Topical Corticosteroids and Fungal Keratitis: A Review of the Literature and Case Series
Abstract
:1. Introduction
2. Methods
3. Management of Inflammation in Fungal Keratitis: The Role of Steroids
3.1. Role of Steroids in The Medical Management of Fungal Keratitis
3.2. Role of Steroids after Therapeutic Keratoplasty for Fungal Keratitis
3.3. Discontinuation of Steroids in Patients Affected by Fungal Keratitis
4. Case Series: Detrimental Effects of Abrupt Discontinuation of Topical Steroids in Fungal Keratitis
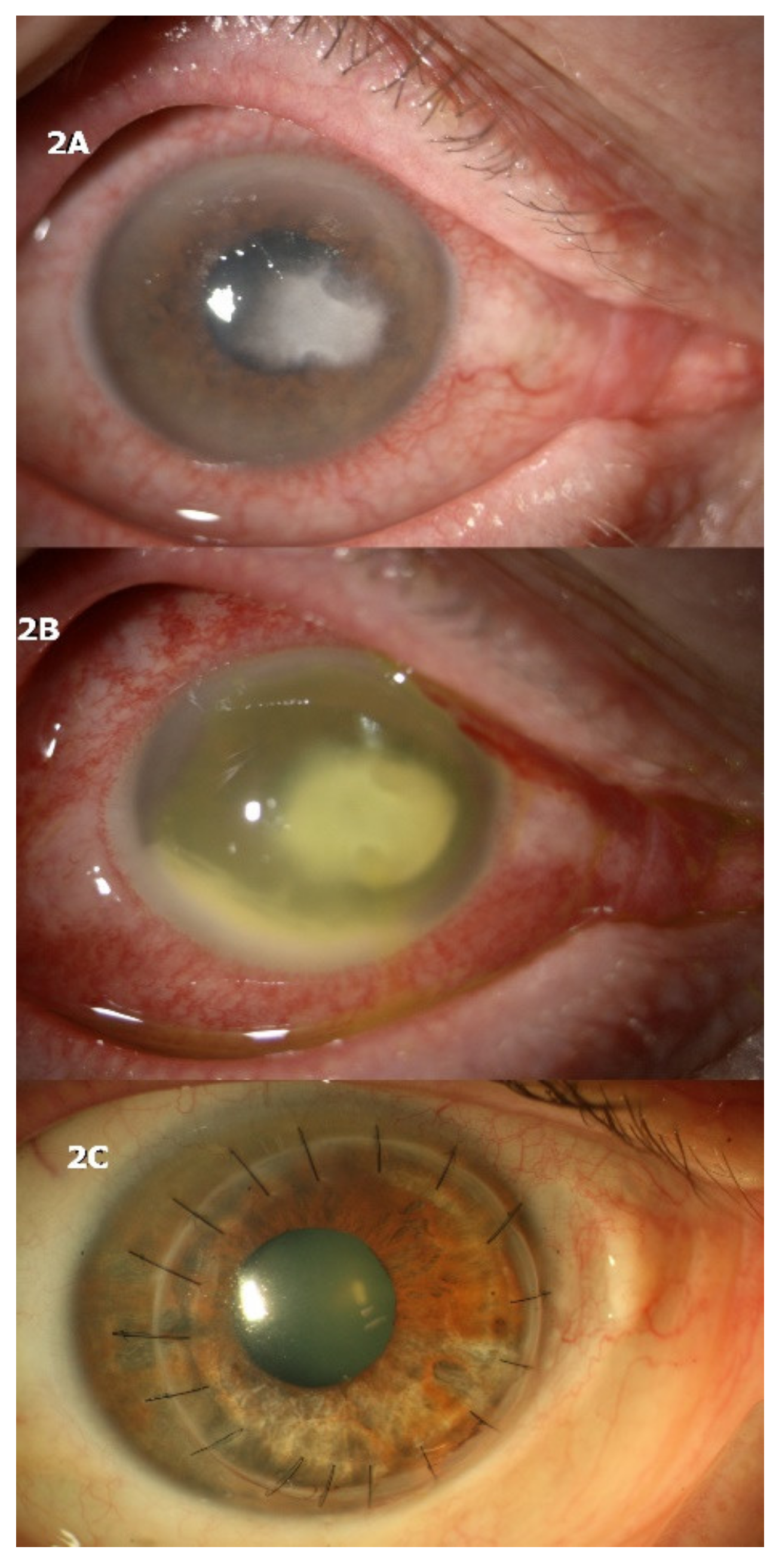
4.1. CASE 1
4.2. CASE 2
4.3. CASE 3
5. Discussion (Concluding Remarks)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Srinivasan, M. Fungal keratitis. Curr. Opin. Ophthalmol. 2004, 15, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Srinivasan, M.; George, C. The current status of Fusarium species in mycotic keratitis in South India. J. Med. Microbiol. 1993, 1, 140–147. [Google Scholar]
- Lin, C.C.; Prajna, L.; Srinivasan, M.; Prajna, N.V.; McLeod, S.D.; Acharya, N.R.; Lietman, T.M.; Porco, T.C. Seasonal trends of microbial keratitis in South India. Cornea 2012, 31, 1123–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoudi, S.; Masoomi, A.; Ahmadikia, K.; Tabatabaei, S.A.; Soleimani, M.; Rezaie, S.; Ghahvechian, H.; Banafsheafshan, A. Fungal keratitis: An overview of clinical and laboratory aspects. Mycoses 2018, 61, 916–930. [Google Scholar] [CrossRef]
- Hagan, M.; Wright, E.; Newman, M.; Dolin, P.; Johnson, G. Causes of suppurative keratitis in Ghana. Br. J. Ophthalmol. 1995, 79, 1024–1028. [Google Scholar] [CrossRef]
- Liesegang, T.J.; Forster, R.K. Spectrum of microbial keratitis in South Florida. Am. J. Ophthalmol. 1980, 90, 38–47. [Google Scholar] [CrossRef]
- Ritterband, D.C.; Seedor, J.A.; Shah, M.K.; Koplin, R.S.; McKormick, S.A. Fungal keratitis at the new york eye and ear infirmary. Cornea 2006, 25, 264–267. [Google Scholar] [CrossRef]
- Galarreta, D.J.; Tuft, S.J.; Ramsay, A.; Dart, J. Fungal keratitis in London: Microbiological and clinical evaluation. Cornea 2007, 26, 1082–1086. [Google Scholar] [CrossRef]
- Wong, T.Y.; Ng, T.P.; Fong, K.S.; Tan, D.T. Risk Factors and clinical outcomes between fungal and bacterial keratitis: A comparative study. CLAO J. 1997, 23, 275–281. [Google Scholar] [PubMed]
- O’Day, D.M.; Head, W.S.; Robinson, R.D.; Clanton, J.A. Corneal penetration of topical amphothericin b and natamycin. Curr. Eye Res. 1986, 5, 877–882. [Google Scholar] [CrossRef]
- Thomas, P.A. Fungal infections of the cornea. Eye 2003, 17, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Hahn, Y.H.; Lee, D.J.; Kim, M.S.; Choi, S.H.; Kim, J.D. Epidemiology of fungal keratitis in Korea: A multi-center study. J. Korean Ophthalmol. Soc. 2000, 41, 499–508. [Google Scholar]
- Kaur, I.P.; Rana, C.; Singh, H. Development of effective ocular preparations of antifungal agents. J. Ocul. Pharmacol. Ther. 2008, 24, 481–493. [Google Scholar] [CrossRef]
