Predictors and Early Markers of Response to Biological Therapies in Inflammatory Bowel Diseases
Abstract
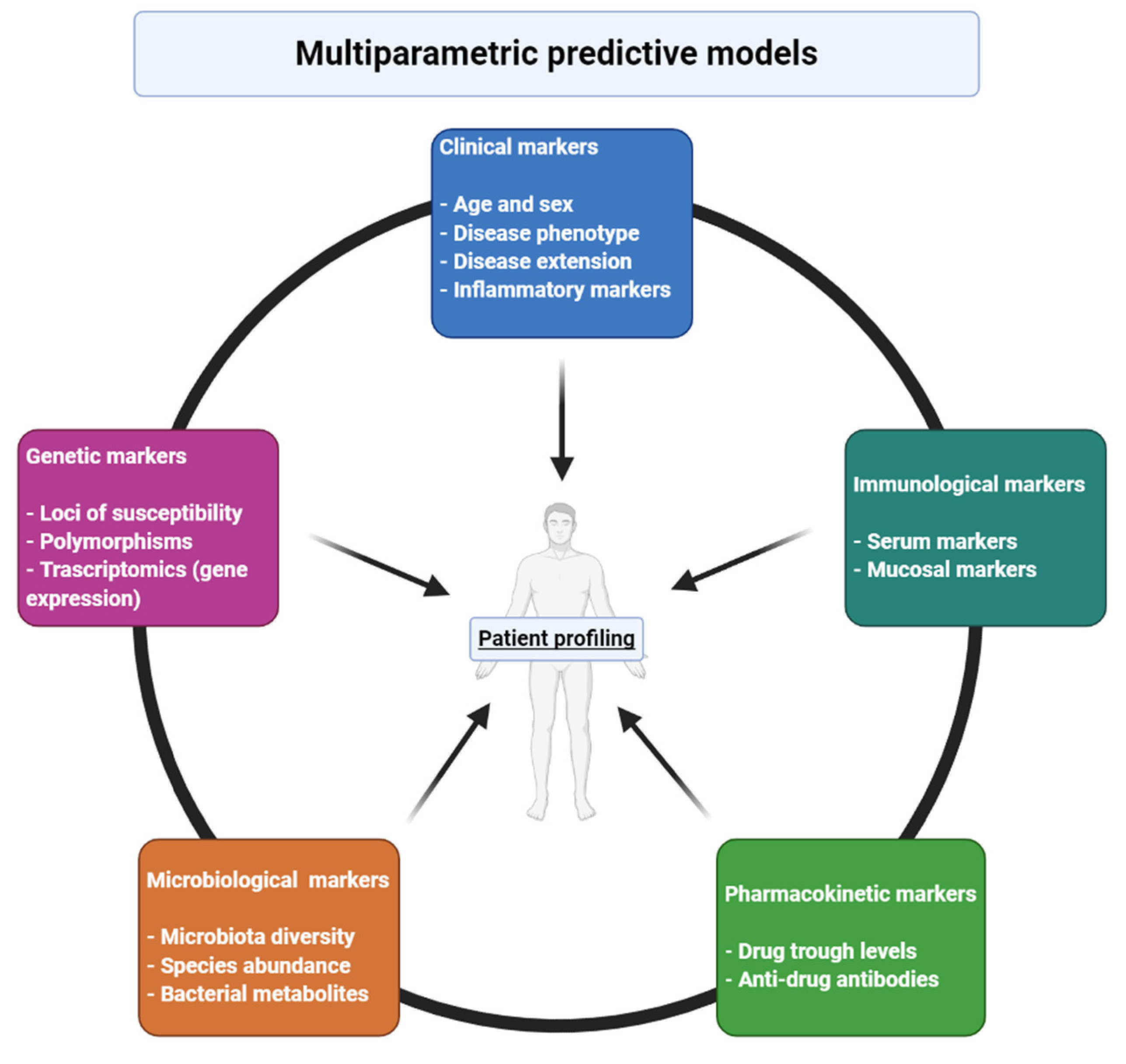
:1. Introduction
2. Traditional Markers
3. Genetic Markers
4. Other Markers: Transcriptomics, Proteomics and Immunological Markers
5. Therapeutic Drug Monitoring
6. Gut Microbiota
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef]
- Park, K.T.; Ehrlich, O.G.; Allen, J.I.; Meadows, P.; Szigethy, E.M.; Henrichsen, K.; Kim, S.C.; Lawton, R.C.; Murphy, S.M.; Regueiro, M.; et al. The Cost of Inflammatory Bowel Disease: An Initiative from the Crohn’s & Colitis Foundation. Inflamm. Bowel Dis. 2020, 26, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Harbord, M.; Eliakim, R.; Bettenworth, D.; Karmiris, K.; Katsanos, K.; Kopylov, U.; Kucharzik, T.; Molnár, T.; Raine, T.; Sebastian, S.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J. Crohn’s Colitis 2017, 11, 769–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohn’s Colitis 2020, 14, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Su, C.; Sands, B.E.; D’Haens, G.R.; Vermeire, S.; Schreiber, S.; Danese, S.; Feagan, B.G.; Reinisch, W.; Niezychowski, W.; et al. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2017, 376, 1723–1736. [Google Scholar] [CrossRef]
- Verdier, J.; Bègue, B.; Cerf-Bensussan, N.; Ruemmele, F.M. Compartmentalized Expression of Th1 and Th17 Cytokines in Pediatric Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2012, 18, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Atreya, R.; Neurath, M.F. Mechanisms of molecular resistance and predictors of response to biological therapy in inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 2018, 3, 790–802. [Google Scholar] [CrossRef]
- Zorzi, F.; Monteleone, I.; Sarra, M.; Calabrese, E.; Marafini, I.; Cretella, M.; Sedda, S.; Biancone, L.; Pallone, F.; Monteleone, G. Distinct Profiles of Effector Cytokines Mark the Different Phases of Crohn’s Disease. PLoS ONE 2013, 8, e54562. [Google Scholar] [CrossRef]
- Singh, S.; Murad, M.H.; Fumery, M.; Dulai, P.S.; Sandborn, W.J. First- and Second-Line Pharmacotherapies for Patients with Moderate to Severely Active Ulcerative Colitis: An Updated Network Meta-Analysis. Clin. Gastroenterol. Hepatol. 2020, 18, 2179–2191.e6. [Google Scholar] [CrossRef]
- Singh, S.; Fumery, M.; Sandborn, W.J.; Murad, M.H. Systematic review and network meta-analysis: First- and second-line biologic therapies for moderate-severe Crohn’s disease. Aliment. Pharmacol. Ther. 2018, 48, 394–409. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; George, J.; Boland, B.S.; Casteele, N.V.; Sandborn, W.J. Primary Non-Response to Tumor Necrosis Factor Antagonists is Associated with Inferior Response to Second-line Biologics in Patients with Inflammatory Bowel Diseases: A Systematic Review and Meta-analysis. J. Crohn’s Colitis 2018, 12, 635–643. [Google Scholar] [CrossRef]
- Ben-Horin, S.; Kopylov, U.; Chowers, Y. Optimizing anti-TNF treatments in inflammatory bowel disease. Autoimmun. Rev. 2014, 13, 24–30. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Chaparro, M. Predictors of Primary Response to Biologic Treatment [Anti-TNF, Vedolizumab, and Ustekinumab] in Patients with Inflammatory Bowel Disease: From Basic Science to Clinical Practice. J. Crohn’s Colitis 2020, 14, 694–709. [Google Scholar] [CrossRef]
- Hamdeh, S.; Aziz, M.; Altayar, O.; Olyaee, M.; Murad, M.H.; Hanauer, S.B. Early vs Late Use of Anti-TNFa Therapy in Adult Patients with Crohn Disease: A Systematic Review and Meta-Analysis. Inflamm. Bowel Dis. 2020, 26, 1808–1818. [Google Scholar] [CrossRef]
- Ungaro, R.C.; Aggarwal, S.; Topaloglu, O.; Lee, W.-J.; Clark, R.; Colombel, J.-F. Systematic review and meta-analysis: Efficacy and safety of early biologic treatment in adult and paediatric patients with Crohn’s disease. Aliment. Pharmacol. Ther. 2020, 51, 831–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, N.; Rabizadeh, S.; Jossen, J.; Pittman, N.; Check, M.; Hashemi, G.; Phan, B.L.; Hyams, J.S.; Dubinsky, M.C. Multi-Center Experience of Vedolizumab Effectiveness in Pediatric Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2016, 22, 2121–2126. [Google Scholar] [CrossRef] [PubMed]
- Vermeire, S.; Louis, E.; Carbonez, A.; Van Assche, G.; Noman, M.; Belaiche, J.; De Vos, M.; Van Gossum, A.; Pescatore, P.; Fiasse, R.; et al. Demographic and clinical parameters influencing the short-term outcome of anti-tumor necrosis factor (infliximab) treatment in Crohn’s disease. Am. J. Gastroenterol. 2002, 97, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Sprakes, M.B.; Ford, A.C.; Warren, L.; Greer, D.; Hamlin, J. Efficacy, tolerability, and predictors of response to infliximab therapy for Crohn’s disease: A large single centre experience. J. Crohn’s Colitis 2012, 6, 143–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorzi, F.; Zuzzi, S.; Onali, S.; Calabrese, E.; Condino, G.; Petruzziello, C.; Ascolani, M.; Pallone, F.; Biancone, L. Efficacy and safety of infliximab and adalimumab in Crohn’s disease: A single centre study. Aliment. Pharmacol. Ther. 2012, 35, 1397–1407. [Google Scholar] [CrossRef]
- Kiss, L.S.; Szamosi, T.; Molnár, T.; Miheller, P.; Lakatos, L.; Vincze, A.; Palatka, K.; Barta, Z.; Gasztonyi, B.; Salamon, A.; et al. Early clinical remission and normalisation of CRP are the strongest predictors of efficacy, mucosal healing and dose escalation during the first year of adalimumab therapy in Crohn’s disease. Aliment. Pharmacol. Ther. 2011, 34, 911–922. [Google Scholar] [CrossRef] [Green Version]
- Bertani, L.; Tricò, D.; Pugliese, D.; Privitera, G.; Linsalata, G.; Zanzi, F.; Mumolo, M.G.; Barberio, B.; Monzani, F.; Marchi, S.; et al. Serum triiodothyronine-to-thyroxine (T3/T4) ratio predicts therapeutic outcome to biological therapies in elderly IBD patients. Aliment. Pharmacol. Ther. 2020, 53. [Google Scholar] [CrossRef]
- Colombel, J.; Sandborn, W.J.; Rutgeerts, P.; Enns, R.; Hanauer, S.B.; Panaccione, R.; Schreiber, S.; Byczkowski, D.; Li, J.; Kent, J.D.; et al. Adalimumab for Maintenance of Clinical Response and Remission in Patients with Crohn’s Disease: The CHARM Trial. Gastroenterology 2007, 132, 52–65. [Google Scholar] [CrossRef] [Green Version]
- Peters, C.P.; Eshuis, E.J.; Toxopeüs, F.M.; Hellemons, M.E.; Jansen, J.M.; D’Haens, G.R.A.M.; Fockens, P.; Stokkers, P.C.F.; Tuynman, H.A.R.E.; Van Bodegraven, A.A.; et al. Adalimumab for Crohn’s disease: Long-term sustained benefit in a population-based cohort of 438 patients. J. Crohn’s Colitis 2014, 8, 866–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jürgens, M.; John, J.M.M.; Cleynen, I.; Schnitzler, F.; Fidder, H.; Van Moerkercke, W.; Ballet, V.; Noman, M.; Hoffman, I.; Van Assche, G.; et al. Levels of C-reactive Protein Are Associated with Response to Infliximab Therapy in Patients with Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2011, 9, 421–427.e1. [Google Scholar] [CrossRef] [PubMed]
