The Role of Cardiac Magnetic Resonance in Evaluation of Idiopathic Ventricular Arrhythmia in Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Pre-CMR Evaluation
2.3. CMR Imaging and Assessment
2.4. Study Endpoint
2.5. Post CMR Follow-Up
2.6. Ethical Considerations
2.7. Statistical Methods
3. Results
3.1. Baseline Characteristics
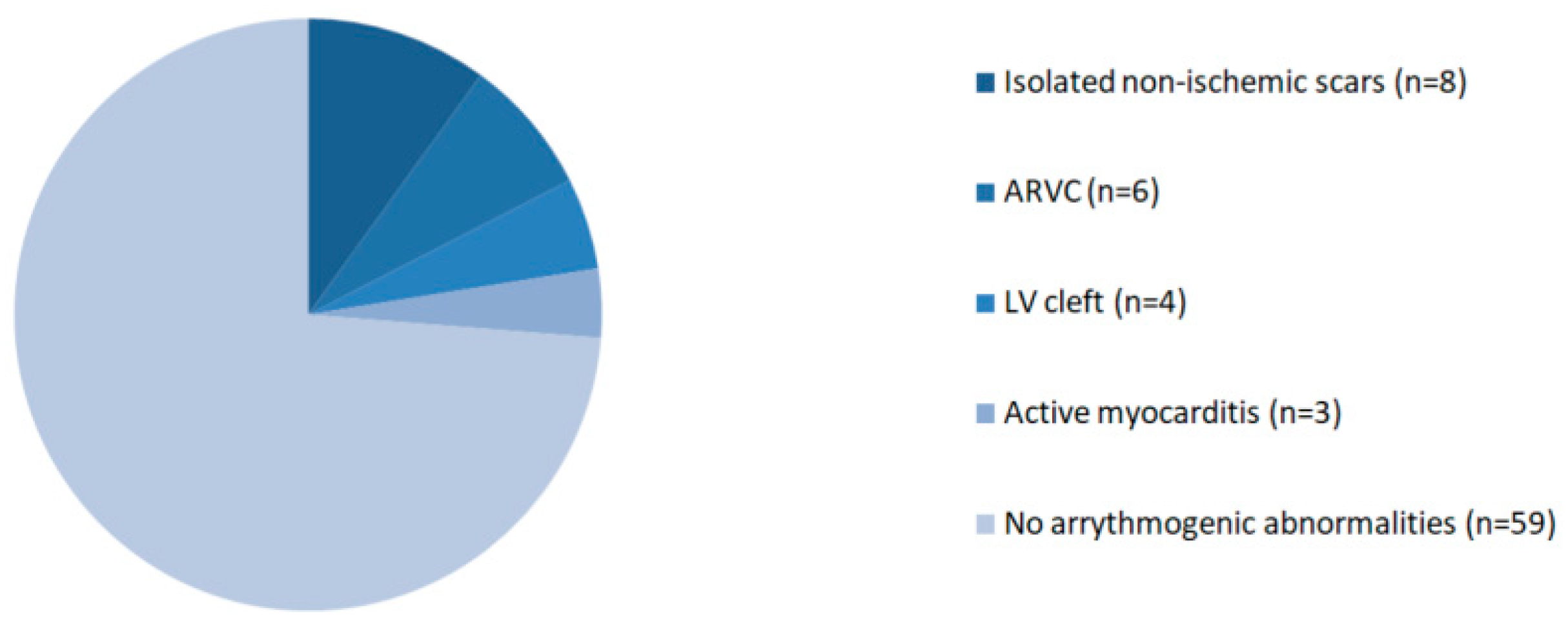
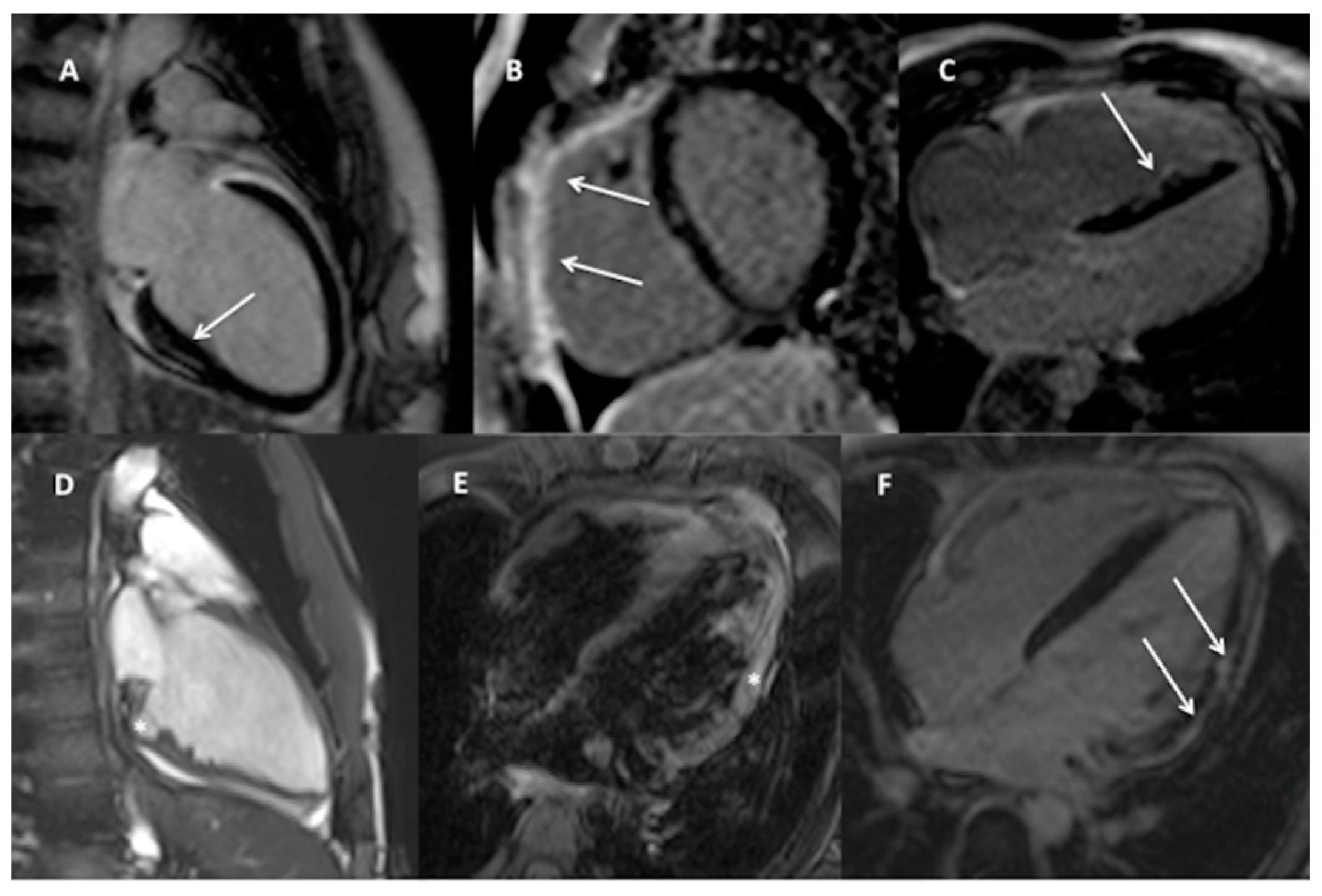
3.2. CMR Findings
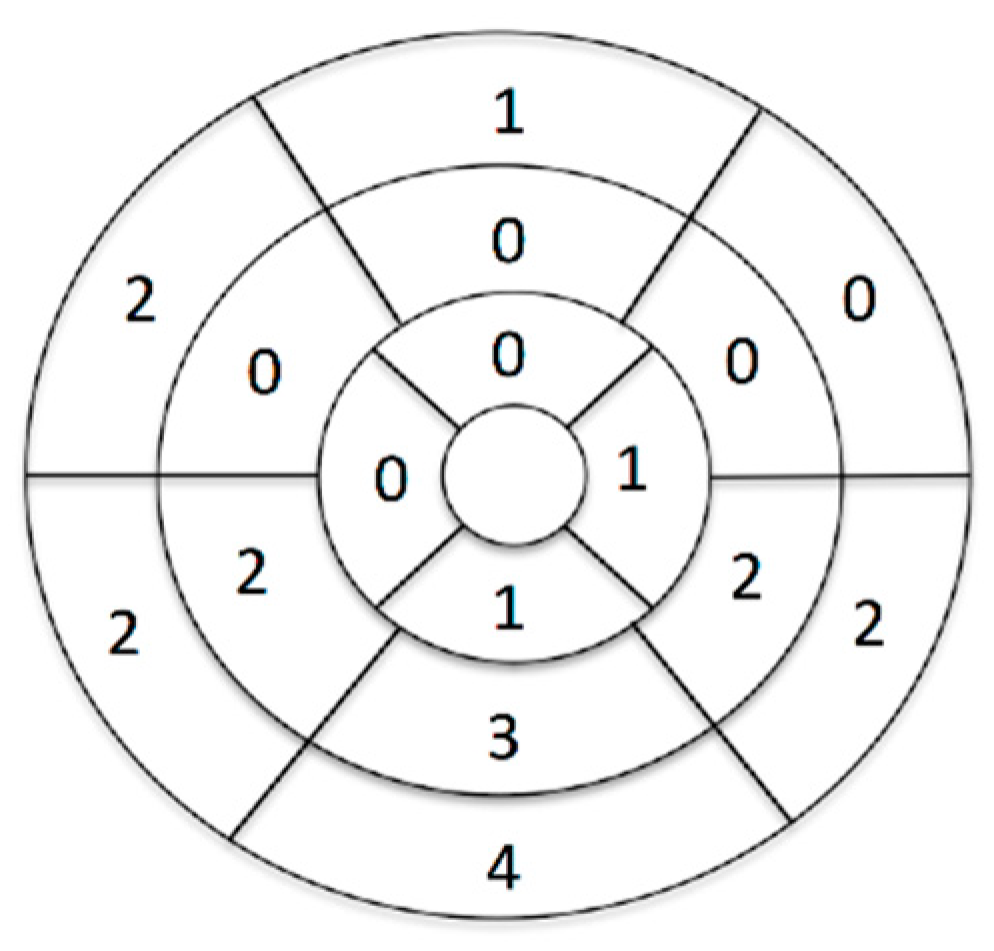
3.3. Reproducibility Analysis
3.4. Follow-Up
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jat, K.R.; Lodha, R.; Kabra, S.K. Arrhythmias in Children. Indian J. Pediatr. 2010, 78, 211–218. [Google Scholar] [CrossRef]
- Do, V.B.; Tsai, W.-C.; Lin, Y.-J.; Higa, S.; Yagi, N.; Chang, S.-L.; Lo, L.-W.; Chung, F.-P.; Liao, J.-N.; Huang, Y.-C.; et al. The Different Substrate Characteristics of Arrhythmogenic Triggers in Idiopathic Right Ventricular Outflow Tract Tachycardia and Arrhythmogenic Right Ventricular Dysplasia: New Insight from Noncontact Mapping. PLoS ONE 2015, 10, e0140167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.H.C.; Bazoukis, G.; Liu, T.; Li, G.; Wu, W.K.K.; Wong, S.H.; Wong, W.T.; Chan, Y.S.; Wong, M.C.S.; Wassilew, K.; et al. Arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) in clinical practice. J. Arrhythmia 2017, 34, 11–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagiub, M.; Carter, K.; Shepard, R. Systematic review of risk stratification of pediatric ventricular arrhythmia in structurally normal and abnormal hearts. Prog. Pediatr. Cardiol. 2017, 45, 55–62. [Google Scholar] [CrossRef]
- Sarvari, S.I.; Haugaa, K.H.; Anfinsen, O.-G.; Leren, T.P.; Smiseth, O.A.; Kongsgaard, E.; Amlie, J.P.; Edvardsen, T. Right ventricular mechanical dispersion is related to malignant arrhythmias: A study of patients with arrhythmogenic right ventricular cardiomyopathy and subclinical right ventricular dysfunction. Eur. Heart J. 2011, 32, 1089–1096. [Google Scholar] [CrossRef] [Green Version]
- Femia, G.; Semsarian, C.; McGuire, M.; Sy, R.W.; Puranik, R. Long term CMR follow up of patients with right ventricular abnormality and clinically suspected arrhythmogenic right ventricular cardiomyopathy (ARVC). J. Cardiovasc. Magn. Reson. 2019, 21, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreu, D.; Ortiz-Pérez, J.T.; Boussy, T.; Fernández-Armenta, J.; De Caralt, T.M.; Perea, R.J.; Prat-González, S.; Mont, L.; Brugada, J.; Berruezo, A. Usefulness of contrast-enhanced cardiac magnetic resonance in identifying the ventricular arrhythmia substrate and the approach needed for ablation. Eur. Heart J. 2014, 35, 1316–1326. [Google Scholar] [CrossRef] [Green Version]
- Andreini, D.; Russo, A.D.; Pontone, G.; Mushtaq, S.; Conte, E.; Perchinunno, M.; Guglielmo, M.; Santos, A.C.; Magatelli, M.; Baggiano, A.; et al. CMR for Identifying the Substrate of Ventricular Arrhythmia in Patients With Normal Echocardiography. JACC Cardiovasc. Imaging 2020, 13, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Pontone, G.; Guaricci, A.I.; Andreini, D.; Solbiati, A.; Guglielmo, M.; Mushtaq, S.; Baggiano, A.; Beltrama, V.; Fusini, L.; Rota, C.; et al. Prognostic Benefit of Cardiac Magnetic Resonance Over Transthoracic Echocardiography for the Assessment of Ischemic and Nonischemic Dilated Cardiomyopathy Patients Referred for the Evaluation of Primary Prevention Implantable Cardioverter-Defibrillator Therapy. Circ. Cardiovasc. Imaging 2016, 9, e004956. [Google Scholar] [CrossRef] [Green Version]
