Prognostic Role of Tumor Budding in Breast Cancer Patients Receiving Neo-Adjuvant Therapy
Abstract
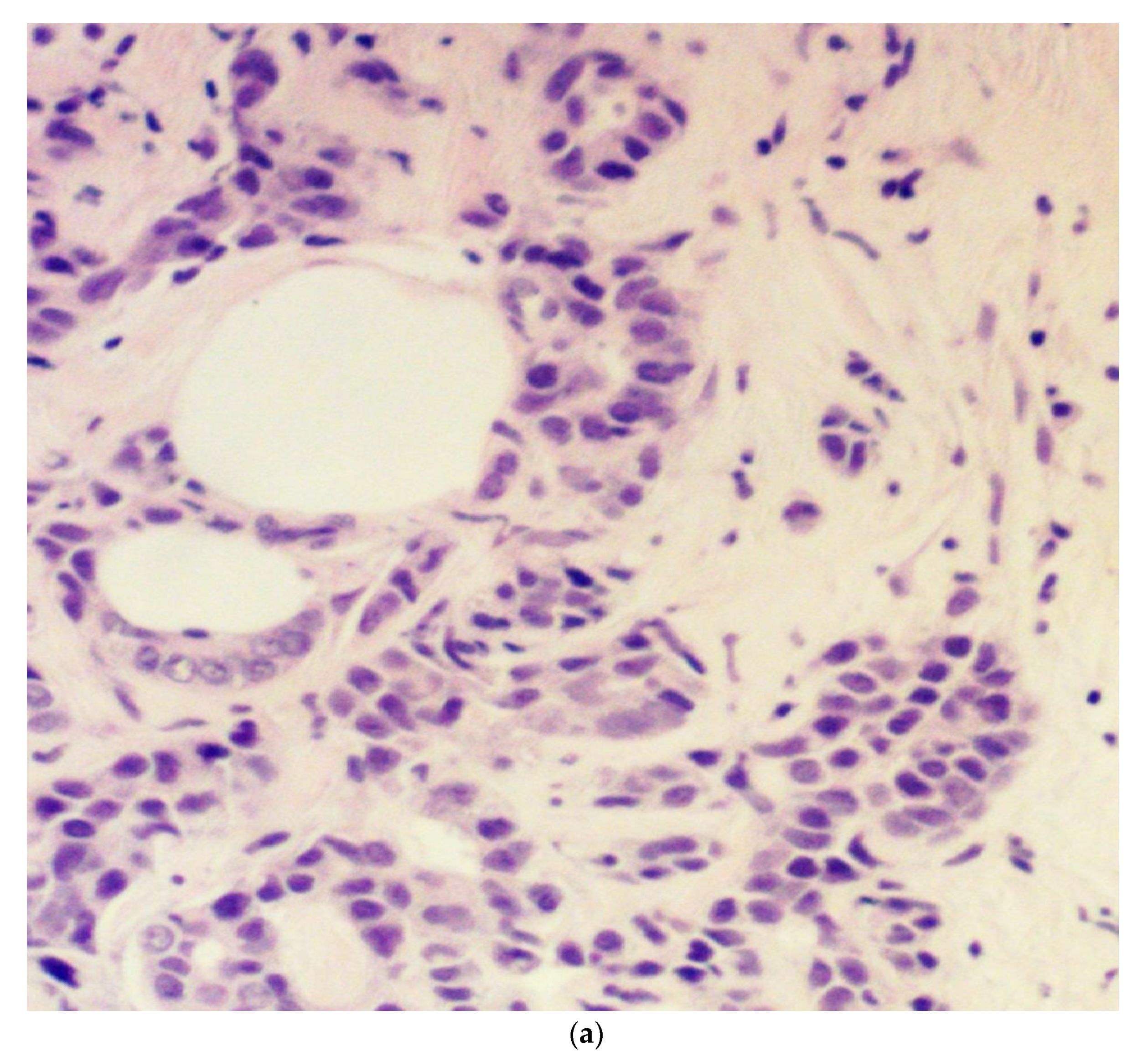
:1. Introduction
2. Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Breast cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Cady, B.; Fulton, J.P. 57% decline in Rhode Island invasive breast cancer mortality between 1987 and 2017: Mammography predominates in preventing mortality. Breast Cancer Res. Treat. 2020, 184, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Battisti, N.M.L.; Tong, D.; Ring, A.; Smith, I. Long-term outcome with targeted therapy in advanced/metastatic HER2-positive breast cancer: The Royal Marsden experience. Breast Cancer Res. Treat. 2019, 178, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Houssami, N.; Macaskill, P.; von Minckwitz, G.; Marinovich, M.L.; Mamounas, E. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur. J. Cancer 2012, 48, 3342–3354. [Google Scholar] [CrossRef] [PubMed]
- Rouzier, R.; Perou, C.M.; Symmans, W.F.; Ibrahim, N.; Cristofanilli, M.; Anderson, K.; Hess, K.R.; Stec, J.; Ayers, M.; Wagner, P.; et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin. Cancer Res. 2005, 11, 5678–5685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voutsadakis, I.A. Prognostic role of tumor budding in breast cancer. World J. Exp. Med. 2018, 8, 12–17. [Google Scholar] [CrossRef]
- Dawson, H.; Lugli, A. Molecular and pathogenetic aspects of tumor budding in colorectal cancer. Front. Oncol. 2015, 2, 11. [Google Scholar] [CrossRef] [Green Version]
- Canguçu, A.L.; Valério, E.; Peixoto, R.B.P.; Felismino, T.C.; de Mello, C.A.L.; Neotti, T.; Calsavara, V.F.; de Macedo, M.P.; Júnior, S.A.; Riechelmann, R. The prognostic influence of tumour budding in Western patients with stage II colorectal cancer. Ecancermedicalscience 2020, 14, 1130. [Google Scholar] [CrossRef]
- Petrelli, F.; Pezzica, E.; Cabiddu, M.; Coinu, A.; Borgonovo, K.; Ghilardi, M.; Lonati, V.; Corti, D.; Barni, S. Tumour budding and survival in stage II colorectal cancer: A systematic review and pooled analysis. J. Gastrointest. Cancer 2015, 46, 212–218. [Google Scholar] [CrossRef]
- Rogers, A.C.; Gibbons, D.; Hanly, A.M.; Hyland, J.M.; O’Connell, P.R.; Winter, D.C.; Sheahan, K. Prognostic significance of tumor budding in rectal cancer biopsies before neoadjuvant therapy. Mod. Pathol. 2014, 27, 156–162. [Google Scholar] [CrossRef] [Green Version]
