Integrative Biology of Diabetic Retinal Disease: Lessons from Diabetic Kidney Disease
Abstract
:1. Introduction
2. DRD: More Than a Vasculopathy
3. DKD: Another Frequent Comorbidity in Diabetes
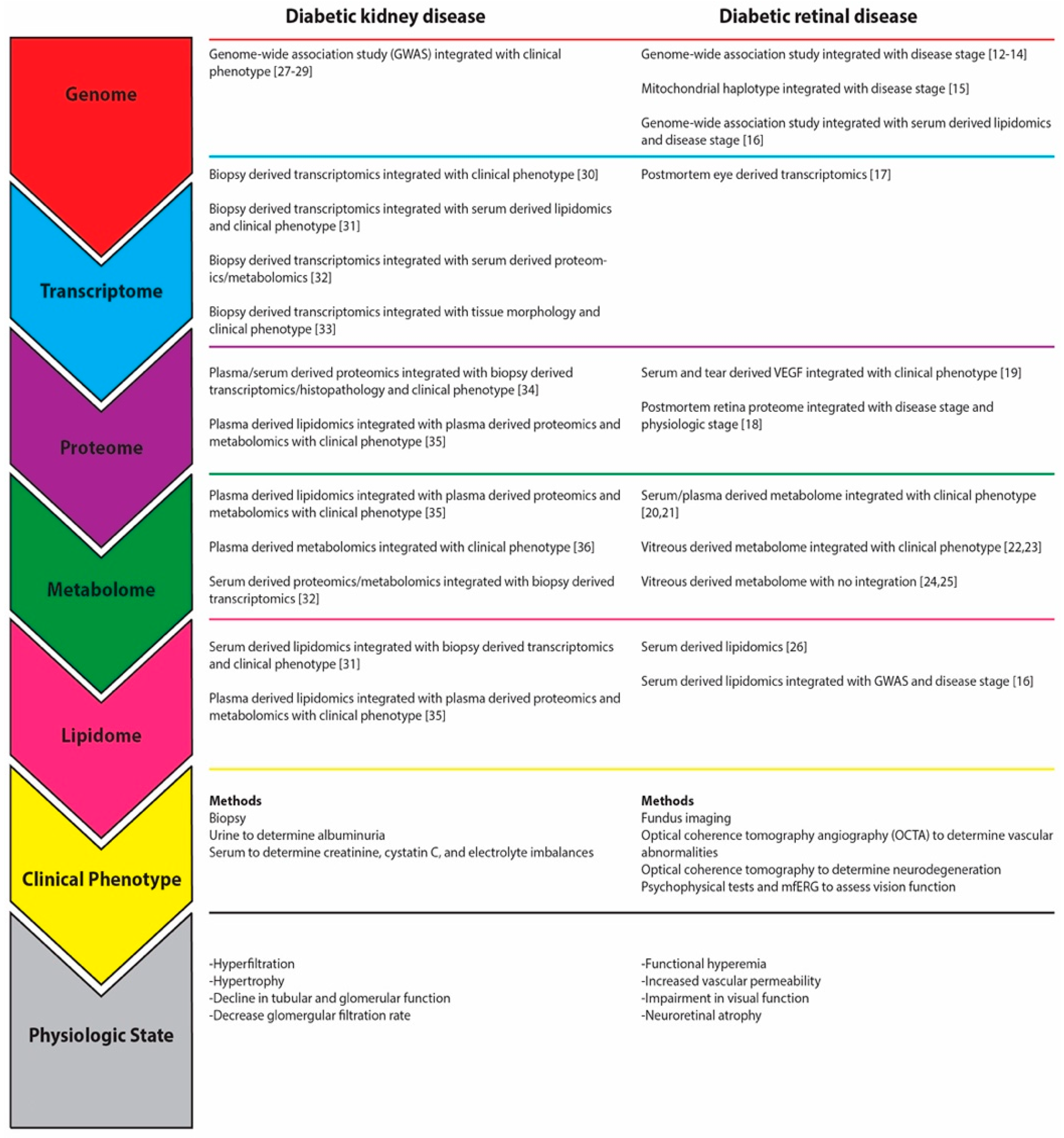
4. Systems Biology Yields Insights into Pathomechanisms of DKD
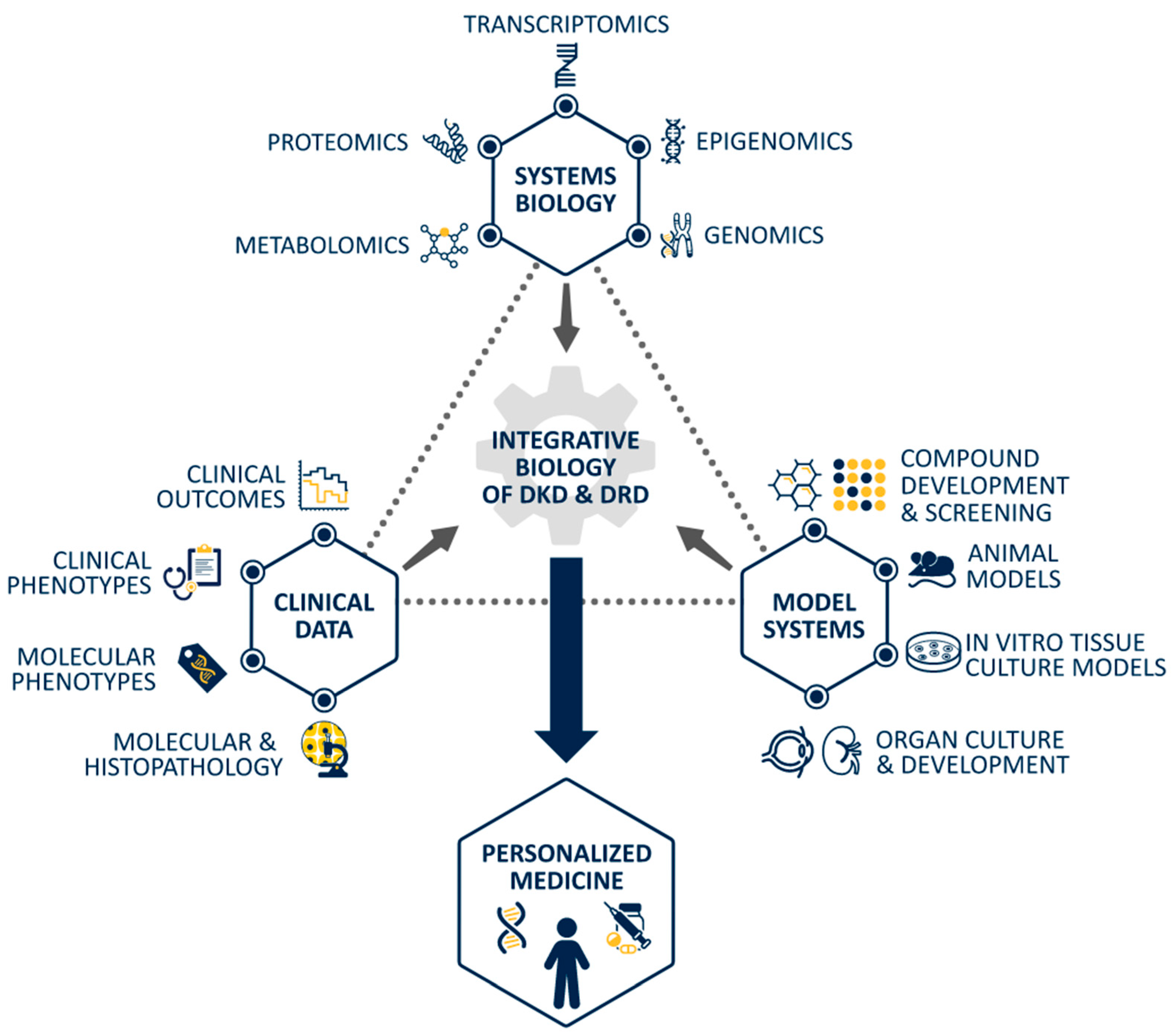
5. Shared Pathophysiology of DRD and DKD: An Avenue for Further Investigation
6. Conclusions: Where Do We Go from Here?
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yau, J.W.Y.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saran, R.; Robinson, B.; Abbott, K.C.; Bragg-Gresham, J.; Chen, X.; Gipson, D.; Gu, H.; Hirth, R.A.; Hutton, D.; Jin, Y.; et al. US Renal Data System 2019 Annual Data Report: Epidemiology of Kidney Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2019. [Google Scholar]
- World Health Organization. Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 12 January 2021).
- Internation Diabetes Federation. IDF Diabetes Atlas; International Diabetes Federation: Brussels, Belgium, 2019; ISBN 9782930229874. [Google Scholar]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [Green Version]
- Diabetic Retinopathy Clinical Research Network; Googe, J.; Brucker, A.J.; Bressler, N.M.; Qin, H.; Aiello, L.P.; Antoszyk, A.; Beck, R.W.; Bressler, S.B.; Ferris, F.L.; et al. Randomized trial evaluating short-term effects of intravitreal ranibizumab or triamcinolone acetonide on macular edema after focal/grid laser for diabetic macular edema in eyes also receiving panretinal photocoagulation. Retina 2011, 31, 1009–1027. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Brown, D.M.; Marcus, D.M.; Boyer, D.S.; Patel, S.; Feiner, L.; Gibson, A.; Sy, J.; Rundle, A.C.; Hopkins, J.J.; et al. Ranibizumab for diabetic macular edema: Results from 2 phase iii randomized trials: RISE and RIDE. Ophthalmology 2012, 119, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Bandello, F.; Schmidt-Erfurth, U.; Lang, G.E.; Massin, P.; Schlingemann, R.O.; Sutter, F.; Simader, C.; Burian, G.; Gerstner, O.; et al. The RESTORE study: Ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 2011, 118, 615–625. [Google Scholar] [CrossRef] [Green Version]
- Gillies, M.C.; Lim, L.L.; Campain, A.; Quin, G.J.; Salem, W.; Li, J.; Goodwin, S.; Aroney, C.; McAllister, I.L.; Fraser-Bell, S. A randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone for diabetic macular edema: The BEVORDEX study. Ophthalmology 2014, 121, 2473–2481. [Google Scholar] [CrossRef]
- Olivares, A.M.; Althoff, K.; Chen, G.F.; Wu, S.; Morrisson, M.A.; DeAngelis, M.M.; Haider, N. Animal Models of Diabetic Retinopathy. Curr. Diab. Rep. 2017, 17, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harder, J.L.; Hodgin, J.B.; Kretzler, M. Integrative Biology of Diabetic Kidney Disease. Kidney Dis. 2015, 1, 194–203. [Google Scholar] [CrossRef]
- Liu, E.; Kaidonis, G.; McComish, B.J.; Gillies, M.C.; Abhary, S.; Essex, R.W.; Chang, J.H.; Pal, B.; Daniell, M.; Lake, S.; et al. MicroRNA-related genetic variants are associated with diabetic retinopathy in type 1 diabetes mellitus. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3937–3942. [Google Scholar] [CrossRef] [Green Version]
- Pollack, S.; Igo, R.P.; Jensen, R.A.; Christiansen, M.; Li, X.; Cheng, C.Y.; Ng, M.C.Y.; Smith, A.V.; Rossin, E.J.; Segrè, A.V.; et al. Multiethnic genome-wide association study of diabetic retinopathy using liability threshold modeling of duration of diabetes and glycemic control. Diabetes 2019, 68, 441–456. [Google Scholar] [CrossRef] [Green Version]