- Reinisch, W.; Wang, Y.; Oddens, B.J.; Link, R. C-reactive protein, an indicator for maintained response or remission to infliximab in patients with Crohn’s disease: A post-hoc analysis from ACCENT I. Aliment. Pharmacol. Ther. 2012, 35, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, M.; Vermeire, S.; Fidder, H.; Schnitzler, F.; Noman, M.; Van Assche, G.; De Hertogh, G.; Hoffman, I.; D’Hoore, A.; Van Steen, K.; et al. Long-term outcome after infliximab for refractory ulcerative colitis. J. Crohn’s Colitis 2008, 2, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Arias, M.T.; Casteele, N.V.; Vermeire, S.; Overstraeten, A.D.B.V.; Billiet, T.; Baert, F.; Wolthuis, A.; Van Assche, G.; Noman, M.; Hoffman, I.; et al. A Panel to Predict Long-term Outcome of Infliximab Therapy for Patients with Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2015, 13, 531–538. [Google Scholar] [CrossRef]
- Reinisch, W.; Sandborn, W.J.; Hommes, D.W.; D’Haens, G.; Hanauer, S.; Schreiber, S.; Panaccione, R.; Fedorak, R.N.; Tighe, M.B.; Huang, B.; et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: Results of a randomised controlled trial. Gut 2011, 60, 780–787. [Google Scholar] [CrossRef]
- Guidi, L.; Marzo, M.; Andrisani, G.; Felice, C.; Pugliese, D.; Mocci, G.; Nardone, O.; De Vitis, I.; Papa, A.; Rapaccini, G.; et al. Faecal calprotectin assay after induction with anti-Tumour Necrosis Factor α agents in inflammatory bowel disease: Prediction of clinical response and mucosal healing at one year. Dig. Liver Dis. 2014, 46, 974–979. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Marín, A.C.; McNicholl, A.G.; Chaparro, M. Systematic review with meta-analysis: The efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment. Pharmacol. Ther. 2015, 41, 613–623. [Google Scholar] [CrossRef]
- Casanova, M.J.; Chaparro, M.; Mínguez, M.; Ricart, E.; Taxonera, C.; García-López, S.; Guardiola, J.; Román, A.L.-S.; Iglesias, E.; Beltrán, B.; et al. Effectiveness and Safety of the Sequential Use of a Second and Third Anti-TNF Agent in Patients with Inflammatory Bowel Disease: Results from the Eneida Registry. Inflamm. Bowel Dis. 2020, 26, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, K.; Rivals-Lerebours, O.; Billiet, T.; Casteele, N.V.; Gils, A.; Ferrante, M.; Van Assche, G.; Rutgeerts, P.; Mantzaris, G.J.; Peyrin-Biroulet, L.; et al. Long-Term Outcome of Patients with Ulcerative Colitis and Primary Non-response to Infliximab. J. Crohn’s Colitis 2016, 10, 1015–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.C.; Jeen, Y.T. Genetic Studies of Inflammatory Bowel Disease-Focusing on Asian Patients. Cells 2019, 8, 404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bek, S.; Nielsen, J.V.; Bojesen, A.B.; Franke, A.; Bank, S.; Vogel, U.; Andersen, V. Systematic review: Genetic biomarkers associated with anti-TNF treatment response in inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2016, 44, 554–567. [Google Scholar] [CrossRef]
- Tong, Q.; Zhao, L.; Qian, X.-D.; Zhang, L.-L.; Xu, X.; Dai, S.-M.; Cai, Q.; Zhao, D. Association ofTNF-α polymorphism with prediction of response to TNF blockers in spondyloarthritis and inflammatory bowel disease: A meta-analysis. Pharmacogenomics 2013, 14, 1691–1700. [Google Scholar] [CrossRef] [Green Version]
- Bank, S.; Andersen, P.S.; Burisch, J.; Pedersen, N.; Roug, S.; Galsgaard, J.; Turino, S.Y.; Brodersen, J.B.; Rashid, S.; Rasmussen, B.K.; et al. Associations between functional polymorphisms in the NFκB signaling pathway and response to anti-TNF treatment in Danish patients with inflammatory bowel disease. Pharm. J. 2014, 14, 526–534. [Google Scholar] [CrossRef] [Green Version]
- Jürgens, M.; Laubender, R.P.; Hartl, F.; Weidinger, M.; Seiderer, J.; Wagner, J.; Wetzke, M.; Beigel, F.; Pfennig, S.; Stallhofer, J.; et al. Disease Activity, ANCA, and IL23R Genotype Status Determine Early Response to Infliximab in Patients with Ulcerative Colitis. Am. J. Gastroenterol. 2010, 105, 1811–1819. [Google Scholar] [CrossRef]
- Sazonovs, A.; Kennedy, N.A.; Moutsianas, L.; Heap, G.A.; Rice, D.L.; Reppell, M.; Bewshea, C.M.; Chanchlani, N.; Walker, G.J.; Perry, M.H.; et al. HLA-DQA1*05 Carriage Associated with Development of Anti-Drug Antibodies to Infliximab and Adalimumab in Patients with Crohn’s Disease. Gastroenterology 2020, 158, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Billiet, T.; Casteele, N.V.; Van Stappen, T.; Princen, F.; Singh, S.; Gils, A.; Ferrante, M.; Van Assche, G.; Cleynen, I.; Vermeire, S. Immunogenicity to infliximab is associated with HLA-DRB1. Gut 2015, 64, 1344–1345. [Google Scholar] [CrossRef]
- Louis, E.; El Ghoul, Z.; Vermeire, S.; Dall’Ozzo, S.; Rutgeerts, P.; Paintaud, G.; Belaiche, J.; De Vos, M.; Van Gossum, A.; Colombel, J.F.; et al. Association between polymorphism in IgG Fc receptor IIIa coding gene and biological response to infliximab in Crohn’s disease. Aliment. Pharmacol. Ther. 2004, 19, 511–519. [Google Scholar] [CrossRef]
- Niess, J.H.; Klaus, J.; Stephani, J.; Pflüger, C.; Degenkolb, N.; Spaniol, U.; Mayer, B.; Lahr, G.; Von Boyen, G.B.T. NOD2 Polymorphism Predicts Response to Treatment in Crohn’s Disease—First Steps to a Personalized Therapy. Dig. Dis. Sci. 2012, 57, 879–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juanola, O.; Moratalla, A.; Gutierrez, A.; Sempere, L.; Zapater, P.; Giménez, P.; Almenta, I.; Peiró, G.; González-Navajas, J.M.; Such, J.F.; et al. Anti-TNF-alpha loss of response is associated with a decreased percentage of FoxP3+ T cells and a variant NOD2 genotype in patients with Crohn’s disease. J. Gastroenterol. 2015, 50, 758–768. [Google Scholar] [CrossRef]
- Schäffler, H.; Geiss, D.; Gittel, N.; Rohde, S.; Huth, A.; Glass, Ä; Brandhorst, G.; Jaster, R.; Lamprecht, G. Mutations in theNOD2gene are associated with a specific phenotype and lower anti-tumor necrosis factor trough levels in Crohn’s disease. J. Dig. Dis. 2018, 19, 678–684. [Google Scholar] [CrossRef]
- Koder, S.; Repnik, K.; Ferkolj, I.; Pernat, C.; Skok, P.; Weersma, R.; Potočnik, U. Genetic polymorphism inATG16L1gene influences the response to adalimumab in Crohn’s disease patients. Pharmacogenomics 2015, 16, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Hlavaty, T.; Ferrante, M.; Henckaerts, L.; Pierik, M.; Rutgeerts, P.; Vermeire, S. Predictive Model for the Outcome of Infliximab Therapy in Crohn’s Disease Based on Apoptotic Pharmacogenetic Index and Clinical Predictors. Inflamm. Bowel Dis. 2007, 13, 372–379. [Google Scholar] [CrossRef]
- Barber, G.E.; Yajnik, V.; Khalili, H.; Giallourakis, C.; Garber, J.; Xavier, R.; Ananthakrishnan, A.N. Genetic Markers Predict Primary Non-Response and Durable Response to Anti-TNF Biologic Therapies in Crohn’s Disease. Am. J. Gastroenterol. 2016, 111, 1816–1822. [Google Scholar] [CrossRef] [Green Version]
- Burke, K.E.; Khalili, H.; Garber, J.; Haritunians, T.; McGovern, D.P.; Xavier, R.J.; Ananthakrishnan, A.N. Genetic Markers Predict Primary Nonresponse and Durable Response to Anti–Tumor Necrosis Factor Therapy in Ulcerative Colitis. Inflamm. Bowel Dis. 2018, 24, 1840–1848. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-H.; Friton, J.J.; Raffals, L.E.; Leighton, J.A.; Pasha, S.F.; Picco, M.F.; Cushing, K.C.; Monroe, K.; Nix, B.D.; Newberry, R.D.; et al. Novel Genetic Risk Variants Can Predict Anti-TNF Agent Response in Patients with Inflammatory Bowel Disease. J. Crohn’s Colitis 2019, 13, 1036–1043. [Google Scholar] [CrossRef]