- Grigore, A.D.; Jolly, M.K.; Jia, D.; Farach-Carson, M.C.; Levine, H. Tumor budding: The name is EMT. Partial, EMT. J. Clin. Med. 2016, 5, 51. [Google Scholar]
- Li, X.; Wei, B.; Sonmez, C.; Li, Z.; Peng, L. High tumor budding count is associated with adverse clinicopathologic features and poor prognosis in breast carcinoma. Hum. Pathol. 2017, 66, 222–229. [Google Scholar] [CrossRef]
- Liang, F.; Cao, W.; Wang, Y.; Li, L.; Zhang, G.; Wang, Z. The prognostic value of tumor budding in invasive breast cancer. Pathol. Res. Pract. 2013, 209, 269–275. [Google Scholar] [CrossRef]
- Gujam, F.J.A.; McMillan, D.C.; Mohammed, Z.M.A.; Edwards, J.; Going, J.J. The relationship between tumour budding, the tumour microenvironment and survival in patients with invasive ductal breast cancer. Br. J. Cancer 2015, 113, 1066–1074. [Google Scholar] [CrossRef] [Green Version]
- Salhia, B.; Trippel, M.; Pfaltz, K.; Cihoric, N.; Grogg, A.; Lädrach, C.; Zlobec, I.; Tapia, C. High tumor budding stratifies breast cancer with metastatic properties. Breast Cancer Res. Treat. 2015, 150, 363–371. [Google Scholar] [CrossRef] [Green Version]
- Lugli, A.; Kirsch, R.; Ajioka, Y.; Bosman, F.; Cathomas, G.; Dawson, H.; El Zimaity, H.; Fléjou, J.F.; Hansen, T.P.; Hartmann, A.; et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol. 2017, 30, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voutsadakis, I.A. Epithelial-Mesenchymal Transition (EMT) and regulation of EMT factors by steroid nuclear receptors in breast cancer: A review and in silico investigation. J. Clin. Med. 2016, 5, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging biologic principles of metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voutsadakis, I.A. The Ubiquitin–Proteasome System and signal transduction pathways regulating Epithelial Mesenchymal transition of cancer. J. Biomed. Sci. 2012, 19, 67. [Google Scholar] [CrossRef] [Green Version]
- Voutsadakis, I.A. The network of pluripotency, epithelial-mesenchymal transition, and prognosis of breast cancer. Breast Cancer 2015, 7, 303–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morel, A.P.; Lièvre, M.; Thomas, C.; Hinkal, G.; Ansieau, S.; Puisieux, A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS ONE 2008, 3, e2888. [Google Scholar] [CrossRef] [PubMed]