- Vuori, N.; Sandholm, N.; Kumar, A.; Hietala, K.; Syreeni, A.; Forsblom, C.; Juuti-Uusitalo, K.; Skottman, H.; Imamura, M.; Maeda, S.; et al. CaCNB2 is a novel susceptibility gene for diabetic retinopathy in type 1 diabetes. Diabetes 2019, 68, 2165–2174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, S.L.; Neininger, A.C.; Bruce, C.N.; Chocron, I.M.; Bregman, J.A.; Estopinal, C.B.; Muhammad, A.; Umfress, A.C.; Jarrell, K.L.; Warden, C.; et al. Mitochondrial haplogroups modify the effect of diabetes duration and HbA1c on proliferative diabetic retinopathy risk in patients with type 2 diabetes. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6481–6488. [Google Scholar] [CrossRef] [Green Version]
- Sobrin, L.; Chong, Y.H.; Fan, Q.; Gan, A.; Stanwyck, L.K.; Kaidonis, G.; Craig, J.E.; Kim, J.; Liao, W.L.; Huang, Y.C.; et al. Genetically determined plasma lipid levels and risk of diabetic retinopathy: A mendelian randomization study. Diabetes 2017, 66, 3130–3141. [Google Scholar] [CrossRef] [Green Version]
- Platania, C.B.M.; Leggio, G.M.G.M.; Drago, F.; Salomone, S.; Bucolo, C. Computational systems biology approach to identify novel pharmacological targets for diabetic retinopathy. Biochem. Pharmacol. 2018, 158. [Google Scholar] [CrossRef] [PubMed]
- Sundstrom, J.M.; Hernández, C.; Weber, S.R.; Zhao, Y.; Dunklebarger, M.; Tiberti, N.; Laremore, T.; Simó-Servat, O.; Garcia-Ramirez, M.; Barber, A.J.; et al. Proteomic analysis of early diabetic retinopathy reveals mediators of neurodegenerative brain diseases. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2264–2274. [Google Scholar] [CrossRef] [Green Version]
- Ang, W.J.; Zunaina, E.; Norfadzillah, A.J.; Raja-Norliza, R.O.; Julieana, M.; Ab-Hamid, S.A.; Mahaneem, M. Evaluation of vascular endothelial growth factor levels in tears and serum among diabetic patients. PLoS ONE 2019. [Google Scholar] [CrossRef]
- Li, X.; Luo, X.; Lu, X.; Duan, J.; Xu, G. Metabolomics study of diabetic retinopathy using gas chromatography-mass spectrometry: A comparison of stages and subtypes diagnosed by Western and Chinese medicine. Mol. Biosyst. 2011, 7, 2228–2237. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cheng, C.Y.; Choi, H.; Ikram, M.K.; Sabanayagam, C.; Tan, G.S.W.; Tian, D.; Zhang, L.; Venkatesan, G.; Tai, E.S.; et al. Plasma metabonomic profiling of diabetic retinopathy. Diabetes 2016, 65, 1099–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomita, Y.; Cagnone, G.; Fu, Z.; Cakir, B.; Kotoda, Y.; Asakage, M.; Wakabayashi, Y.; Hellström, A.; Joyal, J.-S.; Talukdar, S.; et al. Vitreous metabolomics profiling of proliferative diabetic retinopathy. Diabetologia 2020. [Google Scholar] [CrossRef]
- Haines, N.R.; Manoharan, N.; Olson, J.L.; D’Alessandro, A.; Reisz, J.A. Metabolomics Analysis of Human Vitreous in Diabetic Retinopathy and Rhegmatogenous Retinal Detachment. J. Proteome Res. 2018, 17, 2421–2427. [Google Scholar] [CrossRef] [PubMed]
- Barba, I.; Garcia-Ramírez, M.; Hernández, C.; Alonso, M.A.; Masmique, L.; García-Dorado, D.; Simó, R. Metabolic fingerprints of proliferative diabetic retinopathy: An 1H-NMR-based metabonomic approach using vitreous humor. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4416–4421. [Google Scholar] [CrossRef] [PubMed]
- Paris, L.P.; Johnson, C.H.; Aguilar, E.; Usui, Y.; Cho, K.; Hoang, L.T.; Feitelberg, D.; Benton, H.P.; Westenskow, P.D.; Kurihara, T.; et al. Global metabolomics reveals metabolic dysregulation in ischemic retinopathy. Metabolomics 2016, 12, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xuan, Q.; Zheng, F.; Yu, D.; Ouyang, Y.; Zhao, X.; Hu, C.; Xu, G. Rapid lipidomic profiling based on ultra-high performance liquid chromatography–mass spectrometry and its application in diabetic retinopathy. Anal. Bioanal. Chem. 2020, 412, 3585–3594. [Google Scholar] [CrossRef]