- Prajna, N.V.; Krishnan, T.; Mascarenhas, J.; Rajaraman, R.; Prajna, L.; Srinivasan, M.; Raghavan, A.; Oldenburg, C.E.; Ray, K.J.; Zegans, M.E.; et al. Mycotic Ulcer Treatment Trial Group. The mycotic ulcer treatment trial: A randomized trial comparing natamycin vs voriconazole. JAMA Ophthalmol. 2013, 131, 422–429. [Google Scholar] [CrossRef]
- Qu, L.; Li, L.; Xie, H. Corneal and aqueous humor concentrations of amphotericin B using three different routes of administration in a rabbit model. Ophthalmic Res. 2010, 43, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Avunduk, A.M.; Beuerman, R.W.; Warnel, E.D.; Kaufman, H.E.; Greer, D. Comparison of efficacy of topical and oral fluconazole treatment in experimental Aspergillus keratitis. Curr. Eye Res. 2003, 26, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Ahuja, R.; Biswas, N.R.; Satpathy, G.; Khokhar, S. Role of 0.02% polyhexamethylene biguanide and 1% povidone iodine in experimental Aspergillus keratitis. Cornea 2003, 22, 138–141. [Google Scholar] [CrossRef]
- Sharma, N.; Chacko, J.; Velpandian, T.; Titiyal, J.S.; Sinha, R.; Satpathy, G.; Tandon, R.; Vajpayee, R.B. Comparative evaluation of topical versus intrastromal voriconazole as an adjunct to natamycin in recalcitrant fungal keratitis. Ophthalmology 2013, 120, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Mimouni, M.; Tam, G.; Paitan, Y.; Kidron, D.; Segev, F. Safety and efficacy of intrastromal injection of 5% natamycin in experimental fusarium keratitis. J. Ocul. Pharmacol. Ther. 2014, 30, 543–547. [Google Scholar] [CrossRef]
- Kalaiselvi, G.; Narayana, S.; Krishnan, T.; Sengupta, S. Intrastromal voriconazole for deep recalcitrant fungal keratitis: A case series. Br. J. Ophthalmol. 2015, 99, 195–198. [Google Scholar] [CrossRef]
- Sharma, B.; Kataria, P.; Anand, R.; Gupta, R.; Kumar, K.; Kumar, S.; Gupta, R. Efficacy profile of intracameral amphotericin B. The often forgotten step. Asia Pac. J. Ophthalmol. 2015, 4, 360–366. [Google Scholar] [CrossRef]
- Sharma, N.; Sankaran, P.; Agarwal, T.; Arora, T.; Chawla, B.; Titiyal, J.S.; Tandon, R.; Satapathy, G.; Vajpayee, R.B. Evaluation of intracameral amphotericin B in the management of fungal keratitis: Randomized controlled trial. Ocul. Immunol. Inflamm. 2015, 4, 1–5. [Google Scholar] [CrossRef]
- Garg, P.; Roy, A.; Roy, S. Update on fungal keratitis. Curr. Opin. Ophthalmol. 2016, 27, 333–339. [Google Scholar] [CrossRef]
- Panda, A.; Vajpayee, R.B.; Kumar, T.S. Critical evaluation of therapeutic keratoplasty in cases of keratomycosis. Ann. Ophthalmol. 1991, 23, 373–376. [Google Scholar] [PubMed]
- Xie, L.; Dong, X.; Shi, W. Treatment of fungal keratitis by penetrating keratoplasty. Br. J. Ophthalmol. 2001, 85, 1070–1074. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.F.; Zhang, Y.M.; Zhou, P.; Zhang, B.; Qiu, W.Y.; Tseng, S.C. Therapeutic penetrating keratoplasty in severe fungal keratitis using cryopreserved donor corneas. Br. J. Ophthalmol. 2003, 87, 543–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopinathan, U.; Garg, P.; Fernandes, M.; Sharma, S.; Athmanathan, S.; Rao, G.N. The epidemiological features and laboratory results of fungal keratitis: A 10-year review at a referral eye care center in South India. Cornea 2002, 21, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, M.J.; Ramakrishnan, R.; Vasu, S.; Meenakshi, R.; Palaniappan, R. Epidemiological characteristics and laboratory diagnosis of fungal keratitis: A three-year study. Indian J. Ophthalmol. 2003, 51, 315–321. [Google Scholar] [PubMed]