- Kadota, K.; Yeh, Y.C.; Villena-Vargas, J.; Cherkassky, L.; Drill, E.N.; Sima, C.S.; Jones, D.R.; Travis, W.D.; Adusumilli, P.S. Tumor budding correlates with the protumor immune microenvironment and is an independent prognostic factor for recurrence of stage I lung adenocarcinoma. Chest 2015, 148, 711–721. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, S.; Miyazaki, A.; Sonoda, T.; Koike, K.; Ogi, K.; Kobayashi, J.; Kaneko, T.; Igarashi, T.; Ueda, M.; Dehari, H.; et al. Tumor budding is an independent prognostic marker in early stage oral squamous cell carcinoma: With special reference to the mode of invasion and worst pattern of invasion. PLoS ONE 2018, 13, e0195451. [Google Scholar] [CrossRef] [Green Version]
- Lugli, A.; Zlobec, I.; Berger, M.D.; Kirsch, R.; Nagtegaal, I.D. Tumour budding in solid cancers. Nat. Rev. Clin. Oncol. 2020, 8. [Google Scholar] [CrossRef]
- Sun, Y.; Liang, F.; Cao, W.; Wang, K.; He, J.; Wang, H.; Wang, Y. Prognostic value of poorly differentiated clusters in invasive breast cancer. World J. Surg. Oncol. 2014, 12, 310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lloyd, A.J.; Ryan, É.J.; Boland, M.R.; Elwahab, S.A.; Malone, C.; Sweeney, K.J.; Barry, K.M.; McLaughlin, R.; Kerin, M.J.; Lowery, A.J. The histopathological and molecular features of breast carcinoma with tumour budding-a systematic review and meta-analysis. Breast Cancer Res Treat. 2020, 183, 503–514. [Google Scholar] [CrossRef]
- Agarwal, R.; Khurana, N.; Singh, T.; Agarwal, P.N. Tumor budding in infiltrating breast carcinoma: Correlation with known clinicopathological parameters and hormone receptor status. Indian J. Pathol. Microbiol. 2019, 62, 222–225. [Google Scholar] [CrossRef]


| Category | Total (%) (n = 75) | Tumor Budding Absent (n = 25) | Tumor Budding Present (n = 50) | p Value | |
|---|---|---|---|---|---|
| AGE | Mean | 58.9 | 58.9 | 59 | p = 0.9 |
| ≤ 65 | 52 (69.3%) | 19 (76%) | 33 (66%) | p = 0.43 | |
| > 65 | 23 (30.7%) | 6 (24%) | 17 (34%) | ||
| MENOPAUSE STATUS | Pre-/perimenopausal | 19 (25.3%) | 7 (28%) | 12 (24%) | p = 0.78 |
| Post-menopausal | 56 (74.7%) | 18 (72%) | 38 (76%) | ||
| CLINICAL STAGE | I | 3 (4%) | 1 (4%) | 2 (4%) | p = 0.26 (Stage I/II versus stage III) |
| II | 52 (69.3%) | 15 (60%) | 37 (74%) | ||
| III | 20 (26.7%) | 9 (36%) | 11 (22%) | ||
| ER | positive | 58 (77.3%) | 20 (80%) | 38 (76%) | p = 0.77 |