- Atreya, I.; Atreya, R.; Neurath, M.F. NF-κB in inflammatory bowel disease. J. Intern. Med. 2008, 263, 591–596. [Google Scholar] [CrossRef]
- Vermeire, S.; Gils, A.; Accossato, P.; Lula, S.; Marren, A. Immunogenicity of biologics in inflammatory bowel disease. Ther. Adv. Gastroenterol. 2018, 11. [Google Scholar] [CrossRef] [Green Version]
- Colombel, J.-F.; Adedokun, O.J.; Gasink, C.; Gao, L.-L.; Cornillie, F.J.; D’Haens, G.R.; Rutgeerts, P.J.; Reinisch, W.; Sandborn, W.J.; Hanauer, S.B. Combination Therapy with Infliximab and Azathioprine Improves Infliximab Pharmacokinetic Features and Efficacy: A Post Hoc Analysis. Clin. Gastroenterol. Hepatol. 2019, 17, 1525–1532.e1. [Google Scholar] [CrossRef] [Green Version]
- Arijs, I.; Li, K.; Toedter, G.; Quintens, R.; Van Lommel, L.; Van Steen, K.; Leemans, P.; De Hertogh, G.; Lemaire, K.; Ferrante, M.; et al. Mucosal gene signatures to predict response to infliximab in patients with ulcerative colitis. Gut 2009, 58, 1612–1619. [Google Scholar] [CrossRef]
- Verstockt, B.; Verstockt, S.; Creyns, B.; Tops, S.; Van Assche, G.; Gils, A.; Ceuppens, J.L.; Vermeire, S.; Ferrante, M.; Breynaert, C. Mucosal IL13RA2 expression predicts nonresponse to anti-TNF therapy in Crohn’s disease. Aliment. Pharmacol. Ther. 2019, 49, 572–581. [Google Scholar] [CrossRef]
- Ogura, Y.; Bonen, D.K.; Inohara, N.; Nicolae, D.L.; Chen, F.F.; Ramos, R.; Britton, H.; Moran, T.; Karaliuskas, R.; Duerr, R.H.; et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nat. Cell Biol. 2001, 411, 603–606. [Google Scholar] [CrossRef]
- Weersma, R.K.; Stokkers, P.C.F.; Van Bodegraven, A.A.; Van Hogezand, R.A.; Verspaget, H.W.; De Jong, D.J.; Van Der Woude, C.J.; Oldenburg, B.; Linskens, R.K.; Festen, E.A.M.; et al. Molecular prediction of disease risk and severity in a large Dutch Crohn’s disease cohort. Gut 2009, 58, 388–395. [Google Scholar] [CrossRef] [Green Version]
- Vermeire, S.; O’Byrne, S.; Keir, M.; Williams, M.; Lu, T.T.; Mansfield, J.C.; Lamb, C.A.; Feagan, B.G.; Panes, J.; Salas, A.; et al. Etrolizumab as induction therapy for ulcerative colitis: A randomised, controlled, phase 2 trial. Lancet 2014, 384, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Tew, G.W.; Hackney, J.A.; Gibbons, D.; Lamb, C.A.; Luca, D.; Egen, J.G.; Diehl, L.; Anderson, J.E.; Vermeire, S.; Mansfield, J.C.; et al. Association Between Response to Etrolizumab and Expression of Integrin αE and Granzyme A in Colon Biopsies of Patients with Ulcerative Colitis. Gastroenterology 2016, 150, 477–487.e9. [Google Scholar] [CrossRef] [Green Version]
- Gaujoux, R.; Starosvetsky, E.; Maimon, N.; Vallania, F.; Bar-Yoseph, H.; Pressman, S.; Weisshof, R.; Goren, I.; Rabinowitz, K.; Waterman, M.; et al. Cell-centred meta-analysis reveals baseline predictors of anti-TNFα non-response in biopsy and blood of patients with IBD. Gut 2019, 68, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Verstockt, B.; Verstockt, S.; Dehairs, J.; Ballet, V.; Blevi, H.; Wollants, W.-J.; Breynaert, C.; Van Assche, G.; Vermeire, S.; Ferrante, M. Low TREM1 expression in whole blood predicts anti-TNF response in inflammatory bowel disease. EBioMedicine 2019, 40, 733–742. [Google Scholar] [CrossRef] [Green Version]
- Atreya, R.; Neumann, H.; Neufert, C.; Waldner, M.J.; Billmeier, U.; Zopf, Y.; Willma, M.; App, C.; Münster, T.; Kessler, H.; et al. In vivo imaging using fluorescent antibodies to tumor necrosis factor predicts therapeutic response in Crohn’s disease. Nat. Med. 2014, 20, 313–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jessen, B.; Rodriguez-Sillke, Y.; Sonnenberg, E.; Schumann, M.; Kruglov, A.; Freise, I.; Schmidt, F.; Maul, J.; Kühl, A.A.; Glauben, R.; et al. Level of Tumor Necrosis Factor Production by Stimulated Blood Mononuclear Cells Can Be Used to Predict Response of Patients with Inflammatory Bowel Diseases to Infliximab. Clin. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- West, N.R.; Hegazy, A.N.; Owens, B.M.J.; Bullers, S.J.; Linggi, B.; Buonocore, S.; Coccia, M.; Görtz, D.; This, S.; Stockenhuber, K.; et al. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor–neutralizing therapy in patients with inflammatory bowel disease. Nat. Med. 2017, 23, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Bertani, L.; Fornai, M.; Fornili, M.; Antonioli, L.; Benvenuti, L.; Tapete, G.; Svizzero, G.B.; Ceccarelli, L.; Mumolo, M.G.; Baglietto, L.; et al. Serum oncostatin M at baseline predicts mucosal healing in Crohn’s disease patients treated with infliximab. Aliment. Pharmacol. Ther. 2020, 52, 284–291. [Google Scholar] [CrossRef]
- Ungar, B.; Kopylov, U.; Yavzori, M.; Fudim, E.; Picard, O.; Lahat, A.; Coscas, D.; Waterman, M.; Haj-Natour, O.; Orbach-Zingboim, N.; et al. Association of Vedolizumab Level, Anti-Drug Antibodies, and α4β7 Occupancy with Response in Patients with Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2018, 16, 697–705.e7. [Google Scholar] [CrossRef] [Green Version]
- Boden, E.K.; Shows, D.M.; Chiorean, M.V.; Lord, J.D. Identification of Candidate Biomarkers Associated with Response to Vedolizumab in Inflammatory Bowel Disease. Dig. Dis. Sci. 2018, 63, 2419–2429. [Google Scholar] [CrossRef] [PubMed]
- Rath, T.; Bojarski, C.; Neurath, M.; Atreya, R. Molecular imaging of mucosal α4β7 integrin expression with the fluorescent anti-adhesion antibody vedolizumab in Crohn’s disease. Gastrointest. Endosc. 2017, 86, 406–408. [Google Scholar] [CrossRef]
- Allner, C.; Melde, M.; Becker, E.; Fuchs, F.; Mühl, L.; Klenske, E.; Müller, L.; Morgenstern, N.; Fietkau, K.; Hirschmann, S.; et al. Baseline levels of dynamic CD4+ T cell adhesion to MAdCAM-1 correlate with clinical response to vedolizumab treatment in ulcerative colitis: A cohort study. BMC Gastroenterol. 2020, 20, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soendergaard, C.; Seidelin, J.B.; Steenholdt, C.; Nielsen, O.H. Putative biomarkers of vedolizumab resistance and underlying inflammatory pathways involved in IBD. BMJ Open Gastroenterol. 2018, 5, e000208. [Google Scholar] [CrossRef]
- Bertani, L.; Caviglia, G.P.; Antonioli, L.; Pellicano, R.; Sharmila, F.; Astegiano, M.; Saracco, G.M.; Bugianesi, E.; Blandizzi, C.; Costa, F.; et al. Serum Interleukin-6 and -8 as Predictors of Response to Vedolizumab in Inflammatory Bowel Diseases. J. Clin. Med. 2020, 9, 1323. [Google Scholar] [CrossRef] [PubMed]
- Battat, R.; Dulai, P.S.; Casteele, N.V.; Evans, E.; Hester, K.D.; Webster, E.; Jain, A.; Proudfoot, J.A.; Mairalles, A.; Neill, J.; et al. Biomarkers Are Associated with Clinical and Endoscopic Outcomes with Vedolizumab Treatment in Ulcerative Colitis. Inflamm. Bowel Dis. 2019, 25, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Holmer, A.K.; Battat, R.; Dulai, P.S.; Casteele, N.V.; Nguyen, N.; Jain, A.; Miralles, A.; Neill, J.; Le, H.; Singh, S.; et al. Biomarkers are associated with clinical and endoscopic outcomes with vedolizumab treatment in Crohn’s disease. Ther. Adv. Gastroenterol. 2020, 13. [Google Scholar] [CrossRef]
