Ex-Vivo Pharmacological Defatting of the Liver: A Review
Abstract
1. Introduction
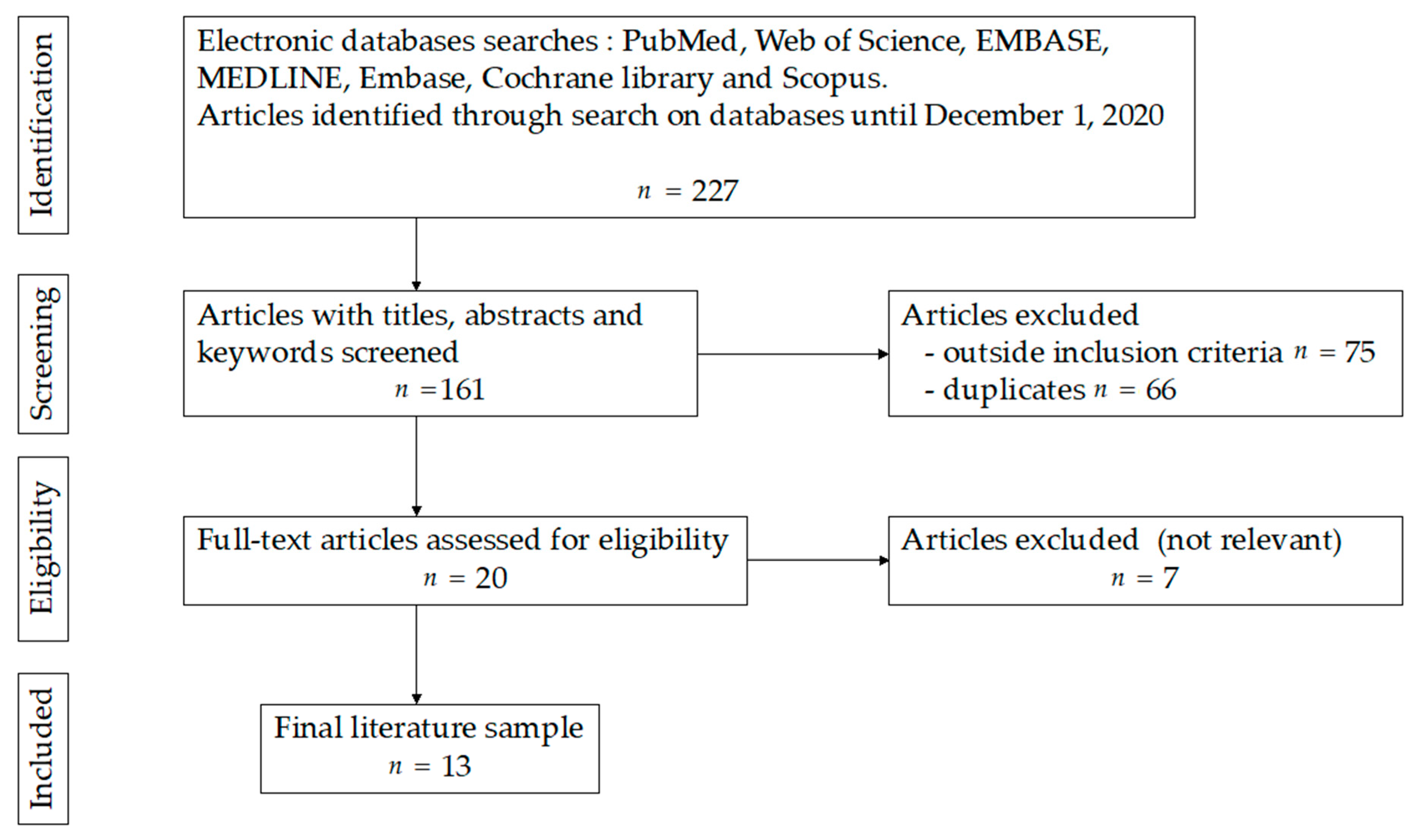
2. Materials and Methods
3. Results
3.1. Steatosis Physiopathology and Its Consequences
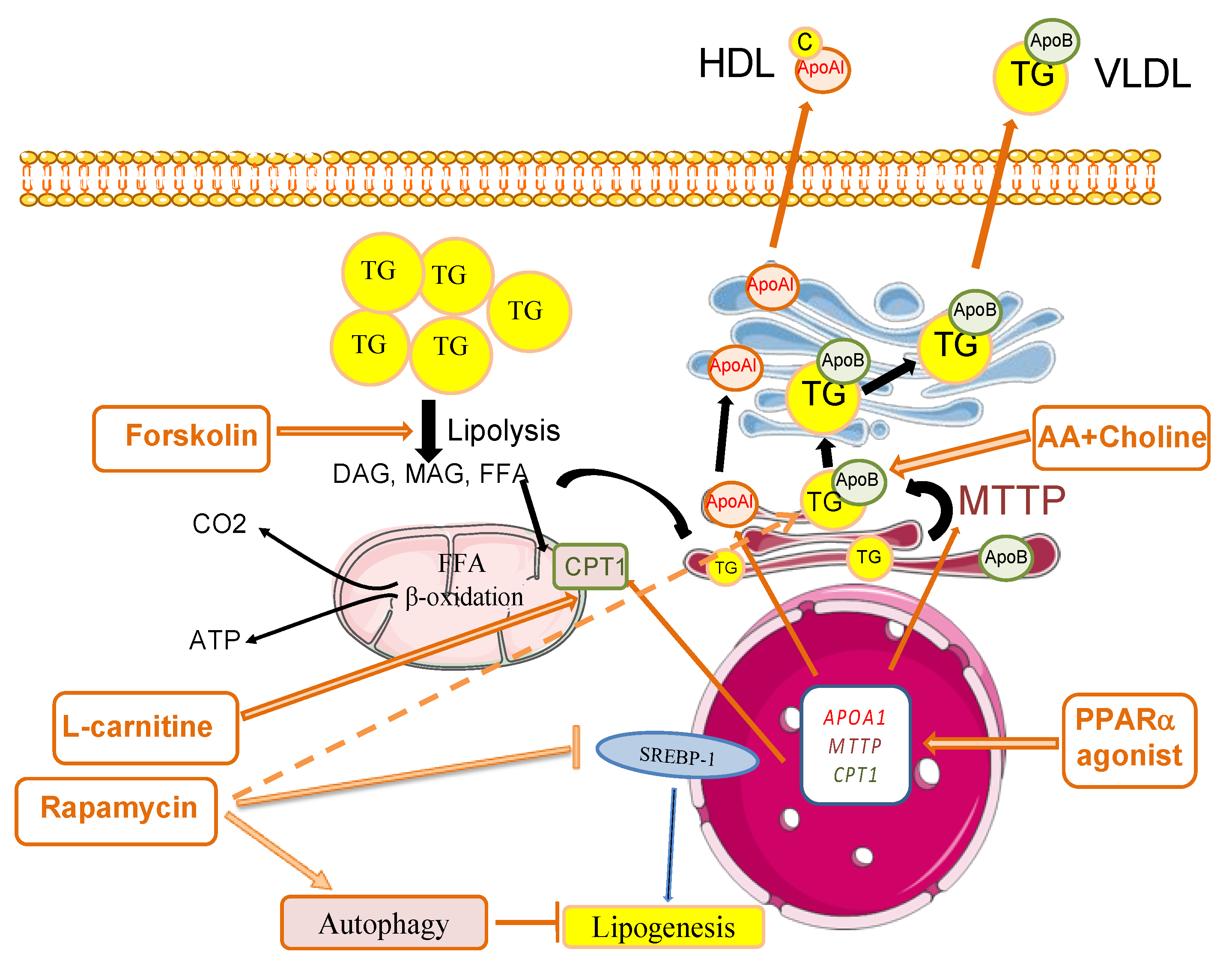
3.2. Pharmacological Therapies for Liver Defatting: In Vitro and Animal Models
3.2.1. Animal Hepatocyte Models
3.2.2. Human In Vitro Models
| Model | n | Defatting Agents | Main Outcomes | |
|---|---|---|---|---|
| Pégorier et al., 1989 [16] | rat hepatocytes | 8 | glucagon, forskolin and c-AMP | induction of ketogenesis |
| Berthiaume et al., 2009 [8] | rat hepatocytes | 14 | None | steatosis reversal |
| Nagrath et al., 2009 [18] | Rat hepatocytes | 4 | combination of visfatin, forskolin, hypericin and nuclear receptor ligands (GW7, GW5, scoparone) | reduction of intracellular TGcontent promotion of lipid export |
| Nativ et al., 2014 [19] | rat hepatocytes | 6 | defatting cocktail | reduction of intrahepatic TG reduction of large lipid droplets |
| Yarmush et al., 2015 [21] | HepG2 cells | 3 | combination of visfatin, forskolin, hypericin and nuclear receptor ligands (GW7, GW5, scoparone) | decrease of TG content |
| Boteon et al., 2018 [23] | human hepatocytes from discarded donor livers HIEC, cholangiocytes | 4 | combination of visfatin, forskolin, hypericin, L-carnithine, PPARα ligand and nuclear receptor ligands (GW7, GW5, scoparone) | reduction of intracellular TG induction of fatty acids β-oxidation |
| Aoudjehane et al., 2020 [24] | human hepatocytes from fatty livers | 6 | forskolin, L-carnitine and PPARα agonist | reduction of intracellular TG |
| Madji et al., 2020 [20] | mice livers human steatotic hepatocytes | 10 10 | RIPA-56 | decrease of intracelliular lipid droplets and TG content decreased inflammation and liver injury |
| Pégorier et al., 1989 [16] | rat hepatocytes | 8 | glucagon, forskolin and c-AMP | induction of ketogenesis |
| Berthiaume et al., 2009 [8] | rat hepatocytes | 14 | NA | reversing of steatosis |
| Nativ et al., 2014 [19] | rat hepatocytes | 6 | defatting cocktail | reduction of intrahepatic TG reduction of large lipid droplets |
| Yarmush et al., 2015 [21] | HepG2 cells | 3 | combination of visfatin, forskolin, hypericin and nuclear receptor ligands (GW7, GW5, scoparone) | decrease of TG content |
| Boteon et al., 2018 [23] | human hepatocytes from discarded donor livers HIEC, cholangiocytes | 4 | combination of visfatin, forskolin, hypericin, L-carnithine, PPARα ligand and nuclear receptor ligands (GW7, GW5, scoparone) | reduction of intracellular TG induction of fatty acids β-oxidation |
