Role of Narrow Band Imaging Technology in the Diagnosis and Follow up of Laryngeal Lesions: Assessment of Diagnostic Accuracy and Reliability in a Large Patient Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of the Study and Population
2.2. Instruments and Endoscopic Evaluation
2.3. Diagnostic Procedures
2.4. Statistical Analysis
- NBI: lesions classified in NBI, suspected as high-grade dysplasia or carcinoma (1), were considered as positive; lesions classified as non-malignant (0) as negative.
- WLE 1 + 2: lesions classified in WLE as uncertain (1) or clearly malignant (2) were considered positive; lesions classified as benign (0) as negative.
- WLE 2: only lesions classified in WLE as clearly malignant (2) were considered positive; lesions classified as benign (0) or as uncertain (1) were considered negative.
- WLE 1 + 2 + NBI: lesions classified in NBI, suspected as high-grade dysplasia or carcinoma (1), and at the same time classified in WLE as uncertain (1) or clearly malignant (2) were considered as positive; lesions classified in white light as benign (0) or classified in NBI as non-malignant (0) were considered as negative.
3. Results
3.1. Group A
- benign (0), n = 25 (35.7%) (Figure 1)
- low-grade dysplasia (1), n = 5 (7.1%)
- high-grade dysplasia (2), n = 12 (17.1%)
- carcinoma in situ or invasive (3), n = 28 (40.1%)
3.2. Group B
- benign (0), n = 8 (27.6%)
- low-grade dysplasia (1), n = 4 (13.8%) (Figure 3)
- high-grade dysplasia (2), n = 6 (20.7%)
- carcinoma in situ or invasive (3), n = 11 (37.9%)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vilaseca, I.; Valls-Mateus, M.; Nogués, A.; Lehrer, E.; López-Chacón, M.; Avilés-Jurado, F.X.; Blanch, J.L.; Bernal-Sprekelsen, M. Usefulness of office examination with narrow band imaging for the diagnosis of head and neck squamous cell carcinoma and follow-up of premalignant lesions. Head Neck 2017, 39, 1854–1863. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Taniguchi, M.; Tsujie, H.; Hosokawa, M.; Fujita, M.; Sasaki, S. The value of narrow band imaging for early detection of laryngeal cancer. Eur. Arch. Oto Rhino Laryngol. 