- Sands, B.E.; Chen, J.; Feagan, B.G.; Penney, M.; Rees, W.A.; Danese, S.; Higgins, P.D.; Newbold, P.; Faggioni, R.; Patra, K.; et al. Efficacy and Safety of MEDI2070, an Antibody Against Interleukin 23, in Patients with Moderate to Severe Crohn’s Disease: A Phase 2a Study. Gastroenterology 2017, 153, 77–86.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitrev, N.; Casteele, N.V.; Seow, C.H.; Andrews, J.M.; Connor, S.J.; Moore, G.T.; Barclay, M.; Begun, J.; Bryant, R.; Chan, W.; et al. Review article: Consensus statements on therapeutic drug monitoring of anti-tumour necrosis factor therapy in inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2017, 46, 1037–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guidi, L.; Pugliese, D.; Tonucci, T.P.; Berrino, A.; Tolusso, B.; Basile, M.; Cantoro, L.; Balestrieri, P.; Civitelli, F.; Bertani, L.; et al. Therapeutic Drug Monitoring is More Cost-Effective than a Clinically Based Approach in the Management of Loss of Response to Infliximab in Inflammatory Bowel Disease: An Observational Multicentre Study. J. Crohn’s Colitis 2018, 12, 1079–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steenholdt, C.; Brynskov, J.; Thomsen, O. Østergaard; Munck, L.K.; Fallingborg, J.; Christensen, L.A.; Pedersen, G.; Kjeldsen, J.; Jacobsen, B.A.; Oxholm, A.S.; et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: A randomised, controlled trial. Gut 2014, 63, 919–927. [Google Scholar] [CrossRef]
- Argollo, M.; Kotze, P.G.; Kakkadasam, P.; D’Haens, G. Optimizing biologic therapy in IBD: How essential is therapeutic drug monitoring? Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 1–9. [Google Scholar] [CrossRef]
- De Cruz, P.; Kamm, M.A.; Prideaux, L.; Allen, P.B.; Moore, G. Mucosal Healing in Crohn’s Disease. Inflamm. Bowel Dis. 2013, 19, 429–444. [Google Scholar] [CrossRef]
- Papamichael, K.; Rakowsky, S.; Rivera, C.; Cheifetz, A.S.; Osterman, M.T. Infliximab trough concentrations during maintenance therapy are associated with endoscopic and histologic healing in ulcerative colitis. Aliment. Pharmacol. Ther. 2018, 47, 478–484. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.C.; Colombel, J.-F.; Sands, B.E.; Narula, N. Mucosal Healing Is Associated with Improved Long-term Outcomes of Patients with Ulcerative Colitis: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 1245–1255.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pugliese, D.; Felice, C.; Papa, A.; Gasbarrini, A.; Rapaccini, G.L.; Guidi, L.; Armuzzi, A. Anti TNF-α therapy for ulcerative colitis: Current status and prospects for the future. Expert Rev. Clin. Immunol. 2017, 13, 223–233. [Google Scholar] [CrossRef]
- Aharie, D.; Filippi, J.; Roblin, X.; Nancey, S.; Chevaux, J.-B.; Hébuterne, X.; Flourié, B.; Capdepont, M.; Peyrin-Biroulet, L. Impact of mucosal healing on long-term outcomes in ulcerative colitis treated with infliximab: A multicenter experience. Aliment. Pharmacol. Ther. 2013, 37, 998–1004. [Google Scholar] [CrossRef]
- Adedokun, O.J.; Sandborn, W.J.; Feagan, B.G.; Rutgeerts, P.; Xu, Z.; Marano, C.W.; Johanns, J.; Zhou, H.; Davis, H.M.; Cornillie, F.; et al. Association Between Serum Concentration of Infliximab and Efficacy in Adult Patients with Ulcerative Colitis. Gastroenterology 2014, 147, 1296–1307.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarur, A.J.; Kubiliun, M.J.; Czul, F.; Sussman, D.A.; Quintero, M.A.; Jain, A.; Drake, K.A.; Hauenstein, S.I.; Lockton, S.; Deshpande, A.R.; et al. Concentrations of 6-Thioguanine Nucleotide Correlate with Trough Levels of Infliximab in Patients with Inflammatory Bowel Disease on Combination Therapy. Clin. Gastroenterol. Hepatol. 2015, 13, 1118–1124.e3. [Google Scholar] [CrossRef] [PubMed]
- Roblin, X.; Marotte, H.; Rinaudo, M.; Del Tedesco, E.; Moreau, A.; Phelip, J.M.; Genin, C.; Peyrin-Biroulet, L.; Paul, S. Association Between Pharmacokinetics of Adalimumab and Mucosal Healing in Patients with Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2014, 12, 80–84.e2. [Google Scholar] [CrossRef] [PubMed]
- Bodini, G.; Giannini, E.G.; Savarino, V.; Del Nero, L.; Pellegatta, G.; De Maria, C.; Baldissarro, I.; Savarino, E. Adalimumab trough serum levels and anti-adalimumab antibodies in the long-term clinical outcome of patients with Crohn’s disease. Scand. J. Gastroenterol. 2016, 51, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Del Tedesco, E.; Marotte, H.; Rinaudo-Gaujous, M.; Moreau, A.; Phelip, J.M.; Genin, C.; Peyrin-Biroulet, L.; Roblin, X. Therapeutic Drug Monitoring of Infliximab and Mucosal Healing in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2013, 19, 2568–2576. [Google Scholar] [CrossRef]
- Kennedy, N.A.; Heap, G.A.; Green, H.D.; Hamilton, B.; Bewshea, C.; Walker, G.J.; Thomas, A.; Nice, R.; Perry, M.H.; Bouri, S.; et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: A prospective, multicentre, cohort study. Lancet Gastroenterol. Hepatol. 2019, 4, 341–353. [Google Scholar] [CrossRef] [Green Version]
- Adedokun, O.J.; Xu, Z.; Marano, C.W.; Strauss, R.; Zhang, H.; Johanns, J.; Zhou, H.; Davis, H.M.; Reinisch, W.; Feagan, B.G.; et al. Pharmacokinetics and Exposure-response Relationship of Golimumab in Patients with Moderately-to-Severely Active Ulcerative Colitis: Results from Phase 2/3 PURSUIT Induction and Maintenance Studies. J. Crohn’s Colitis 2017, 11, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Samaan, M.A.; Cunningham, G.; Tamilarasan, A.G.; Beltran, L.; Pavlidis, P.; Ray, S.; Mawdsley, J.; Sanderson, J.D.; Arkir, Z.; Irving, P.M. Therapeutic thresholds for golimumab serum concentrations during induction and maintenance therapy in ulcerative colitis: Results from the GO-LEVEL study. Aliment. Pharmacol. Ther. 2020, 52, 292–302. [Google Scholar] [CrossRef]
- Magro, F.; Lopes, S.; Silva, M.; Coelho, R.; Portela, F.; Branquinho, D.; Correia, L.; Fernandes, S.; Cravo, M.; Caldeira, P.; et al. Low Golimumab Trough Levels at Week 6 Are Associated with Poor Clinical, Endoscopic and Histological Outcomes in Ulcerative Colitis Patients: Pharmacokinetic and Pharmacodynamic Sub-analysis of the Evolution Study. J. Crohn’s Colitis 2019, 13, 1387–1393. [Google Scholar] [CrossRef]
- Boland, K.; Greener, T.; Kabakchiev, B.; Stempak, J.; Tessolini, J.; Li, R.; Soriano, J.; Croitoru, K.; Nguyen, G.; Steinhart, A.H.; et al. Identification of Target Golimumab Levels in Maintenance Therapy of Crohn’s Disease and Ulcerative Colitis Associated with Mucosal Healing. Inflamm. Bowel Dis. 2019, 26, 766–773. [Google Scholar] [CrossRef]
- Dreesen, E.; Kantasiripitak, W.; Detrez, I.; Stefanović, S.; Vermeire, S.; Ferrante, M.; Bouillon, T.; Drobne, D.; Gils, A. A Population Pharmacokinetic and Exposure-Response Model of Golimumab for Targeting Endoscopic Remission in Patients with Ulcerative Colitis. Inflamm. Bowel Dis. 2019, 26, 570–580. [Google Scholar] [CrossRef]