- Sandholm, N.; Forsblom, C.; Mäkinen, V.P.; McKnight, A.J.; Österholm, A.M.; He, B.; Harjutsalo, V.; Lithovius, R.; Gordin, D.; Parkkonen, M.; et al. Genome-wide association study of urinary albumin excretion rate in patients with type 1 diabetes. Diabetologia 2014, 57, 1143–1153. [Google Scholar] [CrossRef]
- Salem, R.M.; Todd, J.N.; Sandholm, N.; Cole, J.B.; Chen, W.M.; Andrews, D.; Pezzolesi, M.G.; Mc, P.M.K.; Hiraki, L.T.; Qiu, C.; et al. Genome-Wide association study of diabetic kidney disease highlights biology involved in glomerular basement membrane collagen. J. Am. Soc. Nephrol. 2019, 30, 2000–2016. [Google Scholar] [CrossRef] [Green Version]
- Sandholm, N.; Van Zuydam, N.; Ahlqvist, E.; Juliusdottir, T.; Deshmukh, H.A.; Rayner, N.W.; Di Camillo, B.; Forsblom, C.; Fadista, J.; Ziemek, D.; et al. The genetic landscape of renal complications in type 1 diabetes. J. Am. Soc. Nephrol. 2017, 28, 557–574. [Google Scholar] [CrossRef] [Green Version]
- Ju, W.; Nair, V.; Smith, S.; Zhu, L.; Shedden, K.; Song, P.X.K.; Mariani, L.H.; Eichinger, F.H.; Berthier, C.C.; Randolph, A.; et al. Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci. Transl. Med. 2015, 7. [Google Scholar] [CrossRef] [Green Version]
- Afshinnia, F.; Nair, V.; Lin, J.; Rajendiran, T.M.; Soni, T.; Byun, J.; Sharma, K.; Fort, P.E.; Gardner, T.W.; Looker, H.C.; et al. Increased lipogenesis and impaired B-oxidation predict type 2 diabetic kidney disease progression in American Indians. JCI Insight 2019, 4, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Mulder, S.; Hammarstedt, A.; Nagaraj, S.B.; Nair, V.; Ju, W.; Hedberg, J.; Greasley, P.J.; Eriksson, J.W.; Oscarsson, J.; Heerspink, H.J.L. A metabolomics-based molecular pathway analysis of how the sodium-glucose co-transporter-2 inhibitor dapagliflozin may slow kidney function decline in patients with diabetes. Diabetes Obes. Metab. 2020, 22, 1157–1166. [Google Scholar] [CrossRef]
- Nair, V.; Komorowsky, C.V.; Weil, E.J.; Yee, B.; Hodgin, J.; Harder, J.L.; Godfrey, B.; Ju, W.; Boustany-Kari, C.M.; Schwarz, M.; et al. A molecular morphometric approach to diabetic kidney disease can link structure to function and outcome. Kidney Int. 2018, 93, 439–449. [Google Scholar] [CrossRef]
- Niewczas, M.A.; Pavkov, M.E.; Skupien, J.; Smiles, A.; Md Dom, Z.I.; Wilson, J.M.; Park, J.; Nair, V.; Schlafly, A.; Saulnier, P.J.; et al. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat. Med. 2019, 25, 805–813. [Google Scholar] [CrossRef]
- Kammer, M.; Heinzel, A.; Willency, J.A.; Duffin, K.L.; Mayer, G.; Simons, K.; Gerl, M.J.; Klose, C.; Heinze, G.; Reindl-Schwaighofer, R.; et al. Integrative analysis of prognostic biomarkers derived from multiomics panels helps discrimination of chronic kidney disease trajectories in people with type 2 diabetes. Kidney Int. 2019, 96, 1381–1388. [Google Scholar] [CrossRef]
- Tofte, N.; Vogelzangs, N.; Mook-Kanamori, D.; Brahimaj, A.; Nano, J.; Ahmadizar, F.; van Dijk, K.W.; Frimodt-Møller, M.; Arts, I.; Beulens, J.W.J.; et al. Plasma Metabolomics Identifies Markers of Impaired Renal Function: A Meta-analysis of 3089 Persons with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2020, 105, 2275–2287. [Google Scholar] [CrossRef] [PubMed]
- Treatment, E.; Retinopathy, D. Classification of Diabetic Retinopathy from Fluorescein Angiograms: ETDRS Report Number 11. Ophthalmology 1991, 98, 807–822. [Google Scholar] [CrossRef]
- Treatment, E.; Retinopathy, D. Fundus Photographic Risk Factors for Progression of Diabetic Retinopathy: ETDRS Report Number 12. Ophthalmology 1991, 98, 823–833. [Google Scholar] [CrossRef]
- Treatment, E.; Retinopathy, D. Grading Diabetic Retinopathy from Stereoscopic Color Fundus Photographs—An Extension of the Modified Airlie House Classification: ETDRS Report Number 10. Ophthalmology 1991, 98, 786–806. [Google Scholar] [CrossRef]