| Aoudjehane et al., 2020 [24] | human hepatocytes from fatty livers | 6 | forskolin, L-carnitine and PPARα agonist | reduction of intracellular TG |
| Madji et al., 2020 [20] | mice livers human steatotic hepatocytes | 10 10 | RIPA-56 | decrease of intracelliular lipid droplets and TG content decreased inflammation and liver injury |
3.2.3. Animal NMP Models
3.3. Defatting and Ex-Vivo Human Liver Machine Perfusion
| Model | n | Perfusion | Length of Perfusion (hours) | Defatting Agents | Main Outcomes | |
|---|---|---|---|---|---|---|
| Nagrath et al., 2009 [18] | rat | 7 | NMP | 3 | combination of visfatin, forskolin, hypericin and nuclear receptor ligands (GW7, GW5, scoparone) | decrease of TG rate improvement of bile production |
| Liu et al., 2013 [25] | rat | NA | SNMP | 6 | combination of amino acids, visfatin, forskolin, hypericin and nuclear receptor ligands (GW7, GW5, scoparone) | higher rate of TG (non significance) |
| Raigani et al., 2020 [26] | rat | 6 | NMP | 6 | combination of amino acids, visfatin, forskolin, hypericin and nuclear receptor ligands (GW7, GW5, scoparone) | decrease of pro inflammatory markers (NF- κB, TNF-α, IL-6) decrease of pro-apoptotic markers (CASP3, CD95) decrease of combined VLDL/LDL level |
| Taba Taba Vakili et al., 2016 [27] | mice | 4 | NMP | 4 | glial cell line–derived neurotrophic factor | reduction of TG content in liver no liver damage (no increase of apoptosis) |
| Banan et al., 2016 [31] | human discarded livers | 2 | NMP | 8 | L-carnithine and exendin-4 | decrease of TG and LDL level in the perfusate reduction of Mas |
| Boteon et al., 2019 [32] | human livers discarded | 5 | NMP | 12 | combination of L-carnithine, visfatin, forskolin, hypericin and nuclear receptor ligands (GW7, GW5, scoparone) | decrease of T-TG level decrease of MaS increase of P-TG level higher bile production |
3.4. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| ATP | adenosine triphosphate |
| AMP | cyclic adenosine monophosphate |
| CAR | constitutive androstane receptor |
| CIT | cold ischemia time |
| CoA | coenzyme A |
| CPT1A | carnitine palmitoyltransferase form 1A |
| FA | fatty acid |
| GGT | gamma-glutamyl transpeptidase |
| HDL | high-density lipoprotein |
| IRI | ischemia/reperfusion injury |
| IL | interleukin |
| LT | liver transplantation |
| NMP | normothermic machine perfusion |
| PCO2 | partial pressure of carbon dioxide |
| PPAR | peroxisome proliferator-activated receptor |
| TG | triglyceride |
| PXR | pregnane X receptor |
| ROS | reactive oxygen species |
| miRNA | MicroRNA |
| TNF-α | tumor necrosis factor α |
| VLDL | very low-density lipoprotein |
References