- Bouguen, G.; Siproudhis, L.; Gizard, E.; Wallenhorst, T.; Billioud, V.; Bretagne, J.-F.; Bigard, M.-A.; Peyrin-Biroulet, L. Long-term Outcome of Perianal Fistulizing Crohn’s Disease Treated with Infliximab. Clin. Gastroenterol. Hepatol. 2013, 11, 975–981.e4. [Google Scholar] [CrossRef] [Green Version]
- Davidov, Y.; Ungar, B.; Bar-Yoseph, H.; Carter, D.; Haj-Natour, O.; Yavzori, M.; Chowers, Y.; Eliakim, R.; Ben-Horin, S.; Kopylov, U. Association of Induction Infliximab Levels with Clinical Response in Perianal Crohn’s Disease. J. Crohn’s Colitis 2016, 11, 549–555. [Google Scholar] [CrossRef]
- Yarur, A.J.; Kanagala, V.; Stein, D.J.; Czul, F.; Quintero, M.A.; Agrawal, D.; Patel, A.; Best, K.; Fox, C.; Idstein, K.; et al. Higher infliximab trough levels are associated with perianal fistula healing in patients with Crohn’s disease. Aliment. Pharmacol. Ther. 2017, 45, 933–940. [Google Scholar] [CrossRef] [Green Version]
- Gibson, D.J.; Heetun, Z.S.; Redmond, C.E.; Nanda, K.S.; Keegan, D.; Byrne, K.; Mulcahy, H.E.; Cullen, G.; Doherty, G.A. An Accelerated Infliximab Induction Regimen Reduces the Need for Early Colectomy in Patients with Acute Severe Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2015, 13, 330–335.e1. [Google Scholar] [CrossRef] [PubMed]
- Laharie, D.; Rivière, P. Letter: Should we intensify infliximab in acute severe ulcerative colitis? Aliment. Pharmacol. Ther. 2020, 51, 186–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papamichael, K.; Cheifetz, A.S.; Melmed, G.Y.; Irving, P.M.; Casteele, N.V.; Kozuch, P.L.; Raffals, L.E.; Baidoo, L.; Bressler, B.; Devlin, S.M.; et al. Appropriate Therapeutic Drug Monitoring of Biologic Agents for Patients with Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2019, 17, 1655–1668.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papamichael, K.; Van Stappen, T.; Casteele, N.V.; Gils, A.; Billiet, T.; Tops, S.; Claes, K.; Van Assche, G.; Rutgeerts, P.; Vermeire, S.; et al. Infliximab Concentration Thresholds During Induction Therapy Are Associated with Short-term Mucosal Healing in Patients with Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2016, 14, 543–549. [Google Scholar] [CrossRef] [Green Version]
- Papamichael, K.; Baert, F.; Tops, S.; Van Assche, G.; Rutgeerts, P.; Vermeire, S.; Gils, A.; Ferrante, M. Post-Induction Adalimumab Concentration is Associated with Short-Term Mucosal Healing in Patients with Ulcerative Colitis. J. Crohn’s Colitis 2017, 11, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Rosario, M.; French, J.L.; Dirks, N.L.; Sankoh, S.; Parikh, A.; Yang, H.; Danese, S.; Colombel, J.-F.; Smyth, M.; Sandborn, W.J.; et al. Exposure–efficacy Relationships for Vedolizumab Induction Therapy in Patients with Ulcerative Colitis or Crohn’s Disease. J. Crohn’s Colitis 2017, 11, 921–929. [Google Scholar] [CrossRef] [Green Version]
- Osterman, M.T.; Rosario, M.; Lasch, K.; Barocas, M.; Wilbur, J.D.; Dirks, N.L.; Gastonguay, M.R. Vedolizumab exposure levels and clinical outcomes in ulcerative colitis: Determining the potential for dose optimisation. Aliment. Pharmacol. Ther. 2019, 49, 408–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guidi, L.; Pugliese, D.; Tonucci, T.P.; Bertani, L.; Costa, F.; Privitera, G.; Tolusso, B.; Di Mario, C.; Albano, E.; Tapete, G.; et al. Early vedolizumab trough levels predict treatment persistence over the first year in inflammatory bowel disease. United Eur. Gastroenterol. J. 2019, 7, 1189–1197. [Google Scholar] [CrossRef] [Green Version]
- Yacoub, W.; Williet, N.; Pouillon, L.; Di-Bernado, T.; Bittencourt, M.D.C.; Nancey, S.; Lopez, A.; Paul, S.; Zallot, C.; Roblin, X.; et al. Early vedolizumab trough levels predict mucosal healing in inflammatory bowel disease: A multicentre prospective observational study. Aliment. Pharmacol. Ther. 2018, 47, 906–912. [Google Scholar] [CrossRef] [Green Version]
- Dreesen, E.; Verstockt, B.; Bian, S.; De Bruyn, M.; Compernolle, G.; Tops, S.; Noman, M.; Van Assche, G.; Ferrante, M.; Gils, A.; et al. Evidence to Support Monitoring of Vedolizumab Trough Concentrations in Patients with Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2018, 16, 1937–1946.e8. [Google Scholar] [CrossRef]
- Pouillon, L.; Rousseau, H.; Busby-Venner, H.; Bittencourt, M.D.C.; Choukour, M.; Gauchotte, G.; Zallot, C.; Danese, S.; Baumann, C.; Peyrin-Biroulet, L. Vedolizumab Trough Levels and Histological Healing During Maintenance Therapy in Ulcerative Colitis. J. Crohn’s Colitis 2019, 13, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.; Morgan, H.; Steel, A.; Wahed, M. P467 Can induction and maintenance serum vedolizumab levels predict long-term clinical outcome in inflammatory bowel disease (IBD)? J. Crohn’s Colitis 2020, 14 (Suppl. S1), S413–S414. [Google Scholar] [CrossRef] [Green Version]
- Vaughn, B.P.; Yarur, A.J.; Graziano, E.; Campbell, J.P.; Bhattacharya, A.; Lee, J.Y.; Gheysens, K.; Papamichael, K.; Osterman, M.T.; Cheifetz, A.S.; et al. Vedolizumab Serum Trough Concentrations and Response to Dose Escalation in Inflammatory Bowel Disease. J. Clin. Med. 2020, 9, 3142. [Google Scholar] [CrossRef] [PubMed]
- Adedokun, O.J.; Xu, Z.; Gasink, C.; Jacobstein, D.; Szapary, P.; Johanns, J.; Gao, L.-L.; Davis, H.M.; Hanauer, S.B.; Feagan, B.G.; et al. Pharmacokinetics and Exposure Response Relationships of Ustekinumab in Patients with Crohn’s Disease. Gastroenterology 2018, 154, 1660–1671. [Google Scholar] [CrossRef] [Green Version]
- Adedokun, O.J.; Xu, Z.; Marano, C.; O’Brien, C.; Szapary, P.; Zhang, H.; Johanns, J.; Leong, R.W.; Hisamatsu, T.; Van Assche, G.; et al. Ustekinumab Pharmacokinetics and Exposure Response in a Phase 3 Randomized Trial of Patients with Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2020, 18, 2244–2255.e9. [Google Scholar] [CrossRef]
- Verstockt, B.; Dreesen, E.; Noman, M.; Outtier, A.; Berghe, N.V.D.; Aerden, I.; Compernolle, G.; Van Assche, G.; Gils, A.; Vermeire, S.; et al. Ustekinumab Exposure-outcome Analysis in Crohn’s Disease Only in Part Explains Limited Endoscopic Remission Rates. J. Crohn’s Colitis 2019, 13, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Soufflet, N.; Boschetti, G.; Roblin, X.; Cuercq, C.; Williet, N.; Charlois, A.-L.; Duclaux-Loras, R.; Danion, P.; Mialon, A.; Faure, M.; et al. Concentrations of Ustekinumab During Induction Therapy Associate with Remission in Patients with Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2019, 17, 2610–2612. [Google Scholar] [CrossRef] [PubMed]
- Battat, R.; Kopylov, U.; Bessissow, T.; Bitton, A.; Cohen, A.; Jain, A.; Martel, M.; Seidman, E.; Afif, W. Association between Ustekinumab Trough Concentrations and Clinical, Biomarker, and Endoscopic Outcomes in Patients with Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2017, 15, 1427–1434.e2. [Google Scholar] [CrossRef]
