous junction using skeletal muscle-derived multipotent stem cell sheet-pellets as a “bio-bond”. PeerJ 2016, 4, e2231. [Google Scholar] [CrossRef] [Green Version]
- Hoshi, A.; Tamaki, T.; Tono, K.; Okada, Y.; Akatsuka, A.; Usui, Y.; Terachi, T. Reconstruction of Radical Prostatectomy-Induced Urethral Damage Using Skeletal Muscle-Derived Multipotent Stem Cells. Transplantation 2008, 85, 1617–1624. [Google Scholar] [CrossRef]
- Nitta, M.; Tamaki, T.; Tono, K.; Okada, Y.; Masuda, M.; Akatsuka, A.; Hoshi, A.; Usui, Y.; Terachi, T. Reconstitution of Experimental Neurogenic Bladder Dysfunction Using Skeletal Muscle-Derived Multipotent Stem Cells. Transplantation 2010, 89, 1043–1049. [Google Scholar] [CrossRef]
- Nakajima, N.; Tamaki, T.; Hirata, M.; Soeda, S.; Nitta, M.; Hoshi, A.; Terachi, T. Purified Human Skeletal Muscle-Derived Stem Cells Enhance the Repair and Regeneration in the Damaged Urethra. Transplantation 2017, 101, 2312–2320. [Google Scholar] [CrossRef] [Green Version]
- Soeda, S.; Tamaki, T.; Hashimoto, H.; Saito, K.; Sakai, A.; Nakajima, N.; Nakazato, K.; Masuda, M.; Terachi, T. Functional Nerve-Vascular Reconstitution of the Bladder-Wall; Application of Patch Transplantation of Skeletal Muscle-Derived Multipotent Stem Cell Sheet-Pellets. J. Stem. Cell. Res. Ther. 2013, 3, 142. [Google Scholar] [CrossRef]
- Nakazato, K.; Tamaki, T.; Hirata, M.; Kazuno, A.; Kohno, M.; Masuda, R.; Iwazaki, M. Three-dimensional reconstitution of nerve blood vessel units on damaged trachea and bronchial stump using hybrid-transplantation of skeletal muscle-derived stem cells and bioabsorbable polyglyconate felt. J. Regen. Med. 2015, 4. [Google Scholar] [CrossRef]
- Saito, K.; Tamaki, T.; Hirata, M.; Hashimoto, H.; Nakazato, K.; Nakajima, N.; Kazuno, A.; Sakai, A.; Iida, M.; Okami, K. Reconstruction of Multiple Facial Nerve Branches Using Skeletal Muscle-Derived Multipotent Stem Cell Sheet-Pellet Transplantation. PLoS ONE 2015, 10, e0138371. [Google Scholar] [CrossRef] [Green Version]
- Kazuno, A.; Maki, D.; Yamato, I.; Nakajima, N.; Seta, H.; Soeda, S.; Ozawa, S.; Uchiyama, Y.; Tamaki, T. Regeneration of Transected Recurrent Laryngeal Nerve Using Hybrid-Transplantation of Skeletal Muscle-Derived Stem Cells and Bioabsorbable Scaffold. J. Clin. Med. 2018, 7, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Group | Body Mass (g) | Muscle Mass (mg) | Tetanus (N 102) | ||
|---|---|---|---|---|---|
| PLT + GAS + SOL | PLT + GAS + SOL | ||||
| Cont-side | Op-side | Cont-side | Op-side | ||
| NT (n = 8) | 20.8 ± 1.7 | 139 ± 9 | 51 ± 5 | 103.4 ± 7.7 | 15.2 ± 2.7 |
| CT (n = 6) | 20.8 ± 1.5 | 138 ± 9 | 57 ± 4 | 105.1 ± 4.8 | * 27.8 ± 4.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maki, D.; Tamaki, T.; Fukuzawa, T.; Natsume, T.; Yamato, I.; Uchiyama, Y.; Saito, K.; Okami, K. Peripheral Nerve Regeneration Using a Cytokine Cocktail Secreted by Skeletal Muscle-Derived Stem Cells in a Mouse Model. J. Clin. Med. 2021, 10, 824. https://doi.org/10.3390/jcm10040824
Maki D, Tamaki T, Fukuzawa T, Natsume T, Yamato I, Uchiyama Y, Saito K, Okami K. Peripheral Nerve Regeneration Using a Cytokine Cocktail Secreted by Skeletal Muscle-Derived Stem Cells in a Mouse Model. Journal of Clinical Medicine. 2021; 10(4):824. https://doi.org/10.3390/jcm10040824
Chicago/Turabian StyleMaki, Daisuke, Tetsuro Tamaki, Tsuyoshi Fukuzawa, Toshiharu Natsume, Ippei Yamato, Yoshiyasu Uchiyama, Kosuke Saito, and Kenji Okami. 2021. "Peripheral Nerve Regeneration Using a Cytokine Cocktail Secreted by Skeletal Muscle-Derived Stem Cells in a Mouse Model" Journal of Clinical Medicine 10, no. 4: 824. https://doi.org/10.3390/jcm10040824