- Cohen, J.C.; Horton, J.D.; Hobbs, H.H. Human fatty liver disease: Old questions and new insights. Science 2011, 332, 1519–1523. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M. Pathology of fatty liver disease. Mod. Pathol. 2007, 20 (Suppl. 1), S40–S48. [Google Scholar] [CrossRef]
- Ratziu, V.; Bellentani, S.; Cortez-Pinto, H.; Day, C.; Marchesini, G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J. Hepatol. 2010, 53, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Karam, V.; Delvart, V.; O’Grady, J.; Mirza, D.; Klempnauer, J.; Castaing, D.; Neuhaus, P.; Jamieson, N.; Salizzoni, M.; et al. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J. Hepatol. 2012, 57, 675–688. [Google Scholar] [CrossRef]
- Boteon, Y.L.; Boteon, A.P.C.S.; Attard, J.; Mergental, H.; Mirza, D.F.; Bhogal, R.H.; Afford, S.C. Ex situ machine perfusion as a tool to recondition steatotic donor livers: Troublesome features of fatty livers and the role of defatting therapies. A systematic review. Am. J. Transplant. 2018, 18, 2384–2399. [Google Scholar] [CrossRef] [PubMed]
- Dengu, F.; Abbas, S.H.; Ebeling, G.; Nasralla, D. Normothermic Machine Perfusion (NMP) of the Liver as a Platform for Therapeutic Interventions during Ex-Vivo Liver Preservation: A Review. J. Clin. Med. 2020, 9, 1046. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Berthiaume, F.; Barbe, L.; Mokuno, Y.; MacDonald, A.D.; Jindal, R.; Yarmush, M.L. Steatosis Reversibly Increases Hepatocyte Sensitivity to Hypoxia-Reoxygenation Injury. J. Surg. Res. 2009, 152, 54–60. [Google Scholar] [CrossRef]
- Krebs, H.A.; Hems, R. Fatty acid metabolism in the perfused rat liver. Biochem. J. 1970, 119, 525–533. [Google Scholar] [CrossRef]
- Seifalian, A.M.; Piasecki, C.; Agarwal, A.; Davidson, B.R. The effect of graded steatosis on flow in the hepatic parenchymal microcirculation. Transplantation 1999, 68, 780–784. [Google Scholar] [CrossRef]
- Caraceni, P.; Domenicali, M.; Vendemiale, G.; Grattagliano, I.; Pertosa, A.; Nardo, B.; Morselli-Labate, A.M.; Trevisani, F.; Palasciano, G.; Altomare, E.; et al. The reduced tolerance of rat fatty liver to ischemia reperfusion is associated with mitochondrial oxidative injury1. J. Surg. Res. 2005, 124, 160–168. [Google Scholar] [CrossRef]
- Spitzer, A.L.; Lao, O.B.; Dick, A.A.S.; Bakthavatsalam, R.; Halldorson, J.B.; Yeh, M.M.; Upton, M.P.; Reyes, J.D.; Perkins, J.D. The biopsied donor liver: Incorporating macrosteatosis into high-risk donor assessment. Liver Transplant. 2010, 16, 874–884. [Google Scholar] [CrossRef]