- Liefferinckx, C.; Fassin, M.; Thomas, D.; Minsart, C.; Cremer, A.; Amininejad, L.; Tafciu, V.; Wambacq, V.; Van Gossum, A.; Franchimont, D. P346 Single-centre experience of ustekinumab: Therapeutic drug monitoring in Crohn’s disease patients. J. Crohn’s Colitis 2020, 14 (Suppl. S1), S331–S333. [Google Scholar] [CrossRef]
- Yanai, H.; Lichtenstein, L.; Assa, A.; Mazor, Y.; Weiss, B.; Levine, A.; Ron, Y.; Kopylov, U.; Bujanover, Y.; Rosenbach, Y.; et al. Levels of Drug and Antidrug Antibodies Are Associated with Outcome of Interventions After Loss of Response to Infliximab or Adalimumab. Clin. Gastroenterol. Hepatol. 2015, 13, 522–530.e2. [Google Scholar] [CrossRef]
- Roblin, X.; Verot, C.; Paul, S.; Duru, G.; Williet, N.; Boschetti, G.; Del Tedesco, E.; Peyrin-Biroulet, L.; Phelip, J.M.; Nancey, S.; et al. Is the Pharmacokinetic Profile of a First Anti-TNF Predictive of the Clinical Outcome and Pharmacokinetics of a Second Anti-TNF? Inflamm. Bowel Dis. 2018, 24, 2078–2085. [Google Scholar] [CrossRef]
- Roblin, X.; Williet, N.; Boschetti, G.; Phelip, J.-M.; Del Tedesco, E.; Berger, A.-E.; Vedrines, P.; Duru, G.; Peyrin-Biroulet, L.; Nancey, S.; et al. Addition of azathioprine to the switch of anti-TNF in patients with IBD in clinical relapse with undetectable anti-TNF trough levels and antidrug antibodies: A prospective randomised trial. Gut 2020, 69, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Casteele, N.V.; Ferrante, M.; Van Assche, G.; Ballet, V.; Compernolle, G.; Van Steen, K.; Simoens, S.; Rutgeerts, P.; Gils, A.; Vermeire, S. Trough Concentrations of Infliximab Guide Dosing for Patients with Inflammatory Bowel Disease. Gastroenterology 2015, 148, 1320–1329.e3. [Google Scholar] [CrossRef]
- D’Haens, G.; Vermeire, S.; Lambrecht, G.; Baert, F.; Bossuyt, P.; Pariente, B.; Buisson, A.; Bouhnik, Y.; Filippi, J.; Woude, J.V.; et al. Increasing Infliximab Dose Based on Symptoms, Biomarkers, and Serum Drug Concentrations Does Not Increase Clinical, Endoscopic, and Corticosteroid-Free Remission in Patients with Active Luminal Crohn’s Disease. Gastroenterology 2018, 154, 1343–1351.e1. [Google Scholar] [CrossRef] [PubMed]
- Assa, A.; Matar, M.; Turner, D.; Broide, E.; Weiss, B.; Ledder, O.; Guz-Mark, A.; Rinawi, F.; Cohen, S.; Topf-Olivestone, C.; et al. Proactive Monitoring of Adalimumab Trough Concentration Associated with Increased Clinical Remission in Children with Crohn’s Disease Compared with Reactive Monitoring. Gastroenterology 2019, 157, 985–996.e2. [Google Scholar] [CrossRef]
- Sparrow, M.P.; Papamichael, K.; Ward, M.G.; Rivière, P.; Laharie, D.; Paul, S.; Roblin, X. Therapeutic Drug Monitoring of Biologics During Induction to Prevent Primary Non-Response. J. Crohn’s Colitis 2020, 14, 542–556. [Google Scholar] [CrossRef]
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Hanauer, S.; Colombel, J.-F.; Sandborn, W.J.; Van Assche, G.; Axler, J.; Kim, H.-J.; Danese, S.; et al. Vedolizumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2013, 369, 699–710. [Google Scholar] [CrossRef] [Green Version]
- Sands, B.E.; Feagan, B.G.; Rutgeerts, P.; Colombel, J.-F.; Sandborn, W.J.; Sy, R.; D’Haens, G.; Ben-Horin, S.; Xu, J.; Rosario, M.; et al. Effects of Vedolizumab Induction Therapy for Patients with Crohn’s Disease in Whom Tumor Necrosis Factor Antagonist Treatment Failed. Gastroenterol. 2014, 147, 618–627.e3. [Google Scholar] [CrossRef] [Green Version]
- Feagan, B.G.; Sandborn, W.J.; Gasink, C.; Jacobstein, D.; Lang, Y.; Friedman, J.R.; Blank, M.A.; Johanns, J.; Gao, L.-L.; Miao, Y.; et al. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2016, 375, 1946–1960. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E.; Sandborn, W.J.; Panaccione, R.; O’Brien, C.D.; Zhang, H.; Johanns, J.; Adedokun, O.J.; Li, K.; Peyrin-Biroulet, L.; Van Assche, G.; et al. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2019, 381, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Verstockt, B.; Mertens, E.; Dreesen, E.; Outtier, A.; Noman, M.; Tops, S.; Schops, G.; Van Assche, G.; Vermeire, S.; Gils, A.; et al. Influence of Drug Exposure on Vedolizumab-Induced Endoscopic Remission in Anti-Tumour Necrosis Factor [TNF] Naïve and Anti-TNF Exposed IBD Patients. J. Crohn’s Colitis 2020, 14, 332–341. [Google Scholar] [CrossRef]
- Alsoud, D.; Vermeire, S.; Verstockt, B. Monitoring vedolizumab and ustekinumab drug levels in patients with inflammatory bowel disease: Hype or hope? Curr. Opin. Pharmacol. 2020, 55, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Sandborn, W.; Sands, B.E.; Reinisch, W.; Bemelman, W.; Bryant, R.V.; D’Haens, G.; Dotan, I.; Dubinsky, M.; Feagan, B.; et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am. J. Gastroenterol. 2015, 110, 1324–1338. [Google Scholar] [CrossRef]
- Kopylov, U.; Mantzaris, G.J.; Katsanos, K.H.; Reenaers, C.; Ellul, P.; Rahier, J.F.; Israeli, E.; Lakatos, P.L.; Fiorino, G.; Cesarini, M.; et al. The efficacy of shortening the dosing interval to once every six weeks in Crohn’s patients losing re-sponse to maintenance dose of infliximab. Aliment. Pharmacol. Ther. 2011, 33, 349–357. [Google Scholar] [CrossRef]
- Lee, M.; Chang, E.B. Inflammatory Bowel Diseases (IBD) and the Microbiome—Searching the Crime Scene for Clues. Gastroenterology 2021, 160, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.D.; Chen, E.Z.; Baldassano, R.N.; Otley, A.R.; Griffiths, A.M.; Lee, D.; Bittinger, K.; Bailey, A.; Friedman, E.S.; Hoffmann, C.; et al. Inflammation, Antibiotics, and Diet as Environmental Stressors of the Gut Microbiome in Pediatric Crohn’s Disease. Cell Host Microbe 2015, 18, 489–500. [Google Scholar] [CrossRef] [Green Version]
- Estevinho, M.M.; Rocha, C.; Correia, L.; Lago, P.; Ministro, P.; Portela, F.; Trindade, E.; Afonso, J.; Peyrin-Biroulet, L.; Magro, F. Features of Fecal and Colon Microbiomes Associate with Responses to Biologic Therapies for Inflammatory Bowel Diseases: A Systematic Review. Clin. Gastroenterol. Hepatol. 2020, 18, 1054–1069. [Google Scholar] [CrossRef] [PubMed]
- Scaldaferri, F.; Gerardi, V.; Pecere, S.; Petito, V.; Lopetuso, L.R.; Zambrano, D.; Schiavoni, E.; D’Ambrosio, D.; Di Agostini, A.; Laterza, L.; et al. Tu1302 Anti-TNF-α Induction Regimen Modulates Gut Microbiota Molecular Composition While Inducing Clinical Response in Crohn’s Disease Patients: Toward a Personalized Medicine. Gastroenterology 2015, 148, S-852. [Google Scholar] [CrossRef]
- Shaw, K.A.; Bertha, M.; Hofmekler, T.; Chopra, P.; Vatanen, T.; Srivatsa, A.; Prince, J.; Kumar, A.; Sauer, C.; Zwick, M.E.; et al. Dysbiosis, inflammation, and response to treatment: A longitudinal study of pediatric subjects with newly diagnosed inflammatory bowel disease. Genome Med. 2016, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Tian, Z.; Feng, R.; Li, M.; Li, T.; Zhou, G.; Qiu, Y.; Chen, B.; He, Y.; Chen, M.; et al. Fecal Microbiota Alterations Associated with Clinical and Endoscopic Response to Infliximab Therapy in Crohn’s Disease. Inflamm. Bowel Dis. 2020, 26, 1636–1647. [Google Scholar] [CrossRef] [PubMed]