2009, 266, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Bertino, G.; Cacciola, S.; Fernandes, W.B.; Fernandes, C.M.; Occhini, A.; Tinelli, C.; Benazzo, M. Effectiveness of narrow band imaging in the detection of premalignant and malignant lesions of the larynx: Validation of a new endoscopic clinical classification. Head Neck 2015, 37, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Piazza, C.; Del Bon, F.; Peretti, G.; Nicolai, P. Narrow band imaging in endoscopic evaluation of the larynx. Curr. Opin. Otolaryngol. Head Neck Surg. 2012, 20, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Arens, C.; Betz, C.; Kraft, M.; Voigt-Zimmermann, S. Narrow band imaging for early diagnosis of epithelial dysplasia and microinvasive tumors in the upper aerodigestive tract. HNO 2017, 65, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.-G.; He, S.; Xu, Z.-G.; Gao, L.; Lu, N.; Yuan, Z.; Lai, S.-Q.; Zhang, Y.-M.; Yi, J.-L.; Wang, X.-L.; et al. Endoscopic diagnosis of laryngeal cancer and precancerous lesions by narrow band imaging. J. Laryngol. Otol. 2011, 125, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Galli, J.; Settimi, S.; Salonna, G.; Mele, D.A.; De Corso, E.; Paludetti, G. Narrow Band Imaging for lingual tonsil hypertrophy and inflammation, in laryngo-pharyngeal reflux disease. Eur. Arch. Oto Rhino Laryngol. 2020, 277, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Galli, J.; Meucci, D.; Salonna, G.; Anzivino, R.; Giorgio, V.; Trozzi, M.; Settimi, S.; Tropiano, M.L.; Paludetti, G.; Bottero, S. Use OF NBI for the assessment of clinical signs of rhino-pharyngo-laryngeal reflux in pediatric age: Preliminary results. Int. J. Pediatr. Otorhinolaryngol. 2020, 128, 109733. [Google Scholar] [CrossRef]
- Popek, B.; Bojanowska-Poźniak, K.; Tomasik, B.; Fendler, W.; Jeruzal-Świątecka, J.; Pietruszewska, W. Clinical experience of narrow band imaging (NBI) usage in diagnosis of laryngeal lesions. Otolaryngol. Pol. 2019, 73, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Hosono, H.; Katada, C.; Okamoto, T.; Ichinoe, M.; Sakamoto, Y.; Matsuba, H.; Kano, K.; Ishido, K.; Tanabe, S.; Koizumi, W.; et al. Usefulness of narrow band imaging with magnifying endoscopy for the differential diagnosis of cancerous and noncancerous laryngeal lesions. Head Neck 2019, 41, 2555–2560. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, A.K.; Chan, J.K.C.; Rubin Grandis, J.; Takata, T.; Slootweg, P.J. International Agency for Research on Cancer. WHO classification of head and neck tumours. The fourth edition of the head and neck World Health Organization blue book: Editors’ perspectives. Hum. Pathol. 2017, 66, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Piazza, C.; Cocco, D.; De Benedetto, L.; Del Bon, F.; Nicolai, P.; Peretti, G. Narrow band imaging and high definition television in the assessment of laryngeal cancer: A prospective study on 279 patients. Eur Arch. Oto Rhino Laryngol. 2010, 267, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Piazza, C.; Cocco, D.; De Benedetto, L.; Bon FDel Nicolai, P.; Peretti, G. Role of narrow-band imaging and high-definition television in the surveillance of head and neck squamous cell cancer after chemo- and/or radiotherapy. Eur. Arch. Oto Rhino Laryngol. 2010, 267, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Zabrodsky, M.; Lukes, P.; Lukesova, E.; Boucek, J.; Plzak, J. The Role of Narrow Band Imaging in the Detection of Recurrent Laryngeal and Hypopharyngeal Cancer after Curative Radiotherapy. Biomed. Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.-G.; Zhang, Q.-Q.; Wang, G.-Q. Classification of nasopharyngeal microvessels detected by narrow band imaging endoscopy and its role in the diagnosis of nasopharyngeal carcinoma. Acta Otolaryngol. 2017, 137, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Wacławek, M.; Miłoński, J.; Olszewski, J. Comparative evaluation of the diagnostic value of biopsy and NBI endoscopy in patients with cancer of the hypopharynx and larynx. Otolaryngol. Pol. 2019, 73, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ansari, U.H.; Wong, E.; Smith, M.; Singh, N.; Palme, C.E.; Smith, M.C.; Riffat, F. Validity of narrow band imaging in the detection of oral and oropharyngeal malignant lesions: A systematic review and meta-analysis. Head Neck 2019, 41, 2430–2440. [Google Scholar] [CrossRef] [PubMed]
- Takano, J.H.; Yakushiji, T.; Kamiyama, I.; Nomura, T.; Katakura, A.; Takano, N.; Shibahara, T. Detecting early oral cancer: Narrowband imaging system observation of the oral mucosa microvasculature. Int. J. Oral Maxillofac. Surg. 2010, 39, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, J.; Yang, Y.; Liao, H.; Xu, Z.; Hamblin, L.T.; Jiang, L.; Depypere, L.; Ang, K.L.; He, J.; et al. White light, autofluorescence and narrow-band imaging bronchoscopy for diagnosing airway pre-cancerous and early cancer lesions: A systematic review and meta-analysis. J. Thorac. Dis. 2016, 8, 3205–3216. [Google Scholar] [CrossRef]