| negative | 17 (22.7%) | 5 (20%) | 12 (24%) | ||
| PR | positive | 47 (62.7%) | 16 (64%) | 31 (62%) | p = 0.99 |
| negative | 28 (37.3%) | 9 (36%) | 19 (38%) | ||
| HER2 | positive | 23 (30.7%) | 7 (28%) | 16 (32%) | p = 0.79 |
| negative | 52 (69.3%) | 18 (72%) | 34 (64%) | ||
| SUB-TYPE | ER+/ HER2− | 41 (54.7%) | 15 (60%) | 26 (52%) | |
| HER2+ | 24 (32%) | 8 (32%) | 16 (32%) | p = 0.6 | |
| Triple Negative | 10 (13.3%) | 2 (8%) | 8 (16%) | ||
| HISTOLOGY (n = 74) | Ductal | 58 (78.4%) | 21 (84%) | 37 (74%) | p = 0.35 (Ductal versus lobular/ mixed) |
| Lobular | 10 (13.5%) | 3 (12%) | 7 (14%) | ||
| Mixed | 5 (6.8%) | 0 | 5 (10%) | ||
| Other | 1 (1.3%) | 1 (4%) | 0 | ||
| RESPONSE (n = 72) | CR | 15 (20.8%) | 4 (16.7%) | 11 (22.9%) | p = 0.8 |
| PR | 20 (27.8%) | 7 (29.2%) | 13 (27.1%) | ||
| NR | 37 (51.4%) | 13 (54.2%) | 24 (50%) |
| Ductal | Lobular/Mixed | |||||
|---|---|---|---|---|---|---|
| Sub-Type | Total (%) (n = 58) | Tumor Budding Absent (n = 21) | Tumor Budding Present (n = 37) | Total (%) (n = 15) | Tumor Budding Absent (n = 3) | Tumor Budding Present (n = 12) |
| ER+/HER2− | 29 (50%) | 11 (52.4%) | 18 (48.7%) | 11 (73.3%) | 3 (100%) | 8 (66.7%) |
| HER2+/ER+ | 13 (22.4%) | 5 (23.8%) | 8 (21.6%) | 3 (20%) | 0 | 3 (25%) |
| HER2+/ ER− | 6 (10.4%) | 3 (14.3%) | 3 (8.1%) | 1 (6.7%) | 0 | 1 (8.3%) |
| TNBC | 10 (17.2%) | 2 (9.5%) | 8 (21.6%) | 0 | 0 | 0 |
| Category | Total (%) (n = 69) | Tumor Budding Absent (n = 24) | Tumor Budding Present (n = 45) | |
|---|---|---|---|---|
| FEC-D (n = 61) | ||||
| RESPONSE | CR | 12 (19.7%) | 3 (14.3%) | 9 (22.5%) |
| PR | 17 (27.9%) | 6 (28.6%) | 11 (27.5%) | |
| NR | 32 (52.4%) | 12 (57.1%) | 20 (50%) | |
| AC-Paclitaxel (n = 4) | ||||
| RESPONSE | CR | 1 (25%) | 1 (100%) | 0 |
| PR | 1 (25%) | 0 | 1 (33.3%) | |
| NR | 2 (50%) | 0 | 2 (66.7%) | |
| Carboplatin-Docetaxel (n = 3) | ||||
| RESPONSE | CR | 0 | 0 | 0 |
| PR | 2 (66.7%) | 1 (50%) | 1 (100%) | |
| NR | 1 (33.3%) | 1 (50%) | 0 | |
| Docetaxel-Cyclophosphamide (n = 1) | ||||
| RESPONSE | CR | 1 (100%) | 0 | 1 (100%) |
| PR | 0 | 0 | 0 | |
| NR | 0 | 0 | 0 |
| Category | Total (%) (n = 75) | Low Tumor Budding (n = 39) | Intermediate/High Tumor Budding (n = 36) | x2 | |
|---|---|---|---|---|---|
| AGE | Mean | 58.9 | 59.1 | 58.8 | p = 0.8 |
| ≤65 | 52 (69.3%) | 28 (71.8%) | 24 (66.7%) | p = 0.8 | |
| >65 | 23 (30.7%) | 11(28.2%) | 12 (33.3%) | ||