- Aden, K.; Rehman, A.; Waschina, S.; Pan, W.-H.; Walker, A.; Lucio, M.; Nunez, A.M.; Bharti, R.; Zimmerman, J.; Bethge, J.; et al. Metabolic Functions of Gut Microbes Associate with Efficacy of Tumor Necrosis Factor Antagonists in Patients with Inflammatory Bowel Diseases. Gastroenterology 2019, 157, 1279–1292.e11. [Google Scholar] [CrossRef] [Green Version]
- Ding, N.S.; McDonald, J.A.K.; Perdones-Montero, A.; Rees, D.N.; Adegbola, S.O.; Misra, R.; Hendy, P.; Penez, L.; Marchesi, J.R.; Holmes, E.; et al. Metabonomics and the Gut Microbiome Associated with Primary Response to Anti-TNF Therapy in Crohn’s Disease. J. Crohn’s Colitis 2020, 14, 1090–1102. [Google Scholar] [CrossRef]
- Seong, G.; Kim, N.; Joung, J.-G.; Kim, E.R.; Chang, D.K.; Chun, J.; Hong, S.N.; Kim, Y.-H. Changes in the Intestinal Microbiota of Patients with Inflammatory Bowel Disease with Clinical Remission during an 8-Week Infliximab Infusion Cycle. Microorganisms 2020, 8, 874. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, Z.Z.; He, Y.; Yang, Y.; Liu, L.; Lin, Q.; Nie, Y.; Li, M.; Zhi, F.; Liu, S.; et al. Gut Microbiota Offers Universal Biomarkers across Ethnicity in Inflammatory Bowel Disease Diagnosis and Infliximab Response Prediction. mSystems 2018, 3, e00188-17. [Google Scholar] [CrossRef] [Green Version]
- Magnusson, M.K.; Strid, H.; Sapnara, M.; Lasson, A.; Bajor, A.; Ung, K.-A.; Öhman, L. Anti-TNF Therapy Response in Patients with Ulcerative Colitis Is Associated with Colonic Antimicrobial Peptide Expression and Microbiota Composition. J. Crohn’s Colitis 2016, 10, 943–952. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, X.; Ghozlane, A.; Hu, H.; Li, X.; Xiao, Y.; Li, D.; Yu, G.; Zhang, T. Characteristics of Faecal Microbiota in Paediatric Crohn’s Disease and Their Dynamic Changes During Infliximab Therapy. J. Crohn’s Colitis 2018, 12, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Luo, C.; Yajnik, V.; Khalili, H.; Garber, J.J.; Stevens, B.W.; Cleland, T.; Xavier, R.J. Gut Microbiome Function Predicts Response to Anti-integrin Biologic Therapy in Inflammatory Bowel Diseases. Cell Host Microbe 2017, 21, 603–610.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doherty, M.K.; Ding, T.; Koumpouras, C.; Telesco, S.E.; Monast, C.; Das, A.; Brodmerkel, C.; Schloss, P.D. Fecal Microbiota Signatures Are Associated with Response to Ustekinumab Therapy among Crohn’s Disease Patients. mBio 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Study | Genetic Markers | Outcomes |
|---|---|---|
| Bek et al. 2016 [34] | Polymorphisms in TLR2, rs11938228, TLR4, TLR9, TNFRSF1A, IFNG, IL6 and IL1B (rs4848306) | Clinical response to anti-TNF in IBD patients |
| Tong et al. 2013 [35] | Polymorphisms in TNF-α promoter (-308 A/G and -857 C/T) | Clinical response to anti-TNF in IBD e SpA patient |
| Bank et al. 2014 [36] | Polymorphisms implicated in NF-kB pathway: TLR2, TLR4, TLR9, LY96 (MD-2), CD14, MAP3K14 (NIK), TNFA, TNFRSF1A, TNFAIP3(A20), IL1B, IL1RN, IL6, IL17A, IFNG | Clinical response to anti-TNF in IBD patients |
| Jürgens et al. 2010 [37] | Polymorphisms in IL23R | Early response to infliximab in UC patients |
| Sazonovs et al. 2020 [38] | HLA-DQA1*05 | Development of ADA against infliximab and adalimumab in CD patients |
| Billiet et al. 2015 [39] | HLA-DRB1 | Development of ADA against infliximab in IBD patients |
| Louis et al. 2004 [40] | Polymorphism in IgG Fc receptor IIIa | Development of ADA against infliximab in CD patients |
| Niess et al. 2012 [41] | Polymorphisms in NOD2 | Clinical response to anti-TNF in CD patients |
| Juanola et al. 2015 [42] | Polymorphisms in NOD2 | Loss of response to anti-TNF in CD patients |
| Schäffler et al. 2018 [43] | Polymorphisms in NOD2 | Lower anti-TNF TLs in CD patients |
| Koder et al. 2015 [44] | Polymorphisms in ATG16L1 | Clinical response to adalimumab in CD patients |
| Hlavaty et al. 2007 [45] | Polymorphisms in Fas, Fas ligand and Caspase 9 (Apoptotic Pharmacogenetic Index) | Clinical response to infliximab in CD patients |
| Barber et al. 2016 [46] | Multiple polymorphisms (Combined clinical-genetic model) | Short- and long-term to anti-TNF in CD patients |
| Burke et al. 2018 [47] | Multiple polymorphisms (Combined clinical-genetic model) | Short- and long-term response to anti-TNF in UC patients |
| Wang et al. 2019 [48] | Polymorphisms in TNFSF4/18, PLIN2, rs762787, rs9572250, rs144256942, rs523781 | Clinical response to anti-TNF in IBD patients |
| Study | Immunological Markers | Outcomes |
|---|---|---|
| Gaujoux et al. 2019 [58] | Higher expression of TREM-1 and CCR2-CCL7 in intestinal biopsies | Nonresponse to anti-TNF treatment |
| Verstockt et al. 2019 [53] | Lower expression of TREM-1 in whole blood and intestinal biopsies, lower concentration in serum | Mucosal healing in patients treated with anti-TNF |
| Atreya et al. 2014 [60] | Higher number of mTNF+ cells in intestinal biopsies | Short term (12 weeks) response to adalimumab |
| Jessen et al. 2020 [61] | TNF production > 500 pg/mL by PBMC stimulated with LPS | Clinical response to infliximab at week 6 |
| West et al. 2017 [62] | Higher expression of OSM in intestinal biopsies | Refractoriness to infliximab (at weeks 8 and 30) and golimumab (at week 6) |
| Bertani et al. 2020 [63] | Lower serum concentration of OSM | Mucosal healing at week 54 in infliximab-treated patients |
| Boden et al. 2018 [65] | Higher expression of α4β7 on T effector memory cells and NK cells | Response to vedolizumab |
| Rath et al. 2017 [66] | Presence of α4β7+ cells in intestinal mucosa | Response to anti-integrin therapy |
| Allner et al. 2020 [67] | Higher dynamic adhesion of peripheral blood CD4+ T cells to MAdCAM-1 and more pronounced reduction of adhesion following treatment | Clinical response to vedolizumab in UC patients |
| Soendergaard et al. 2020 [68] | Higher serum IL6 Higher serum CD40L Higher serum osteocalcin | Nonresponse to vedolizumab in IBD patients Nonresponse to vedolizumab in CD patients Response to vedolizumab in UC patients |
| Bertani et al. 2020 [69] | Higher serum IL6 and IL8, more pronounced decrease of IL6 after 10 weeks | Clinical response to vedolizumab after 12 months |
| Battat et al. 2019 [70] | Increase of serum α4β7 and decrease of serum MAdCAM-1, VCAM-1, ICAM-1 and TNF | Clinical and endoscopic remission ate week 26 in vedolizumab-treated patients |
| Holmer et al. 2020 [71] | Higher serum VCAM-1 and ICAM-1 and lower serum α4β7 | Endoscopic remission in vedolizumab-treated patients |
| Sands et al. 2017 [72] | Higher serum IL22 | Clinical response to brazikumab |
| Study | Cut-Off | Outcomes |
|---|---|---|
| Anti-TNF Agents | ||
| Adedokun et al. 2014 [82] | Infliximab TLs ≥41 μg/mL at week 8 Infliximab TLs ≥3.7 μg/mL during maintenance | Clinical response, clinical remission, mucosa healing in UC patients Clinical response, clinical remission, mucosa healing in UC patients |
| Yarur et al. 2015 [83] | Infliximab TLs ≥8.3 μg/mL during maintenance | Mucosal healing in IBD patients |
| Roblin et al. 2014 [84] | Adalimumab TLs ≥6 μg/mL during maintenance Adalimumab TLs ≥6.5 μg/mL during maintenance | Clinical remission in IBD patients Mucosal healing in IBD patients |
