Predicting Keratoconus Progression and Need for Corneal Crosslinking Using Deep Learning
Abstract
1. Introduction
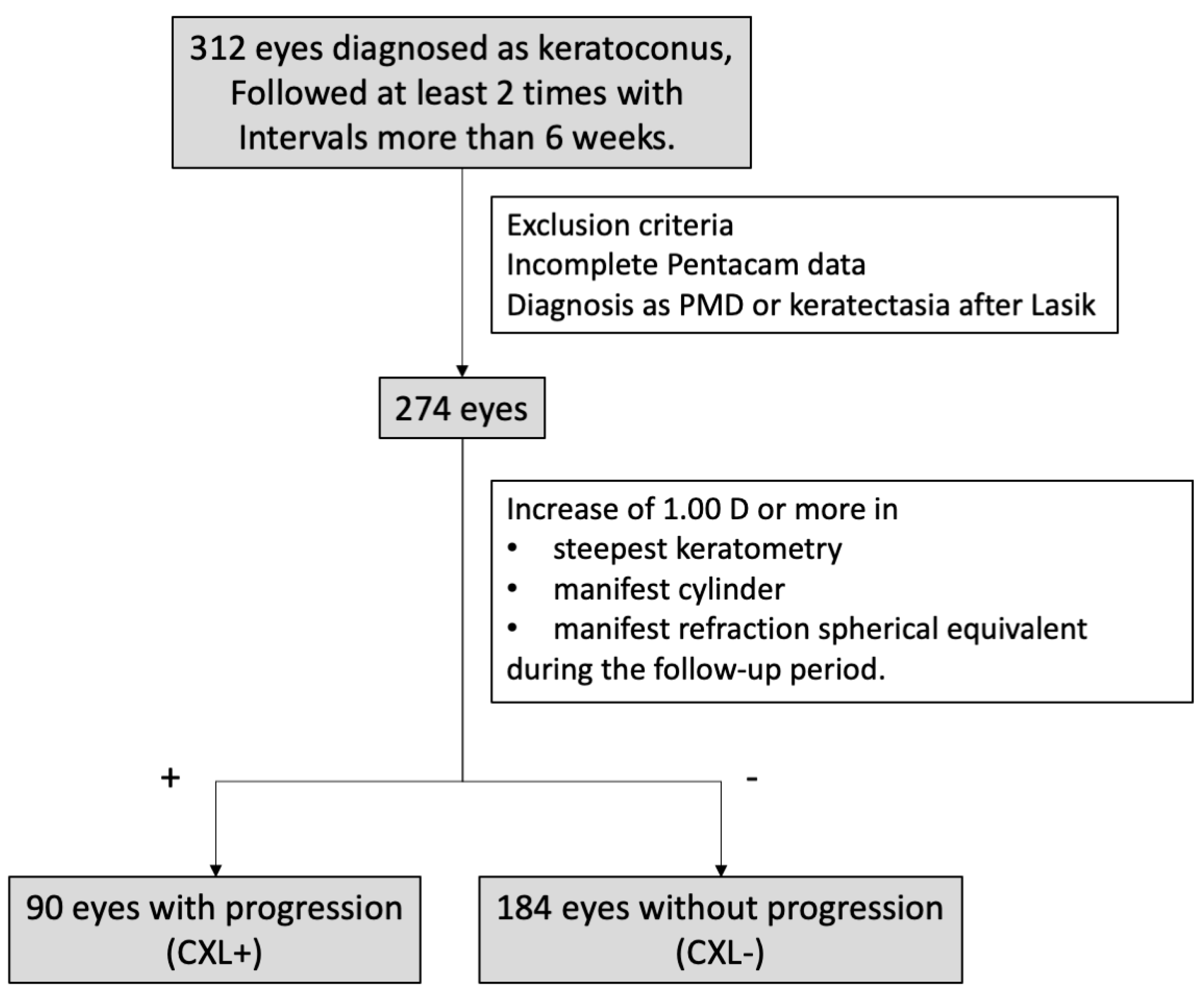
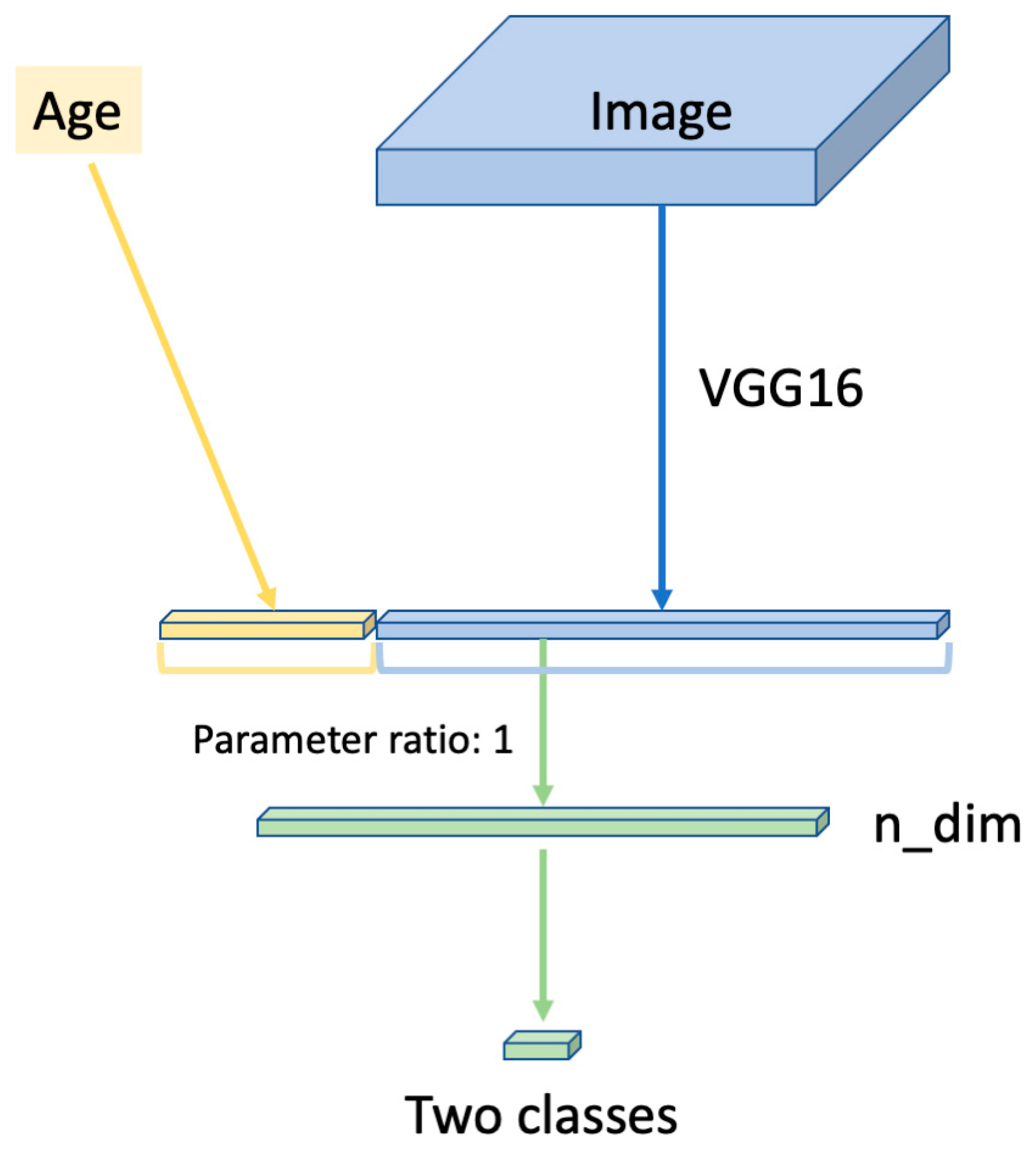
2. Materials and Methods
3. Results
3.1. Background
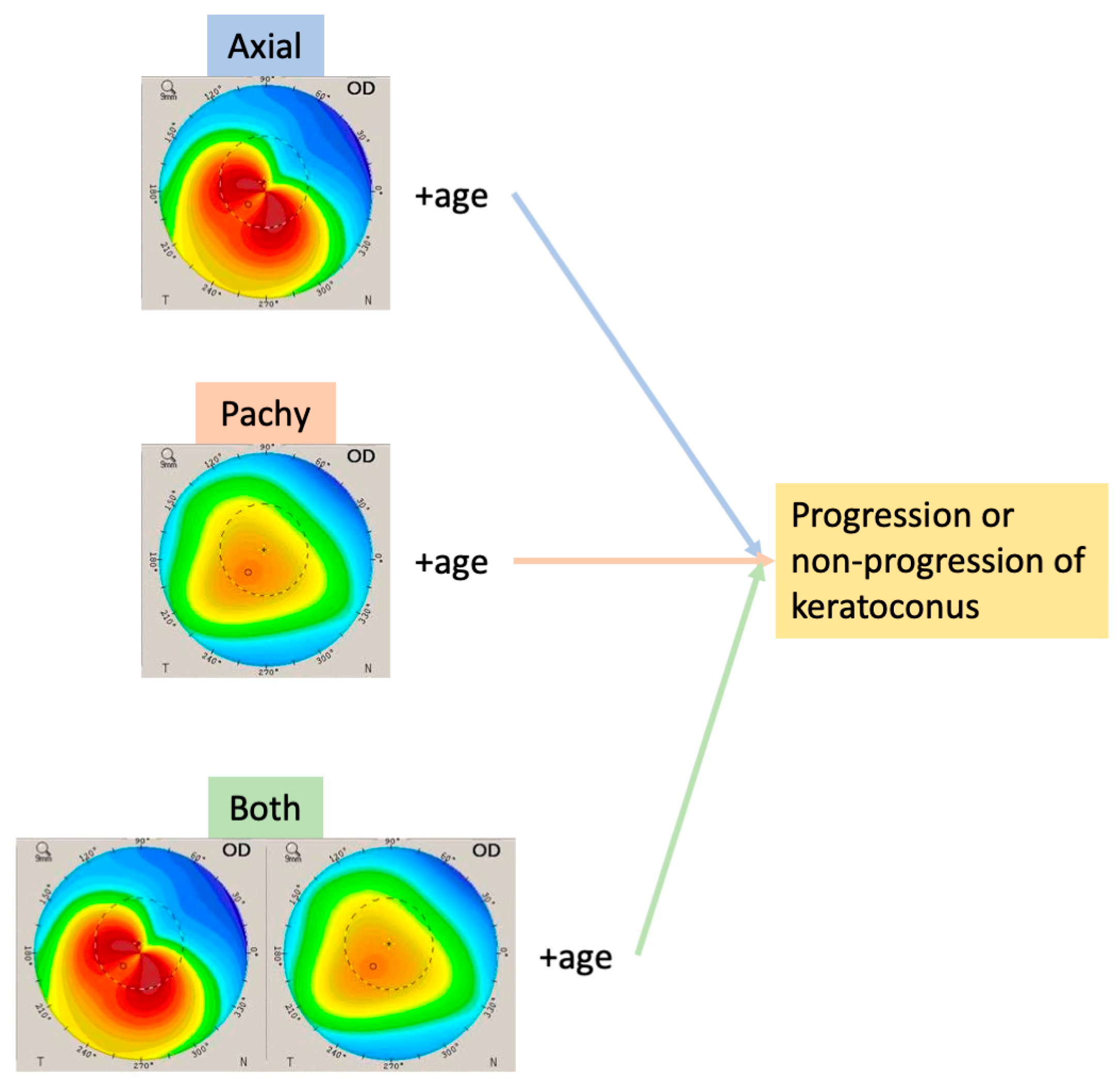
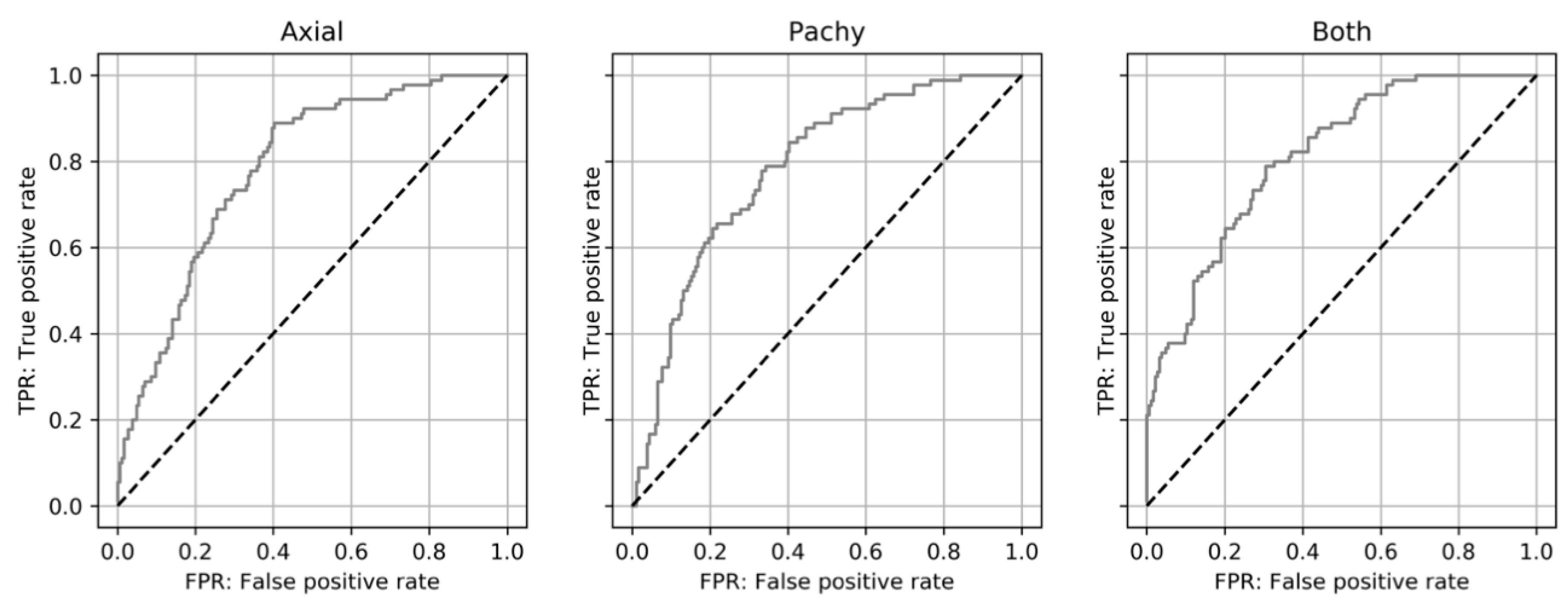
3.2. Evaluation of Keratoconus Progression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wollensak, G.; Spoerl, E.; Seiler, T. Riboflavin/ultraviolet-a–induced collagen crosslinking for the treatment of keratoconus. Am. J. Ophthalmol. 2003, 135, 620–627. [Google Scholar] [CrossRef]
- Khattak, A.; Nakhli, F.R.; Cheema, H.R. Corneal collagen crosslinking for progressive keratoconus in Saudi Arabia: One-year controlled clinical trial analysis. Saudi J. Ophthalmol. 2015, 29, 249–254. [Google Scholar] [CrossRef]
- Alhayek, A.; Lu, P.-R. Corneal collagen crosslinking in keratoconus and other eye disease. Int. J. Ophthalmol. 2015, 8, 407–418. [Google Scholar]
- Asri, D.; Touboul, D.; Fournié, P.; Malet, F.; Garra, C.; Gallois, A.; Malecaze, F.; Colin, J. Corneal collagen crosslinking in progressive keratoconus: Multicenter results from the French National Reference Center for Keratoconus. J. Cataract. Refract. Surg. 2011, 37, 2137–2143. [Google Scholar] [CrossRef] [PubMed]