- Nielsen, S.E.; Nielsen, E.; Julian, H.O.; Lindegaard, J.; Højgaard, K.; Ivarsen, A.; Hjortdal, J.; Heegaard, S. Incidence and clinical characteristics of fungal keratitis in a Danish population from 2000 to 2013. Acta Ophthalmol. 2015, 93, 54–58. [Google Scholar] [CrossRef]
- Ong, H.S.; Fung, S.S.M.; Macleod, D.; Dart, J.K.G.; Tuft, S.J.; Burton, M.J. Altered patterns of fungal keratitis at a London ophthalmic referral hospital: An eight year retroscpective observational study. Am. J. Ophthalmol. 2016, 168, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Keay, L.J.; Gower, E.W.; Iovieno, A.; Oechsler, R.A.; Alfonso, E.C.; Matoba, A.; Colby, K.; Tuli, S.S.; Hammersmith, K.; Cavanagh, D.; et al. Clinical and microbiological characteristics of fungal keratitis in the United States, 2001–2007: A multicenter study. Ophthalmology 2011, 118, 920–926. [Google Scholar] [CrossRef] [Green Version]
- Bhartiya, P.; Daniell, M.; Constantinou, M.; Islam, F.M.; Taylor, H.R. Fungal keratitis in Melbourne. Clin. Exp. Ophthalmol. 2007, 35, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Stern, G.A.; Buttross, M. Use of corticosteroids in combination with antimicrobial drugs in the treatment of infectious corneal disease. Ophthalmology 1991, 98, 847–853. [Google Scholar] [CrossRef]
- Thygeson, P.; Okumoto, M. Keratomycosis: A preventable disease. Trans. Am. Acad. Ophthalmol. Otolaryngol. 1974, 78, 433–439. [Google Scholar] [PubMed]
- Forster, R.K.; Rebell, G. Animal model of Fusarium solani keratitis. Am. J. Ophthalmol. 1975, 79, 510–515. [Google Scholar] [CrossRef]
- Burda, C.D.; Fisher, E. The use of cortisone in establishing experi mental fungal keratitis in rats. A preliminary report. Am. J. Ophthaimol. 1959, 48, 330–335. [Google Scholar] [CrossRef]
- Ley, A.P. Experimental fungus infections of the cornea. A preliminary report. Am. J. Ophthalmol. 1956, 42, 59–71. [Google Scholar] [CrossRef]
- Berson, E.L.; Kobayashi, G.S.; Becker, B.; Rosenbaum, L. Topical corticosteroids and fungal keratitis. Investig. Ophthalmol. 1967, 6, 512–517. [Google Scholar]
- Kaufman, H.E. Use of corticosteroids in corneal disease and external diseases of the eye. Int. Ophthalmol. Clin. 1966, 6, 827–843. [Google Scholar] [CrossRef]
- Mitsui, Y.; Hanabusa, J. Corneal infections after cortisone therapy. Br. J. Ophthalmol. 1955, 39, 244–250. [Google Scholar] [CrossRef] [Green Version]
- O’Day, D.M.; Ray, W.A.; Robinson, R.; Head, W.S. Efficacy of antifungal agents in the cornea. Influence of corticosteroids. Investig. Ophthalmol. Vis. Sci. 1984, 25, 331–335. [Google Scholar]