| Bodini et al. 2016 [85] | Adalimumab TLs ≥10.1 μg/mL at week 48 | Clinical remission in CD patients |
| Paul et al. 2013 [86] | Increase of infliximab TLs >0.5 μg/mL after dose-escalation | Mucosal healing in IBD patients |
| Papamichael et al. 2018 [78] | Infliximab TLs ≥7.5 μg/mL during maintenance Infliximab TLs ≥7.5 μg/mL during maintenance | Endoscopic healing in CD patients Histologic healing in CD patients |
| Kennedy et al. 2019 [87] | Infliximab TLs >7 μg/mL at week 14 Adalimumab TLs >12 μg/mL at week 14 | Clinical remission at weeks 14 and 54 in CD patients Clinical remission at weeks 14 and 54 in CD patients |
| Adedokun et al. 2017 [88] | Golimumab TLs ≥2.5 μg/mL at week 6 Golimumab levels (steady-state) ≥1.4 μg/mL at week 44 | Clinical response at week 6 in UC patients Clinical remission at week 54 in UC patients |
| Samaan et al. 2020 [89] | Golimumab TLs ≥3.8 μg/mL at week 6 Golimumab TLs ≥2.4 μg/mL during maintenance | Combined clinical and biochemical remission at week 6 in UC patients Combined clinical and biochemical remission during maintenance in UC patients |
| Magro et al. 2019 [90] | Golimumab TLs ≥2.9 μg/mL at week 6 | Higher rates of clinical response, lower rates of endoscopic and histologic activity at week 6 in UC patients |
| Boland et al. 2019 [91] | Golimumab TLs ≥8.0 μg/mL during maintenance | Mucosal healing in CD patients during maintenance |
| Dreesen et al. 2019 [92] | Golimumab TLs ≥7.4 and 3.4 μg/mL at weeks 6 and 14 | Endoscopic remission at week 14 in UC patients |
| Vedolizumab | ||
| Rosario et al. 2017 [101] | Median vedolizumab TLs 26.8 μg/mL at week 6 Median vedolizumab TLs 34.7 μg/mL at week 6 | Clinical remission at week 52 in CD patients Clinical remission at week 52 in UC patients |
| Osterman et al. 2019 [102] | Vedolizumab TLs ≥37.1 μg/mL at week 6, ≥18.4 μg/mL at week 14 and ≥12.7 μg/mL during maintenance | Clinical remission at week 52 in UC patients |
| Guidi et al. 2019 [103] | Vedolizumab TLs ≥16.55 μg/mL at week 14 | Vedolizumab persistence in IBD patients |
| Yacoub et al. 2018 [104] | Vedolizumab TLs ≥18 μg/mL at week 6 | Mucosal healing within the first year in IBD patients |
| Dreesen et al. 2018 [105] | Vedolizumab TLs ≥28.9 μg/mL at week 2 Vedolizumab TLs ≥23.4 μg/mL at week 6 Vedolizumab TLs ≥13.9 μg/mL at week 14 Vedolizumab TLs ≥13.5 μg/mL at week 22 | Mucosal healing at week 14 in UC patients Biochemical remission at week 6 in CD patients Mucosal healing at week 14 in UC patients Mucosal healing at week 22 in CD patients |
| Vedolizumab TLs ≥20.9 μg/mL at week 6 and ≥10.1 μg/mL at week 14 Vedolizumab TLs ≥26.2 μg/mL at week 6 and ≥30.1 μg/mL at week 14 | Endoscopic improvement at week 14 Endoscopic remission at 6 months | |
| Pouillon et al. 2019 [106] | Vedolizumab TLs ≥25 μg/mL during maintenance | Endoscopic and histological healing in UC patients |
| Miller et al. 2020 [107] | Vedolizumab TLs ≥27 μg/mL during maintenance | Clinical remission at week 52 in IBD patients |
| Vaughn et al. 2020 [108] | Vedolizumab TLs <7.4 μg/mL before dose-escalation | Response to dose-escalation in IBD patients |
| Ustekinumab | ||
| Adedokun et al. 2018 [109] | Ustekinumab TLs ≥3.3 μg/mL at week 8 Ustekinumab TLs 0.8–1.4 μg/mL during maintenance | Clinical remission at week 8 in CD patients Clinical remission during maintenance in CD patients |
| Adedokun et al. 2020 [110] | Ustekinumab TLs ≥3.7 μg/mL at week 8 Ustekinumab TLs 1.1–1.3 μg/mL during maintenance | Clinical remission at week 8 in UC patients Clinical remission at week 44 in UC patients |
| Verstockt et al. 2019 [111] | Ustekinumab TLs ≥21.8 μg/mL at week 4 and ≥6.6 μg/mL at week 8 Ustekinumab TLs ≥2.7 μg/mL at week 16 and ≥1.9 μg/mL at week 24 | 50% decrease of faecal calprotectin in CD patients Endoscopic response at week 24 |
| Soufflet et al. 2019 [112] | Ustekinumab TLs ≥2 μg/mL at week 8 | Steroid-free clinical remission and biochemical remission at week 16 |
| Battat et al. 2017 [113] | Ustekinumab TLs ≥4.5 μg/mL at week 26 | Endoscopic response from week 26 |
| Liefferinckx et al. 2020 [114] | Median ustekinumab TLs 2.45 μg/mL at week 16 | Need for optimization during maintenance |
| Study | Microbial Markers | Outcomes |
|---|---|---|
| Magnusson et al. 2016 [131] | Lower dysbiosis indexes and higher abundance of F. prausnitizii | Clinical response to anti-TNF in IBD patients Clinical response to anti-TNF in IBD patients |
| Shaw et al. 2016 [132] | Difference in abundance of specific genera (Akkermansia, Coprococcus, Fusobacterium, Veillonella, Faecalibacterium, and Adlercreutzia) | Clinical response to anti-TNF in IBD patients Clinical response to anti-TNF in IBD patients |
| Zhuang et al. 2020 [133] | Increased proportions of Lachnospiraceae and Blautia taxa at week 6 | Clinical and endoscopic response to infliximab in CD patients |
| Wang et al. 2018 [134] | Higher abundance of SCFA-producing bacteria | Sustained response to infliximab in CD patients |
| Aden et al. 2019 [135] | Reduced metabolic interactions Higher levels of SCAFs after anti-TNF initiation | Non-response to anti-TNF in IBD patients Clinical remission in IBD patients |
| Seong et al. 2020 [136] | Increased bacterial diversity, richness and relative abundance of F. prausnitzii | Infliximab TLs >5 μg/mL at week 8 and mucosal healing within 3 months in IBD patients |
| Ananthakrishnan et al. 2017 [137] | Higher α-diversity, higher abundance of Roseburia inulinivorans and of a Burkholderiales species Metabolic pathways associated with microbial functions | Clinical remission in IBD patients treated with vedolizumab Clinical remission in IBD patients treated with vedolizumab |
| Doherty et al. 2018 [138] | Higher diversity and higher abundance of 2 OTUs affiliated with Faecalibacterium and Bacteroides | Clinical remission at week 6 in CD patients treated with ustekinumab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Privitera, G.; Pugliese, D.; Rapaccini, G.L.; Gasbarrini, A.; Armuzzi, A.; Guidi, L. Predictors and Early Markers of Response to Biological Therapies in Inflammatory Bowel Diseases. J. Clin. Med. 2021, 10, 853. https://doi.org/10.3390/jcm10040853
Privitera G, Pugliese D, Rapaccini GL, Gasbarrini A, Armuzzi A, Guidi L. Predictors and Early Markers of Response to Biological Therapies in Inflammatory Bowel Diseases. Journal of Clinical Medicine. 2021; 10(4):853. https://doi.org/10.3390/jcm10040853
Chicago/Turabian StylePrivitera, Giuseppe, Daniela Pugliese, Gian Ludovico Rapaccini, Antonio Gasbarrini, Alessandro Armuzzi, and Luisa Guidi. 2021. "Predictors and Early Markers of Response to Biological Therapies in Inflammatory Bowel Diseases" Journal of Clinical Medicine 10, no. 4: 853. https://doi.org/10.3390/jcm10040853
APA StylePrivitera, G., Pugliese, D., Rapaccini, G. L., Gasbarrini, A., Armuzzi, A., & Guidi, L. (2021). Predictors and Early Markers of Response to Biological Therapies in Inflammatory Bowel Diseases. Journal of Clinical Medicine, 10(4), 853. https://doi.org/10.3390/jcm10040853