- Bursell, S.E.; Cavallerano, J.D.; Cavallerano, A.A.; Clermont, A.C.; Birkmire-Peters, D.; Aiello, L.P.; Aiello, L.M. Stereo nonmydriatic digital-video color retinal imaging compared with Early Treatment Diabetic Retinopathy Study seven standard field 35-mm stereo color photos for determining level of diabetic retinopathy. Ophthalmology 2001, 108, 572–585. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Lou, Y.; Erginay, A.; Clarida, W.; Amelon, R.; Folk, J.C.; Niemeijer, M. Improved automated detection of diabetic retinopathy on a publicly available dataset through integration of deep learning. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5200–5206. [Google Scholar] [CrossRef] [Green Version]
- Gulshan, V.; Peng, L.; Coram, M.; Stumpe, M.C.; Wu, D.; Narayanaswamy, A.; Venugopalan, S.; Widner, K.; Madams, T.; Cuadros, J.; et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA—J. Am. Med. Assoc. 2016, 316, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Abramoff, M.D.; Garvin, M.K.; Sonka, M. Retinal imaging and image analysis. IEEE Rev. Biomed. Eng. 2010, 3, 169–208. [Google Scholar] [CrossRef] [Green Version]
- Antonetti, D.A.; Klein, R.; Gardner, T.W. Diabetic retinopathy. N. Engl. J. Med. 2012, 366, 1227–1239. [Google Scholar] [CrossRef] [Green Version]
- Simó, R.; Stitt, A.W.; Gardner, T.W. Neurodegeneration in diabetic retinopathy: Does it really matter? Diabetologia 2018, 61, 1902–1912. [Google Scholar] [CrossRef] [Green Version]
- Jackson, G.R.; Barber, A.J. Visual dysfunction associated with diabetic retinopathy. Curr. Diab. Rep. 2010, 10, 380–384. [Google Scholar] [CrossRef]
- Trento, M.; Durando, O.; Lavecchia, S.; Charrier, L.; Cavallo, F.; Costa, M.A.; Hernández, C.; Simó, R.; Porta, M. Vision related quality of life in patients with type 2 diabetes in the EUROCONDOR trial. Endocrine 2017, 57, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Wolff, B.E.; Bearse, M.A.; Schneck, M.E.; Dhamdhere, K.; Harrison, W.W.; Barez, S.; Adams, A.J. Color vision and neuroretinal function in diabetes. Doc. Ophthalmol. 2015, 130, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Joltikov, K.A.; de Castro, V.M.; Davila, J.R.; Anand, R.; Khan, S.M.; Farbman, N.; Jackson, G.R.; Johnson, C.A.; Gardner, T.W. Multidimensional Functional and Structural Evaluation Reveals Neuroretinal Impairment in Early Diabetic Retinopathy. Invest. Ophthalmol. Vis. Sci. 2017, 58, BIO277–BIO290. [Google Scholar] [CrossRef]
- Reis, A.; Mateus, C.; Melo, P.; Figueira, J.; Cunha-Vaz, J.; Castelo-Branco, M. Neuroretinal dysfunction with intact blood-retinal barrier and absent vasculopathy in type 1 diabetes. Diabetes 2014, 63, 3926–3937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juen, S.; Kieselbach, G.F. Electrophysiological Changes in Juvenile Diabetics Without Retinopathy. Arch. Ophthalmol. 2011, 108, 372. [Google Scholar] [CrossRef]
- Di Leo, M.A.S.; Caputo, S.; Falsini, B.; Porciatti, V.; Greco, A.V.; Ghirlanda, G. Presence and further development of retinal dysfunction after 3-year follow up in IDDM patients without angiographically documented vasculopathy. Diabetologia 1994, 37, 911–916. [Google Scholar] [CrossRef]
- Tyrberg, M.; Lindblad, U.; Melander, A.; Lövestam-Adrian, M.; Ponjavic, V.; Andréasson, S. Electrophysiological studies in newly onset type 2 diabetes without visible vascular retinopathy. Doc. Ophthalmol. 2011, 123, 193–198. [Google Scholar] [CrossRef]