- Tirelli, G.; Piovesana, M.; Bonini, P.; Gatto, A.; Azzarello, G.; Boscolo Nata, F. Follow-up of oral and oropharyngeal cancer using narrow-band imaging and high-definition television with rigid endoscope to obtain an early diagnosis of second primary tumors: A prospective study. Eur. Arch. Oto Rhino Laryngol. 2017, 274, 2529–2536. [Google Scholar] [CrossRef]
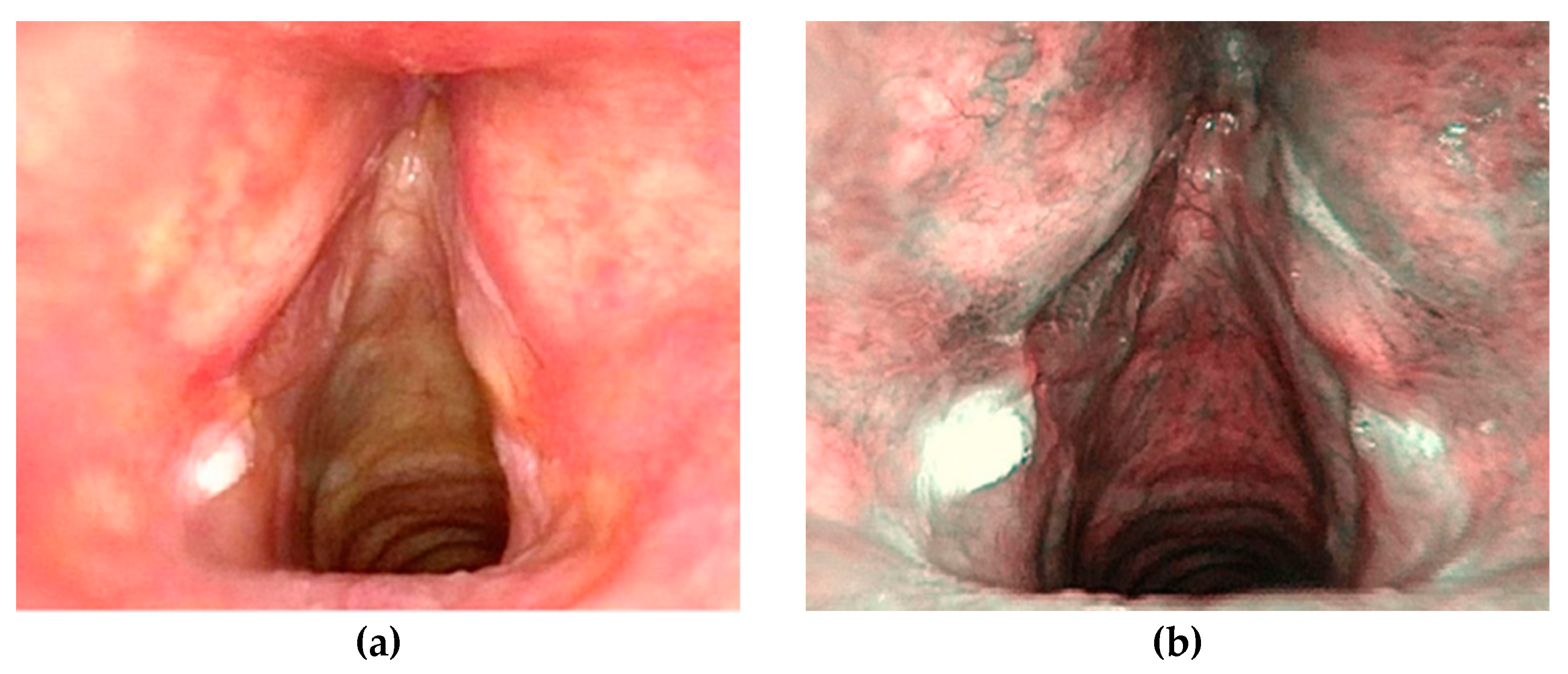


| Overall (n = 196) | Group A (n = 156) | Group B (n = 40) | |
|---|---|---|---|
| Age ± SD (range) | 60.5 ± 12.3 (26–93) | 57.3 ± 13.4 (26–87) | 62.8 ± 11.5 (32–93) |
| Sex (M–F) | 145–51 | 112–39 | 33–12 |
| Pts with >1 lesion | 50 | 39 | 11 |
| Total lesions | 246 | 195 | 51 |
| Indicator | NBI | WLE 1 + 2 | WLE 2 | WLE 1 + 2 and NBI |
|---|---|---|---|---|
| Sensitivity | 95.0 % | 95.0 % | 77.5 % | 90.0 % |
| Specificity | 96.8 % | 40.6 % | 89.0 % | 94.2 % |
| PPV | 88.3 % | 29.2 % | 64.6 % | 80.0 % |
| NPV | 98.7 % | 96.9 % | 93.9 % | 97.3 % |
| Accuracy | 96.4 % | 51.8 % | 86.7 % | 93.3 % |
| Positive likelihood ratio | 29.68 | 1.59 | 7.04 | 15.51 |
| Negative likelihood ratio | 0.05 | 0.13 | 0.25 | 0.10 |
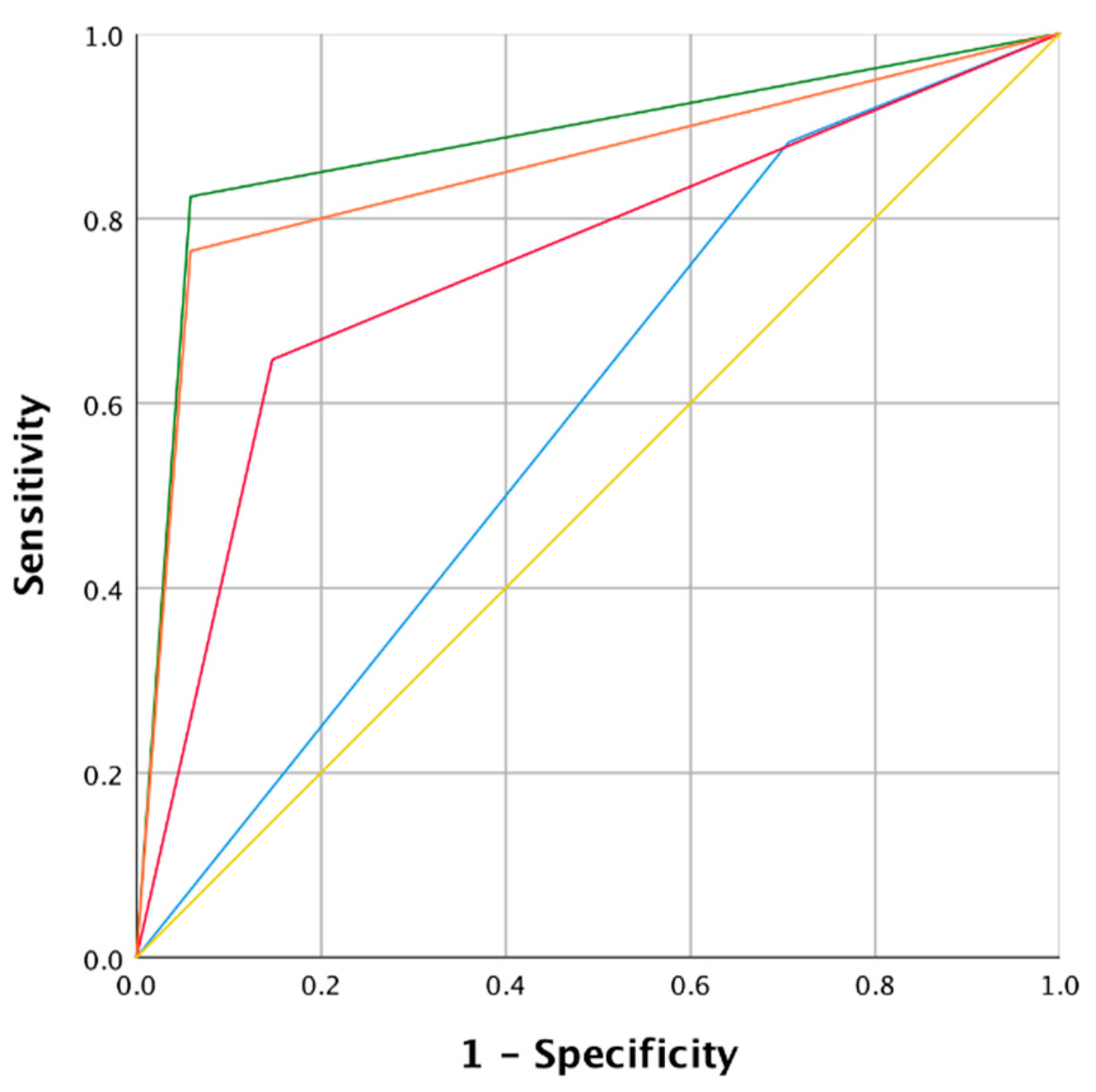
| Mode | Area | Lower limit 95% CI | Upper limit 95% CI | p |
| NBI | 0.96 | 0.91 | 0.99 | <0.0001 |
| WLE 1 + 2 | 0.67 | 0.59 | 0.75 | <0.0001 |
| WLE 2 | 0.83 | 0.75 | 0.91 | <0.0001 |
| WLE 1 + 2 and NBI | 0.92 | 0.86 | 0.97 | <0.0001 |
| Mode comparison | z | Lower limit 95% CI | Upper limit 95% CI | p |
| WLE 1 + 2 vs. WLE 2 | −4.168 | −0.227 | −0.082 | <0.0001 |
| WLE 1 + 2 vs. NBI | −8.699 | −0.344 | −0.217 | <0.0001 |
| WLE 1 + 2 vs. WLE + NBI | −6.608 | −0.315 | −0.171 | <0.0001 |
| WLE 2 vs. NBI | −3.420 | −0.0199 | −0.054 | <0.001 |
| WLE 2 vs. WLE + NBI | −2.091 | −0.171 | −0.006 | 0.037 |
| NBI vs. WLE + NBI | 2.040 | 0.001 | 0.074 | 0.041 |
| Indicator | NBI | WLE 1 + 2 | WLE 2 | WLE 1 + 2 and NBI |
|---|---|---|---|---|
| Sensitivity | 82.4 % | 88.3 % | 64.7 % | 76.5 % |
| Specificity | 94.1 % | 29.4 % | 85.3 % | 94.1 % |
| PPV | 87.5 % | 38.4 % | 68.7 % | 86.7 % |
| NPV | 91.4 % | 83.3 % | 82.8 % | 88.9 % |