| MENOPAUSE STATUS | Pre-/perimenopausal | 19 (25.3%) | 11 (28.2%) | 8 (22.2%) | p = 0.7 |
| Post-menopausal | 56 (74.7%) | 28 (71.8%) | 28 (77.8%) | ||
| CLINICAL STAGE | I | 3 (4%) | 1 (2.6%) | 2 (5.6%) | p = 0.6 |
| II | 52 (69.3%) | 26 (66.7%) | 26 (72.2%) | ||
| III | 20 (26.7%) | 12 (30.7%) | 8 (22.2%) | ||
| ER | positive | 58 (77.3%) | 29 (74.4%) | 29 (80.6%) | p = 0.7 |
| negative | 17 (22.7%) | 10 (25.6%) | 7 (19.4%) | ||
| PR | positive | 47 (62.7%) | 24 (61.5%) | 23 (63.9%) | p = 0.8 |
| negative | 28 (37.3%) | 15 (38.5%) | 13 (36.1%) | ||
| HER2 | positive | 23 (30.7%) | 12 (30.7%) | 11 (30.6%) | p = 0.9 |
| negative | 52 (69.3%) | 27 (69.3%) | 25 (69.4%) | ||
| SUB-TYPE | ER+/ HER2− | 41 (54.7%) | 22 (56.4%) | 19 (52.7%) | |
| HER2+ | 24 (32%) | 13 (33.3%) | 11 (30.6%) | p = 0.7 | |
| Triple Negative | 10 (13.3%) | 4 (10.3%) | 6 (16.7%) | ||
| HISTOLOGY (n = 74) | Ductal | 58 (78.4%) | 32 (82%) | 26 (74.3%) | |
| Lobular | 10 (13.5%) | 4 (10.3%) | 6 (17.1%) | p = 0.7 | |
| Mixed | 5 (6.8%) | 2 (5.1%) | 3 (8.6%) | ||
| Other | 1 (1.3%) | 1 (2.6%) | 0 | ||
| RESPONSE (n = 72) | CR | 15 (20.8%) | 8 (21%) | 7 (20.6%) | p = 0.69 |
| PR | 20 (27.8%) | 9 (23.7%) | 11 (32.3%) | ||
| NR | 37 (51.4%) | 21 (55.3%) | 16 (47.1%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mozarowski, P.; Rasaiah, B.; Reed, M.; Lewis, A.; Walde, N.; Voutsadakis, I.A. Prognostic Role of Tumor Budding in Breast Cancer Patients Receiving Neo-Adjuvant Therapy. J. Clin. Med. 2021, 10, 827. https://doi.org/10.3390/jcm10040827
Mozarowski P, Rasaiah B, Reed M, Lewis A, Walde N, Voutsadakis IA. Prognostic Role of Tumor Budding in Breast Cancer Patients Receiving Neo-Adjuvant Therapy. Journal of Clinical Medicine. 2021; 10(4):827. https://doi.org/10.3390/jcm10040827
Chicago/Turabian StyleMozarowski, Paul, Bhubendra Rasaiah, Melissa Reed, Alexis Lewis, Natalie Walde, and Ioannis A. Voutsadakis. 2021. "Prognostic Role of Tumor Budding in Breast Cancer Patients Receiving Neo-Adjuvant Therapy" Journal of Clinical Medicine 10, no. 4: 827. https://doi.org/10.3390/jcm10040827
APA StyleMozarowski, P., Rasaiah, B., Reed, M., Lewis, A., Walde, N., & Voutsadakis, I. A. (2021). Prognostic Role of Tumor Budding in Breast Cancer Patients Receiving Neo-Adjuvant Therapy. Journal of Clinical Medicine, 10(4), 827. https://doi.org/10.3390/jcm10040827