- de Graaf, E.L.; Kench, J.; Dilworth, P.; Shackel, N.A.; Strasser, S.I.; Joseph, D.; Pleass, H.; Crawford, M.; McCaughan, G.W.; Verran, D.J. Grade of deceased donor liver macrovesicular steatosis impacts graft and recipient outcomes more than the Donor Risk Index. J. Gastroenterol. Hepatol. 2012, 27, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, D.; Gibbons, G.F. The lipolysis/esterification cycle of hepatic triacylglycerol. Its role in the secretion of very-low-density lipoprotein and its response to hormones and sulphonylureas. Biochem. J. 1992, 284, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Chavin, K.D.; Fiorini, R.N.; Shafizadeh, S.; Cheng, G.; Wan, C.; Evans, Z.; Rodwell, D.; Polito, C.; Haines, J.K.; Baillie, G.M.; et al. Fatty acid synthase blockade protects steatotic livers from warm ischemia reperfusion injury and transplantation. Am. J. Transplant. 2004, 4, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Pégorier, J.P.; Garcia-Garcia, M.V.; Prip-Buus, C.; Duée, P.H.; Kohl, C.; Girard, J. Induction of ketogenesis and fatty acid oxidation by glucagon and cyclic AMP in cultured hepatocytes from rabbit fetuses. Evidence for a decreased sensitivity of carnitine palmitoyltransferase I to malonyl-CoA inhibition after glucagon or cyclic AMP treatment. Biochem. J. 1989, 264, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Vons, C.; Pegorier, J.P.; Girard, J.; Kohl, C.; Ivanov, M.A.; Franco, D. Regulation of fatty-acid metabolism by pancreatic hormones in cultured human hepatocytes. Hepatol. Baltim. Md. 1991, 13, 1126–1130. [Google Scholar] [CrossRef]
- Nagrath, D.; Xu, H.; Tanimura, Y.; Zuo, R.; Berthiaume, F.; Avila, M.; Yarmush, R.; Yarmush, M.L. Metabolic preconditioning of donor organs: Defatting fatty livers by normothermic perfusion ex vivo. Metab. Eng. 2009, 11, 274–283. [Google Scholar] [CrossRef]
- Nativ, N.I.; Yarmush, G.; So, A.; Barminko, J.; Maguire, T.J.; Schloss, R.; Berthiaume, F.; Yarmush, M.L. Elevated sensitivity of macrosteatotic hepatocytes to hypoxia/reoxygenation stress is reversed by a novel defatting protocol. Liver Transplant. 2014, 20, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Majdi, A.; Aoudjehane, L.; Ratziu, V.; Islam, T.; Afonso, M.B.; Conti, F.; Mestiri, T.; Lagouge, M.; Foufelle, F.; Ballenghien, F.; et al. Inhibition of receptor-interacting protein kinase 1 improves experimental non-alcoholic fatty liver disease. J. Hepatol. 2020, 72, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Yarmush, G.; Santos, L.; Yarmush, J.; Koundinyan, S.; Saleem, M.; Nativ, N.; Schloss, R.; Yarmush, M.; Maguire, T.; Berthiaume, F. Metabolic Flux Distribution during Defatting of Steatotic Human Hepatoma (HepG2) Cells. Metabolites 2016, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Yarmush, G.; Santos, L.; Yarmush, J.; Koundinyan, S.; Saleem, M.; Nativ, N.I.; Yarmush, M.L.; Berthiaume, F.; Maguire, T.J.; Guaghan, C. CFD assessment of the effect of convective mass transport on the intracellular clearance of intracellular triglycerides in macrosteatotic hepatocytes. Biomech. Model. Mechanobiol. 2017, 16, 1095–1102. [Google Scholar] [CrossRef]