| Accuracy | 90.2 % | 49.0 % | 78.4 % | 88.2 % |
| Positive likelihood ratio | 13.96 | 1.25 | 4.40 | 12.97 |
| Negative likelihood ratio | 0.18 | 0.40 | 0.41 | 0.25 |
| Mode | Area | Lower limit 95% CI | Upper limit 95% CI | p |
| NBI | 0.88 | 0.78 | 0.98 | <0.0001 |
| WLE 1 + 2 | 0.58 | 0.47 | 0.69 | <0.0001 |
| WLE 2 | 0.75 | 0.62 | 0.88 | <0.0001 |
| WLE 1 + 2 and NBI | 0.85 | 0.74 | 0.96 | <0.0001 |
| Mode comparison | z | Lower limit 95% CI | Upper limit 95% CI | p |
| WLE 1 + 2 vs. WLE 2 | −2.365 | 0.347 | −0.296 | 0.018 |
| WLE 1 + 2 vs. NBI | −3.714 | 0.330 | −0.449 | <0.0001 |
| WLE 1 + 2 vs. WLE + NBI | −3.674 | 0.335 | −0.406 | <0.0001 |
| WLE 2 vs. NBI | −1.842 | 0.342 | −0.273 | 0.066 |
| WLE 2 vs. WLE + NBI | −1.532 | 0.348 | −0.235 | 0.126 |
| NBI vs. WLE + NBI | 1.000 | 0.322 | −0.028 | 0.317 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galli, J.; Settimi, S.; Mele, D.A.; Salvati, A.; Schiavi, E.; Parrilla, C.; Paludetti, G. Role of Narrow Band Imaging Technology in the Diagnosis and Follow up of Laryngeal Lesions: Assessment of Diagnostic Accuracy and Reliability in a Large Patient Cohort. J. Clin. Med. 2021, 10, 1224. https://doi.org/10.3390/jcm10061224
Galli J, Settimi S, Mele DA, Salvati A, Schiavi E, Parrilla C, Paludetti G. Role of Narrow Band Imaging Technology in the Diagnosis and Follow up of Laryngeal Lesions: Assessment of Diagnostic Accuracy and Reliability in a Large Patient Cohort. Journal of Clinical Medicine. 2021; 10(6):1224. https://doi.org/10.3390/jcm10061224
Chicago/Turabian StyleGalli, Jacopo, Stefano Settimi, Dario Antonio Mele, Antonio Salvati, Enrico Schiavi, Claudio Parrilla, and Gaetano Paludetti. 2021. "Role of Narrow Band Imaging Technology in the Diagnosis and Follow up of Laryngeal Lesions: Assessment of Diagnostic Accuracy and Reliability in a Large Patient Cohort" Journal of Clinical Medicine 10, no. 6: 1224. https://doi.org/10.3390/jcm10061224
APA StyleGalli, J., Settimi, S., Mele, D. A., Salvati, A., Schiavi, E., Parrilla, C., & Paludetti, G. (2021). Role of Narrow Band Imaging Technology in the Diagnosis and Follow up of Laryngeal Lesions: Assessment of Diagnostic Accuracy and Reliability in a Large Patient Cohort. Journal of Clinical Medicine, 10(6), 1224. https://doi.org/10.3390/jcm10061224