- van Dijk, H.W.; Verbraak, F.D.; Stehouwer, M.; Kok, P.H.B.; Garvin, M.K.; Sonka, M.; DeVries, J.H.; Schlingemann, R.O.; Abràmoff, M.D. Association of visual function and ganglion cell layer thickness in patients with diabetes mellitus type 1 and no or minimal diabetic retinopathy. Vision Res. 2011, 51, 224–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, A.J.; Bearse, M.A. Retinal neuropathy precedes vasculopathy in diabetes: A function-based opportunity for early treatment intervention? Clin. Exp. Optom. 2012, 95, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Tey, K.Y.; Teo, K.; Tan, A.C.S.; Devarajan, K.; Tan, B.; Tan, J.; Schmetterer, L.; Ang, M. Optical coherence tomography angiography in diabetic retinopathy: A review of current applications. Eye Vis. 2019, 6, 1–10. [Google Scholar] [CrossRef]
- Sohn, E.H.; Van Dijk, H.W.; Jiao, C.; Kok, P.H.B.B.; Jeong, W.; Demirkaya, N.; Garmager, A.; Wit, F.; Kucukevcilioglu, M.; Van Velthoven, M.E.J.J.; et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc. Natl. Acad. Sci. USA 2016, 113, E2655–E2664. [Google Scholar] [CrossRef] [Green Version]
- Chihara, E.; Matsuoka, T.; Ogura, Y.; Matsumura, M. Retinal nerve fiber layer defect as an early manifestation of diabetic retinopathy. Ophthalmology 1993, 100, 1147–1151. [Google Scholar] [CrossRef]
- van Dijk, H.W.; Verbraak, F.D.; Kok, P.H.B.; Garvin, M.K.; Sonka, M.; Lee, K.; Devries, J.H.; Michels, R.P.J.; van Velthoven, M.E.J.; Schlingemann, R.O.; et al. Decreased retinal ganglion cell layer thickness in patients with type 1 diabetes. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3660–3665. [Google Scholar] [CrossRef] [PubMed]
- Abcouwer, S.F.; Gardner, T.W. Diabetic retinopathy: Loss of neuroretinal adaptation to the diabetic metabolic environment. Ann. N. Y. Acad. Sci. 2014, 1311, 174–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, E.J.; Gardner, T.W. Retinal Failure in Diabetes: A Feature of Retinal Sensory Neuropathy. Curr. Diab. Rep. 2015, 15, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Vaz, J.G. Diabetic retinopathy: Need for more research to understand the relative role of neuropathy and microvascular disease. Ophthalmic Res. 2015, 54, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, D.A. The neuroscience of diabetic retinopathy. Vis. Neurosci. 2021, 38, E001. [Google Scholar] [CrossRef]
- Sun, J.K.; Aiello, L.P.; Abràmoff, M.D.; Antonetti, D.A.; Dutta, S.; Pragnell, M.; Levine, S.R.; Gardner, T.W. Updating the Staging System for Diabetic Retinal Disease. Ophthalmology 2020, 1–4. [Google Scholar] [CrossRef]
- Bailey, R.A.; Wang, Y.; Zhu, V.; Rupnow, M.F. Chronic kidney disease in US adults with type 2 diabetes: An updated national estimate of prevalence based on Kidney Disease: Improving Global Outcomes (KDIGO) staging. BMC Res. Notes 2014, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Afkarian, M.; Zelnick, L.R.; Hall, Y.N.; Heagerty, P.J.; Tuttle, K.; Weiss, N.S.; de Boer, I.H. Clinical Manifestations of Kidney Disease Among US Adults with Diabetes, 1988-2014. JAMA 2016, 316, 602–610. [Google Scholar] [CrossRef]
- Selby, J.V.; Friedman, G.D.; Quesenberry, C.P.; Weiss, N.S. TA Case–Control Study of Screening Sigmoidoscopy and Mortality from Colorectal Cancer. N. Engl. J. Med. 1992, 326, 653–657. [Google Scholar] [CrossRef]
- Viberti, G.C.; Jarrett, R.J.; Mahmud, U.; Hill, R.D.; Argyropoulos, A.; Keen, H. Microalbuminuria As a Predictor of Clinical Nephropathy in Insulin-Dependent Diabetes Mellitus. Lancet 1982, 319, 1430–1432. [Google Scholar] [CrossRef]
- Caramori, M.L.; Fioretto, P.; Mauer, M. The need for early predictors of diabetic nephropathy risk: Is albumin excretion rate sufficient? Diabetes 2000, 49, 1399–1408. [Google Scholar] [CrossRef] [Green Version]
- MacIsaac, R.J.; Ekinci, E.I. Progression of diabetic kidney disease in the absence of albuminuria. Diabetes Care 2019, 42, 1842–1844. [Google Scholar] [CrossRef] [Green Version]