- Wittig-Silva, C.; Whiting, M.; Lamoureux, E.; Sullivan, L.J.; Lindsay, R.G.; Snibson, G.R. A Randomized Controlled Trial of Corneal Collagen Cross-linking in Progressive Keratoconus: Preliminary Results. J. Refract. Surg. 2008, 24, S720–S725. [Google Scholar] [CrossRef]
- Hersh, P.S.; Stulting, R.D.; Muller, D.; Durrie, D.S.; Rajpal, R.K.; Binder, P.S.; Donnenfeld, E.D.; Hardten, D.; Price, F.; Schanzlin, D.; et al. United States Multicenter Clinical Trial of Corneal Collagen Crosslinking for Keratoconus Treatment. Ophthalmology 2017, 124, 1259–1270. [Google Scholar] [CrossRef]
- Wisse, R.P.L.; Simons, R.W.P.; Van der Vossen, M.J.B.; Muijzer, M.B.; Soeters, N.; Nuijts, R.M.M.A.; Godefrooij, D.A. Clinical evaluation and validation of the Dutch Crosslinking for Kera-toconus score. JAMA Ophthalmol. 2019, 137, 610–616. [Google Scholar] [CrossRef]
- Romano, V.; Vinciguerra, R.; Arbabi, E.M.; Hicks, N.; Rosetta, P.; Vinciguerra, P.; Kaye, S.B. Progression of Keratoconus in Patients While Awaiting Corneal Cross-linking: A Prospective Clinical Study. J. Refract. Surg. 2018, 34, 177–180. [Google Scholar] [CrossRef]
- Ting, D.S.W.; Pasquale, L.R.; Peng, L.; Campbell, J.P.; Lee, A.Y.; Raman, R.; Tan, G.S.W.; Schmetterer, L.; A Keane, P.; Wong, T.Y. Artificial intelligence and deep learning in ophthalmology. Br. J. Ophthalmol. 2018, 103, 167–175. [Google Scholar] [CrossRef]