- Boteon, Y.L.; Wallace, L.; Boteon, A.P.C.S.; Mirza, D.F.; Mergental, H.; Bhogal, R.H.; Afford, S. An effective protocol for pharmacological defatting of primary human hepatocytes which is non-toxic to cholangiocytes or intrahepatic endothelial cells. PLoS ONE 2018, 13, e0201419. [Google Scholar] [CrossRef]
- Aoudjehane, L.; Gautheron, J.; Le Goff, W.; Goumard, C.; Gilaizeau, J.; Nget, C.S.; Savier, E.; Atif, M.; Lesnik, P.; Morichon, R.; et al. Novel defatting strategies reduce lipid accumulation in primary human culture models of liver steatosis. Dis. Model. Mech. 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Berendsen, T.; Izamis, M.-L.; Uygun, B.; Yarmush, M.L.; Uygun, K. Perfusion Defatting at Subnormothermic Temperatures in Steatotic Rat Livers. Transplant. Proc. 2013, 45, 3209–3213. [Google Scholar] [CrossRef]
- Raigani, S.; Karimian, N.; Huang, V.; Zhang, A.M.; Beijert, I.; Geerts, S.; Nagpal, S.; Hafiz, E.O.A.; Fontan, F.M.; Aburawi, M.M.; et al. Metabolic and lipidomic profiling of steatotic human livers during ex situ normothermic machine perfusion guides resuscitation strategies. PLoS ONE 2020, 15, e0228011. [Google Scholar] [CrossRef] [PubMed]
- Taba Taba Vakili, S.; Kailar, R.; Rahman, K.; Nezami, B.G.; Mwangi, S.M.; Anania, F.A.; Srinivasan, S. Glial cell line-derived neurotrophic factor-induced mice liver defatting: A novel strategy to enable transplantation of steatotic livers: Gdnf and Liver Defatting. Liver Transpl. 2016, 22, 459–467. [Google Scholar] [CrossRef]
- Xu, J.; Buchwald, J.E.; Martins, P.N. Review of Current Machine Perfusion Therapeutics for Organ Preservation. Transplantation 2020, 104, 1792–1803. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, J.E.; Xu, J.; Bozorgzadeh, A.; Martins, P.N. Therapeutics administered during ex vivo liver machine perfusion: An overview. World J. Transplant. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Jamieson, R.W.; Zilvetti, M.; Roy, D.; Hughes, D.; Morovat, A.; Coussios, C.C.; Friend, P.J. Hepatic steatosis and normothermic perfusion-preliminary experiments in a porcine model. Transplantation 2011, 92, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Banan, B.; Watson, R.; Xu, M.; Lin, Y.; Chapman, W. Development of a normothermic extracorporeal liver perfusion system toward improving viability and function of human extended criteria donor livers. Liver Transplant. 2016, 22, 979–993. [Google Scholar] [CrossRef] [PubMed]