- Lewis, E.J.; Hunsicker, L.G.; Clarke, W.R.; Berl, T.; Pohl, M.A.; Lewis, J.B.; Ritz, E.; Atkins, R.C.; Rohde, R.; Raz, I.; et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N. Engl. J. Med. 2001, 345, 851–860. [Google Scholar] [CrossRef] [Green Version]
- Parving, H.H.; Hommel, E.; Jensen, B.R.; Hansen, H.P. Long-term beneficial effect of ACE inhibition on diabetic nephropathy in normotensive type 1 diabetic patients. Kidney Int. 2001, 60, 228–234. [Google Scholar] [CrossRef] [Green Version]
- Kasiske, B.L.; Kalil, R.S.N.; Ma, J.Z.; Liao, M.; Keane, W.F. Effect of antihypertensive therapy on the kidney in patients with diabetes: A meta-regression analysis. Ann. Intern. Med. 1993, 118, 129–138. [Google Scholar] [CrossRef]
- Hebert, L.A.; Bain, R.P.; Verme, D.; Cattran, D.; Whittier, F.C.; Tolchin, N.; Rohde, R.D.; Lewis, E.J. Remission of nephrotic range proteinuria in type I diabetes. Collaborative Study Group. Kidney Int. 1994, 46, 1688–1693. [Google Scholar] [CrossRef] [Green Version]
- Lewis, E.J.; Hunsicker, L.G.; Bain, R.P.; Rohde, R.D. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N. Engl. J. Med. 1993, 329, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Neuen, B.L.; Young, T.; Heerspink, H.J.L.; Neal, B.; Perkovic, V.; Billot, L.; Mahaffey, K.W.; Charytan, D.M.; Wheeler, D.C.; Arnott, C.; et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: A systematic review and meta-analysis. Lancet. Diabetes Endocrinol. 2019, 7, 845–854. [Google Scholar] [CrossRef]
- Tuttle, K.R.; Brosius, F.C.; Adler, S.G.; Kretzler, M.; Mehta, R.L.; Tumlin, J.A.; Tanaka, Y.; Haneda, M.; Liu, J.; Silk, M.E.; et al. JAK1/JAK2 inhibition by baricitinib in diabetic kidney disease: Results from a Phase 2 randomized controlled clinical trial. Nephrol. Dial. Transplant. 2018, 33, 1950–1959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brosius, F.C.; Ju, W. The Promise of Systems Biology for Diabetic Kidney Disease. Adv. Chronic Kidney Dis. 2018, 25, 202–213. [Google Scholar] [CrossRef]
- Bhensdadia, N.M.; Hunt, K.J.; Lopes-Virella, M.F.; Tucker, J.M.; Mataria, M.R.; Alge, J.L.; Neely, B.A.; Janech, M.G.; Arthur, J.M. Veterans Affairs Diabetes Trial (VADT) study group Urine haptoglobin levels predict early renal functional decline in patients with type 2 diabetes. Kidney Int. 2013, 83, 1136–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zürbig, P.; Jerums, G.; Hovind, P.; MacIsaac, R.J.; Mischak, H.; Nielsen, S.E.; Panagiotopoulos, S.; Persson, F.; Rossing, P. Urinary proteomics for early diagnosis in diabetic nephropathy. Diabetes 2012, 61, 3304–3313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satirapoj, B.; Dispan, R.; Radinahamed, P.; Kitiyakara, C. Urinary epidermal growth factor, monocyte chemoattractant protein-1 or their ratio as predictors for rapid loss of renal function in type 2 diabetic patients with diabetic kidney disease. BMC Nephrol. 2018, 19, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Berthier, C.C.; Zhang, H.; Schin, M.; Henger, A.; Nelson, R.G.; Yee, B.; Boucherot, A.; Neusser, M.A.; Cohen, C.D.; Carter-Su, C.; et al. Enhanced expression of janus kinase-signal transducer and activator of transcription pathway members in human diabetic nephropathy. Diabetes 2009, 58, 469–477. [Google Scholar] [CrossRef] [Green Version]
- Woroniecka, K.I.; Park, A.S.D.; Mohtat, D.; Thomas, D.B.; Pullman, J.M.; Susztak, K. Transcriptome analysis of human diabetic kidney disease. Diabetes 2011, 60, 2354–2369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lilly, E. Olumiant (Baricitinib). 2018. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/207924s000lbl.pdf (accessed on 6 January 2021).