- Du, X.-L.; Li, W.-B.; Hu, B.-J. Application of artificial intelligence in ophthalmology. Int. J. Ophthalmol. 2018, 11, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xue, K.; Zhang, K. Current status and future trends of clinical diagnoses via image-based deep learning. Theranostics 2019, 9, 7556–7565. [Google Scholar] [CrossRef]
- Tong, Y.; Lu, W.; Yu, Y.; Shen, Y. Application of machine learning in ophthalmic imaging modalities. Eye Vis. 2020, 7, 1–15. [Google Scholar] [CrossRef]
- Lim, G.; Bellemo, V.; Xie, Y.; Lee, X.Q.; Yip, M.Y.T.; Ting, D.S.W. Different fundus imaging modalities and technical factors in AI screening for diabetic retinopathy: A review. Eye Vis. 2020, 7, 1–13. [Google Scholar] [CrossRef]
- Devalla, S.K.; Liang, Z.; Pham, T.H.; Boote, C.; Strouthidis, N.G.; Thiery, A.H.; A Girard, M.J. Glaucoma management in the era of artificial intelligence. Br. J. Ophthalmol. 2019, 104, 301–311. [Google Scholar] [CrossRef]
- Grzybowski, A.; Brona, P.; Lim, G.; Ruamviboonsuk, P.; Tan, G.S.W.; Abramoff, M.; Ting, D.S.W. Artificial intelligence for diabetic retinopathy screening: A review. Eye 2020, 34, 451–460. [Google Scholar] [CrossRef]
- Wong, T.Y.; Sabanayagam, C. Strategies to Tackle the Global Burden of Diabetic Retinopathy: From Epidemiology to Artificial Intelligence. Ophthalmology 2019, 243, 9–20. [Google Scholar] [CrossRef]
- Yousefi, S.; Takahashi, H.; Hayashi, T.; Tampo, H.; Inoda, S.; Arai, Y.; Tabuchi, H.; Asbell, P. Predicting the likelihood of need for future keratoplasty intervention using artifi-cial intelligence. Ocul. Surf. 2020, 18, 320–325. [Google Scholar] [CrossRef]