- Cho, C.H.; Lee, S.B. Clinical analysis of microbiologically proven fungal keratitis according to prior topical steroid use: A retrospective study in South Korea. BMC Ophthalmol. 2019, 19, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wong, T.Y.; Au Eong, K.G.; Chan, W.K.; Tseng, P.S. Fusarium keratitis following the use of topical antibiotic-corticosteroid therapy in traumatized eyes. Ann. Acad. Med. Singap. 1996, 25, 862–865. [Google Scholar] [PubMed]
- Kalavathy, C.M.; Parmar, P.; Kaliamurthy, J.; Philip, V.R.; Ramalingam, M.D.; Jesudasan, C.A.; Thomas, P.A. Comparison of topical itraconazole 1% with topical natamycin 5% for the treatment of filamentous fungal keratitis. Cornea 2005, 24, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhong, W.; Shi, W.; Sun, S. Spectrum of fungal keratitis in north China. Ophthalmology 2006, 113, 1943–1948. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhai, H.; Shi, W. Penetrating keratoplasty for corneal perforations in fungal keratitis. Cornea 2007, 26, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Shi, W.; Liu, Z.; Li, S. Lamellar keratoplasty for the treatment of fungal keratitis. Cornea 2002, 21, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.L.; Wu, C.Y.; Hu, F.R.; Wang, J.J. Therapeutic penetrating keratoplasty for microbial keratitis in Taiwan from 1987 to 2001. Am. J. Ophthalmol. 2004, 137, 736–743. [Google Scholar] [CrossRef]
- Shi, W.; Wang, T.; Xie, L.; Li, S.; Gao, H.; Liu, J.; Li, H. Risk factors, clinical features, and outcomes of recurrent fungal keratitis after corneal transplantation. Ophthalmology 2010, 17, 890–896. [Google Scholar] [CrossRef]
- Shi, W.; Wang, T.; Zhang, J.; Zhao, J.; Xie, L. Clinical features of immune rejection after corneoscleral transplantation. Am. J. Ophthalmol. 2008, 146, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, G.; Che, C.; Lin, J.; Li, N.; Jia, W.Y.; Zhang, Q.Q.; Jiang, N.; Hu, L.T. Effect of corneal graft diameter on therapeutic penetrating keratoplasty for fungal keratitis. Int. J. Ophthalmol. 2012, 5, 698–703. [Google Scholar] [CrossRef]
- Seedor, J.A.; Stulting, R.D.; Epstein, R.J.; Nay, R.E.; Dreizen, N.G.; Waring, G.O.; Wilson, L.A.; Cavanagh, H.D. Survival of corneal grafts from donors supported by mechanical ventilation. Ophthalmology 1987, 94, 101–108. [Google Scholar] [CrossRef]
- Belliappa, S.; Hade, J.; Kim, S.; Ayres, B.D.; Chu, D.S. Surgical outcomes in cases of contact lens-related Fusarium keratitis. Eye Contact Lens 2010, 36, 190–194. [Google Scholar] [CrossRef]
- Alfonso, E.C.; Rosa, R.H.; Miller, D. Cornea, 2nd ed.; Mosby: Maryland Heights, MO, USA, 2005; pp. 1101–1113. [Google Scholar]
- Gregory, M.E.; Macdonald, E.C.; Lockington, D.; Ramaesh, K. Recurrent fungal keratitis following penetrating keratoplasty: An unusual source of infection. Arch. Ophthalmol. 2010, 128, 1490–1491. [Google Scholar] [CrossRef] [Green Version]
- Jain, V.; Maiti, A.; Shome, D.; Borse, N.; Natarajan, S. Aspergillus-induced malignant glaucoma. Cornea 2007, 26, 762–763. [Google Scholar] [CrossRef] [PubMed]