- Asencio-Duran, M.; Vallejo-Garcia, J.L.; Pastora-Salvador, N.; Fonseca-Sandomingo, A.; Romano, M.R. Vitreous diagnosis in neoplastic diseases. Mediat. Inflamm. 2012, 2012, 930704. [Google Scholar] [CrossRef] [Green Version]
- Ghodasra, D.H.; Fante, R.; Gardner, T.W.; Langue, M.; Niziol, L.M.; Besirli, C.; Cohen, S.R.; Dedania, V.S.; Demirci, H.; Jain, N.; et al. Safety and feasibility of quantitative multiplexed cytokine analysis from Office-Based vitreous aspiration. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3017–3023. [Google Scholar] [CrossRef] [Green Version]
- Wong, T.Y.; Coresh, J.; Klein, R.; Muntner, P.; Couper, D.J.; Sharrett, A.R.; Klein, B.E.K.; Heiss, G.; Hubbard, L.D.; Duncan, B.B. Retinal microvascular abnormalities and renal dysfunction: The Atherosclerosis Risk in Communities Study. J. Am. Soc. Nephrol. 2004, 15, 2469–2476. [Google Scholar] [CrossRef] [Green Version]
- Trevisan, R.; Vedovato, M.; Mazzon, C.; Coracina, A.; Iori, E.; Tiengo, A.; Del Prato, S. Concomitance of diabetic retinopathy and proteinuria accelerates the rate of decline of kidney function in type 2 diabetic patients. Diabetes Care 2002, 25, 2026–2031. [Google Scholar] [CrossRef] [Green Version]
- Manaviat, M.R.; Afkhami, M.; Shoja, M.R. Retinopathy and microalbuminuria in type II diabetic patients. BMC Ophthalmol. 2004, 4, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Dogné, S.; Flamion, B.; Caron, N. Endothelial glycocalyx as a shield against diabetic vascular complications: Involvement of hyaluronan and hyaluronidases. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1427–1439. [Google Scholar] [CrossRef] [PubMed]
- Leskova, W.; Pickett, H.; Eshaq, R.S.; Shrestha, B.; Pattillo, C.B.; Harris, N.R. Effect of diabetes and hyaluronidase on the retinal endothelial glycocalyx in mice. Exp. Eye Res. 2019, 179, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Kumase, F.; Morizane, Y.; Mohri, S.; Takasu, I.; Ohtsuka, A.; Ohtsukri, H. Glycocalyx degradation in retinal and choroidal capillary endothelium in rats with diabetes and hypertension. Acta Med. Okayama 2010, 64, 277–283. [Google Scholar] [CrossRef]
- To, M.; Goz, A.; Camenzind, L.; Oertle, P.; Candiello, J.; Sullivan, M.; Henrich, P.B.; Loparic, M.; Safi, F.; Eller, A.; et al. Diabetes-induced morphological, biomechanical, and compositional changes in ocular basement membranes. Exp. Eye Res. 2013, 116, 298–307. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Zou, Y.; Liu, F. Transforming Growth Factor-Beta1 in Diabetic Kidney Disease. Front. Cell Dev. Biol. 2020, 8, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziyadeh, F.N.; Hoffman, B.B.; Han, D.C.; Iglesias-De La, M.C.C.; Hong, S.W.; Isono, M.; Chen, S.; McGowan, T.A.; Sharma, K. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-β antibody in db/db diabetic mice. Proc. Natl. Acad. Sci. USA 2000, 97, 8015–8020. [Google Scholar] [CrossRef] [Green Version]
- Loeffler, I.; Wolf, G. Transforming growth factor-β and the progression of renal disease. Nephrol. Dial. Transplant. 2014, 29, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Gerhardinger, C.; Dagher, Z.; Sebastiani, P.; Yong, S.P.; Lorenzi, M. The transforming growth factor-β pathway is a common target of drugs that prevent experimental diabetic retinopathy. Diabetes 2009, 58, 1659–1667. [Google Scholar] [CrossRef] [Green Version]
- Pearce, I.; Simó, R.; Lövestam-Adrian, M.; Wong, D.T.; Evans, M. Association between diabetic eye disease and other complications of diabetes: Implications for care. A systematic review. Diabetes Obes. Metab. 2019, 21, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.C.; Wu, H.; Kirita, Y.; Uchimura, K.; Ledru, N.; Rennke, H.G.; Welling, P.A.; Waikar, S.S.; Humphreys, B.D. The single-cell transcriptomic landscape of early human diabetic nephropathy. Proc. Natl. Acad. Sci. USA 2019, 116, 19619–19625. [Google Scholar] [CrossRef] [Green Version]
- Diabetes Control and Complications Trial Research Group; Nathan, D.M.; Genuth, S.; Lachin, J.; Cleary, P.; Crofford, O.; Davis, M.; Rand, L.; Siebert, C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Turner, R. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998, 352, 854–865. [Google Scholar] [CrossRef]