- Yousefi, S.; Yousefi, E.; Takahashi, H.; Hayashi, T.; Tampo, H.; Inoda, S.; Arai, Y.; Asbell, P. Keratoconus severity identification using unsupervised machine learning. PLoS ONE 2018, 13, e0205998. [Google Scholar] [CrossRef]
- Klyce, S.D. The Future of Keratoconus Screening with Artificial Intelligence. Ophthalmology 2018, 125, 1872–1873. [Google Scholar] [CrossRef]
- Kamiya, K.; Ayatsuka, Y.; Kato, Y.; Fujimura, F.; Takahashi, M.; Shoji, N.; Mori, Y.; Miyata, K. Keratoconus detection using deep learning of colour-coded maps with anterior seg-ment optical coherence tomography: A diagnostic accuracy study. BMJ Open 2019, 9, e031313. [Google Scholar] [CrossRef] [PubMed]
- Mosteller, F.; Tukey, J.W. Data analysis, including statistics. In Handbook of Social Psychology: Vol. 2. Research Methods; Lindzey, G., Aronson, E., Eds.; Addison-Wesley Pub. Co: Boston, MA, USA, 1968; pp. 80–203. [Google Scholar]
- Kohavi, R. A study of cross-validation and bootstrap for accuracy estimation and model selection. In Proceedings of the Fourteenth International Joint Conference on Artificial Intelligence, IJCAI 95, Montréal, QC, Canada, 20–25 August 1995; pp. 1137–1145. [Google Scholar]
- Simonyan, K.; Zisserman, A. Very deep convolutional networks for large-scale image recognition. arXiv 2014, arXiv:1409.1556. [Google Scholar]