- Kiryu, H.; Yoshida, S.; Suenaga, Y.; Asahi, M. Invasion and survival of Fusarium solani in the dexamethasone-treated corne of rabbits. J. Med. Vet. Mycol. 1991, 29, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, S.; Gao, H.; Shi, W. Therapeutic dilemma in fungal keratitis: Administration of steroids for immune rejection early after keratoplasty. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 1585–1589. [Google Scholar] [CrossRef] [PubMed]
- Peponis, V.; Herz, J.B.; Kaufman, H.E. The role of corticosteroids in fungal keratitis: A different view. Br. J. Ophthalmol. 2004, 88, 1227. [Google Scholar] [CrossRef] [Green Version]
- Matoba, A.Y.; Barrett, R.; Lehmann, A.E. Cure rate of fungal keratitis with antibacterial therapy. Cornea 2017, 36, 578–580. [Google Scholar] [CrossRef]
- Shukla, P.K.; Kumar, M.; Keshava, G.B. Mycotic keratitis: An overview of diagnosis and therapy. Mycoses 2008, 51, 183–199. [Google Scholar] [CrossRef]
- Piccolella, E.; Vismara, D.; Lombardi, G.; Guerritore, D.; Piantelli, M.; Ranelletti, F.O. Effect of glucocorticoids on the development of suppressive activity in human lymphocyte response to a polysaccharide purified from Candida albicans. J. Immunol. 1985, 134, 1166–1171. [Google Scholar] [PubMed]
- Agrawal, P.K.; Lal, B.; Shukla, P.K.; Khan, Z.A.; Srivastava, O.P. Clinical and experimental keratitis due to Curvularia lunata Wakker Boedijn var. aeria (Batista, Lima and Vasconcelos) Ellis. Sabouraudia 1982, 20, 225–232. [Google Scholar] [CrossRef]
- Schreiber, W.; Olbrisch, A.; Vorwerk, C.K.; König, W.; Behrens-Baumann, W. Combined topical fluconazole and corticosteroid treatment for experimental Candida albicans keratomycosis. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2634–2643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, F.; Huang, X.; Yuan, K.; Zhu, B.; Zhao, Y.; Hu, R.; Wan, T.; Zhu, L.; Jin, X. Glucocorticoids May Exacerbate Fungal Keratitis by Increasing Fungal Aggressivity and Inhibiting the Formation of Neutrophil Extracellular Traps. Curr. Eye Res. 2020, 45, 124–133. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knutsson, K.A.; Iovieno, A.; Matuska, S.; Fontana, L.; Rama, P. Topical Corticosteroids and Fungal Keratitis: A Review of the Literature and Case Series. J. Clin. Med. 2021, 10, 1178. https://doi.org/10.3390/jcm10061178
Knutsson KA, Iovieno A, Matuska S, Fontana L, Rama P. Topical Corticosteroids and Fungal Keratitis: A Review of the Literature and Case Series. Journal of Clinical Medicine. 2021; 10(6):1178. https://doi.org/10.3390/jcm10061178
Chicago/Turabian StyleKnutsson, Karl Anders, Alfonso Iovieno, Stanislav Matuska, Luigi Fontana, and Paolo Rama. 2021. "Topical Corticosteroids and Fungal Keratitis: A Review of the Literature and Case Series" Journal of Clinical Medicine 10, no. 6: 1178. https://doi.org/10.3390/jcm10061178
APA StyleKnutsson, K. A., Iovieno, A., Matuska, S., Fontana, L., & Rama, P. (2021). Topical Corticosteroids and Fungal Keratitis: A Review of the Literature and Case Series. Journal of Clinical Medicine, 10(6), 1178. https://doi.org/10.3390/jcm10061178