- Matthews, D.R.; Stratton, I.M.; Aldington, S.J.; Holman, R.R.; Kohner, E.M. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch. Ophthalmol. 2004, 122, 1631–1640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, R.; Holman, R.; Stratton, I.; Cull, C.; Frighi, V.; Manley, S.; Matthews, D.; Neil, A.; McElroy, H.; Kohner, E.; et al. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 1998, 317, 703–713. [Google Scholar] [CrossRef] [Green Version]
- ACCORD Study Group; ACCORD Eye Study Group; Chew, E.Y.; Ambrosius, W.T.; Davis, M.D.; Danis, R.P.; Gangaputra, S.; Greven, C.M.; Hubbard, L.; Esser, B.A.; et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N. Engl. J. Med. 2010, 363, 233–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson-Berka, J.L. Angiotensin and diabetic retinopathy. Int. J. Biochem. Cell Biol. 2006, 38, 752–765. [Google Scholar] [CrossRef]
- Barnett, A.H.; Bain, S.C.; Bouter, P.; Karlberg, B.; Madsbad, S.; Jervell, J.; Mustonen, J. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N. Engl. J. Med. 2004, 351, 1952–1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Valle, M.L.; Beveridge, C.; Liu, Y.; Sharma, S. Unraveling the role of genetics in the pathogenesis of diabetic retinopathy. Eye 2019, 33, 534–541. [Google Scholar] [CrossRef] [Green Version]
- Usui-Ouchi, A.; Friedlander, M. Anti-VEGF therapy: Higher potency and long-lasting antagonism are not necessarily better. J. Clin. Investig. 2019, 129, 3032–3034. [Google Scholar] [CrossRef] [PubMed]
- Maguire, M.G.; Liu, D.; Glassman, A.R.; Jampol, L.M.; Johnson, C.A.; Baker, C.W.; Bressler, N.M.; Gardner, T.W.; Pieramici, D.; Stockdale, C.R.; et al. Visual Field Changes over 5 Years in Patients Treated with Panretinal Photocoagulation or Ranibizumab for Proliferative Diabetic Retinopathy. JAMA Ophthalmol. 2020, 138, 285–293. [Google Scholar] [CrossRef]
- Nair, P.; Aiello, L.P.; Gardner, T.W.; Jampol, L.M.; Ferris, F.L. Report from the NEI/FDA diabetic retinopathy clinical trial design and endpoints workshop. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5127–5142. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Hernández, C.; Porta, M.; Bandello, F.; Grauslund, J.; Harding, S.P.; Aldington, S.J.; Egan, C.; Frydkjaer-Olsen, U.; García-Arumí, J.; et al. Effects of Topically Administered Neuroprotective Drugs in Early Stages of Diabetic Retinopathy: Results of the EUROCONDOR Clinical Trial. Diabetes 2019, 68, 457–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brigell, M.G.; Chiang, B.; Maa, A.Y.; Davis, C.Q. Enhancing Risk Assessment in Patients with Diabetic Retinopathy by Combining Measures of Retinal Function and Structure. Transl. Vis. Sci. Technol. 2020, 9, 40. [Google Scholar] [CrossRef]
- Foundation, J.D.R. Complications. 2019. Available online: http://grantcenter.jdrf.org/wp-content/uploads/2019/07/Complications-Program-Strategy.pdf (accessed on 12 December 2020).
- Hodgin, J.B.; Nair, V.; Zhang, H.; Randolph, A.; Harris, R.C.; Nelson, R.G.; Weil, E.J.; Cavalcoli, J.D.; Patel, J.M.; Brosius, F.C.; et al. Identification of cross-species shared transcriptional networks of diabetic nephropathy in human and mouse glomeruli. Diabetes 2013, 62, 299–308. [Google Scholar] [CrossRef] [Green Version]
- Fligor, C.M.; Langer, K.B.; Sridhar, A.; Ren, Y.; Shields, P.K.; Edler, M.C.; Ohlemacher, S.K.; Sluch, V.M.; Zack, D.J.; Zhang, C.; et al. Three-Dimensional Retinal Organoids Facilitate the Investigation of Retinal Ganglion Cell Development, Organization and Neurite Outgrowth from Human Pluripotent Stem Cells. Sci. Rep. 2018, 8, 14520. [Google Scholar] [CrossRef] [PubMed]
- NIH. About Tissue Chip. Available online: https://ncats.nih.gov/tissuechip/about (accessed on 12 January 2021).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, W.W.; Gardner, T.W.; Harder, J.L. Integrative Biology of Diabetic Retinal Disease: Lessons from Diabetic Kidney Disease. J. Clin. Med. 2021, 10, 1254. https://doi.org/10.3390/jcm10061254
Pan WW, Gardner TW, Harder JL. Integrative Biology of Diabetic Retinal Disease: Lessons from Diabetic Kidney Disease. Journal of Clinical Medicine. 2021; 10(6):1254. https://doi.org/10.3390/jcm10061254
Chicago/Turabian StylePan, Warren W., Thomas W. Gardner, and Jennifer L. Harder. 2021. "Integrative Biology of Diabetic Retinal Disease: Lessons from Diabetic Kidney Disease" Journal of Clinical Medicine 10, no. 6: 1254. https://doi.org/10.3390/jcm10061254