- Deng, J.; Dong, W.; Socher, R.; Li, L.J.; Li, K.; Li, F.F. Imagenet: A Large-Scale Hierarchical Image Database. In Proceedings of the 2009 IEEE Conference on Computer Vision and Pattern Recognition, Miami, FL, USA, 20–25 June 2009; pp. 248–255. [Google Scholar]
- Lee, C.Y.; Xie, S.; Gallagher, P.; Zhang, Z.; Tu, Z. Deeply-supervised nets. In Proceedings of the 18th International Conference on Artificial Intelligence and Statistics, San Diego, CA, USA, 9–12 May 2015; Volume 38, pp. 562–570. [Google Scholar]
- Russakovsky, O.; Deng, J.; Su, H.; Krause, J.; Satheesh, S.; Ma, S.; Huang, Z.; Karpathy, A.; Khosla, A.; Bernstein, M.; et al. ImageNet Large Scale Visual Recognition Challenge. Int. J. Comput. Vis. 2015, 115, 211–252. [Google Scholar] [CrossRef]
- Glorot, X.; Bordes, A.; Bengio, Y. Deep sparse rectifier neural networks. In Proceedings of the 14th International Conference on Artificial Intelligence and Statistics, Lauderdale, FL, USA, 11–13 April 2011; Volume 15, pp. 315–323. [Google Scholar]
- Scherer, D.; Müller, A.; Behnke, S. Evaluation of Pooling Operations in Convolutional Architectures for Object Recognition. In Proceedings of the Artificial Neural Networks—ICANN 2010—20th International Conference, Thessaloniki, Greece, 15–18 September 2010; pp. 92–101. [Google Scholar]