- Boteon, Y.L.; Attard, J.; Boteon, A.P.C.S.; Wallace, L.; Reynolds, G.; Hubscher, S.; Mirza, D.F.; Mergental, H.; Bhogal, R.H.; Afford, S.C. Manipulation of Lipid Metabolism During Normothermic Machine Perfusion: Effect of Defatting Therapies on Donor Liver Functional Recovery: Liver Transplantation. Liver Transpl. 2019, 25, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Goldaracena, N.; Spetzler, V.N.; Echeverri, J.; Kaths, J.M.; Cherepanov, V.; Persson, R.; Hodges, M.R.; Janssen, H.L.A.; Selzner, N.; Grant, D.R.; et al. Inducing Hepatitis C Virus Resistance After Pig Liver Transplantation-A Proof of Concept of Liver Graft Modification Using Warm Ex Vivo Perfusion. Am. J. Transplant. 2017, 17, 970–978. [Google Scholar] [CrossRef]
- Thijssen, M.F.; Brüggenwirth, I.M.A.; Gillooly, A.; Khvorova, A.; Kowalik, T.F.; Martins, P.N. Gene Silencing With siRNA (RNA Interference): A New Therapeutic Option During Ex Vivo Machine Liver Perfusion Preservation. Liver Transpl. 2019, 25, 140–151. [Google Scholar] [CrossRef]
- Eshmuminov, D.; Becker, D.; Bautista Borrego, L.; Hefti, M.; Schuler, M.J.; Hagedorn, C.; Muller, X.; Mueller, M.; Onder, C.; Graf, R.; et al. An integrated perfusion machine preserves injured human livers for 1 week. Nat. Biotechnol. 2020, 38, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Goumard, C.; Savier, E.; Danion, J.; Pelissie, J.; Legallais, C.; Scatton, O. Cold-to-warm machine perfusion of the liver: A novel circuit for an uninterrupted combined perfusion protocol. HPB 2020. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goumard, C.; Turco, C.; Sakka, M.; Aoudjehane, L.; Lesnik, P.; Savier, E.; Conti, F.; Scatton, O. Ex-Vivo Pharmacological Defatting of the Liver: A Review. J. Clin. Med. 2021, 10, 1253. https://doi.org/10.3390/jcm10061253
Goumard C, Turco C, Sakka M, Aoudjehane L, Lesnik P, Savier E, Conti F, Scatton O. Ex-Vivo Pharmacological Defatting of the Liver: A Review. Journal of Clinical Medicine. 2021; 10(6):1253. https://doi.org/10.3390/jcm10061253
Chicago/Turabian StyleGoumard, Claire, Célia Turco, Mehdi Sakka, Lynda Aoudjehane, Philippe Lesnik, Eric Savier, Filomena Conti, and Olivier Scatton. 2021. "Ex-Vivo Pharmacological Defatting of the Liver: A Review" Journal of Clinical Medicine 10, no. 6: 1253. https://doi.org/10.3390/jcm10061253
APA StyleGoumard, C., Turco, C., Sakka, M., Aoudjehane, L., Lesnik, P., Savier, E., Conti, F., & Scatton, O. (2021). Ex-Vivo Pharmacological Defatting of the Liver: A Review. Journal of Clinical Medicine, 10(6), 1253. https://doi.org/10.3390/jcm10061253