- Agrawal, P.; Girshick, R.; Malik, J. Analyzing the Performance of Multilayer Neural Networks for Object Recognition. In Proceedings of the Constructive Side-Channel Analysis and Secure Design, Paris, France, 13–15 April 2014; Springer International Publishing: Graz, Austria, 2014; pp. 329–344. [Google Scholar]
- Qian, N. On the momentum term in gradient descent learning algorithms. Neural Netw. 1999, 12, 145–151. [Google Scholar] [CrossRef]
- Nesterov, Y. A method for unconstrained convex minimization problem with the rate of convergence O (1/k^2). Proc. USSR Acad. Sci. 1983, 269, 543–547. [Google Scholar]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 32–35. [Google Scholar] [CrossRef]
- Perez-Straziota, C.; Gaster, R.N.; Rabinowitz, Y.S. Corneal cross-linking for pediatric keratoconus review. Cornea 2018, 37, 802–809. [Google Scholar] [CrossRef]
- Olivo-Payne, A.; Abdala-Figuerola, A.; Hernandez-Bogantes, E.; Pedro-Aguilar, L.; Chan, E.; Godefrooij, D. Optimal management of pediatric keratoconus: Challenges and solutions. Clin. Ophthalmol. 2019, 13, 1183–1191. [Google Scholar] [CrossRef]
- Kato, N.; Negishi, K.; Sakai, C.; Tsubota, K. Baseline factors predicting the need for corneal crosslinking in patients with kera-toconus. PLoS ONE 2020, 15, e0231439. [Google Scholar] [CrossRef]
| Progression Group | Non-Progression Group | p-Value | |
|---|---|---|---|
| Age (mean ± SD) | 21.0 ± 5.9 | 31.5 ± 12.4 | p < 0.01 |
| Gender (female ratio) | 24/90 | 55/184 | p = 0.67 |
| AUC | Sensitivity | Specificity | |
|---|---|---|---|
| Axial | 0.783 (0.721–0.845) | 87.8% (79/90) (79.2–93.7) | 59.8% (110/184) (52.3–66.9) |
| Pachy | 0.784 (0.722–0.846) | 77.8% (70/90) (67.8–85.9) | 65.8% (121/184) (58.4–72.6) |
| Both | 0.814 (0.755–0.872) | 77.8% (70/90) (67.8–85.9) | 69.6% (128/184) (62.4–76.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, N.; Masumoto, H.; Tanabe, M.; Sakai, C.; Negishi, K.; Torii, H.; Tabuchi, H.; Tsubota, K. Predicting Keratoconus Progression and Need for Corneal Crosslinking Using Deep Learning. J. Clin. Med. 2021, 10, 844. https://doi.org/10.3390/jcm10040844
Kato N, Masumoto H, Tanabe M, Sakai C, Negishi K, Torii H, Tabuchi H, Tsubota K. Predicting Keratoconus Progression and Need for Corneal Crosslinking Using Deep Learning. Journal of Clinical Medicine. 2021; 10(4):844. https://doi.org/10.3390/jcm10040844
Chicago/Turabian StyleKato, Naoko, Hiroki Masumoto, Mao Tanabe, Chikako Sakai, Kazuno Negishi, Hidemasa Torii, Hitoshi Tabuchi, and Kazuo Tsubota. 2021. "Predicting Keratoconus Progression and Need for Corneal Crosslinking Using Deep Learning" Journal of Clinical Medicine 10, no. 4: 844. https://doi.org/10.3390/jcm10040844
APA StyleKato, N., Masumoto, H., Tanabe, M., Sakai, C., Negishi, K., Torii, H., Tabuchi, H., & Tsubota, K. (2021). Predicting Keratoconus Progression and Need for Corneal Crosslinking Using Deep Learning. Journal of Clinical Medicine, 10(4), 844. https://doi.org/10.3390/jcm10040844