- Hennig, A.; Salel, M.; Sacher, F.; Camaioni, C.; Sridi, S.; Denis, A.; Montaudon, M.; Laurent, F.; Jais, P.; Cochet, H. High-resolution three-dimensional late gadolinium-enhanced cardiac magnetic resonance imaging to identify the underlying substrate of ventricular arrhythmia. Europace 2018, 20, f179–f191. [Google Scholar] [CrossRef] [Green Version]
- Alexis, J.A.; Costello, B.; Iles, L.M.; Ellims, A.H.; Hare, J.L.; Taylor, A.J. Assessment of the accuracy of common clinical thresholds for cardiac morphology and function by transthoracic echocardiography. J. Echocardiogr. 2016, 15, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Badano, L.P.; Boccalini, F.; Muraru, D.; Bianco, L.D.; Peluso, D.; Bellu, R.; Zoppellaro, G.; Iliceto, S. Current clinical applications of transthoracic three-dimensional echocardiog-raphy. J. Cardiovasc. Ultrasound 2012, 20, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Szychta, W.; Werys, K.; Barczuk-Falęcka, M.; Postuła, M.; Małek, Ł.A. Validation of performance of free of charge plugin for the open-source platform to perform cardiac segmentation in magnetic resonance imaging. Heart Beat J. 2019, 3, 83–89. [Google Scholar] [CrossRef]
- Van der Ven, J.P.G.; Sadighy, Z.; Valsangiacomo Buechel, E.R.; Sarikouch, S.; Robbers-Visser, D.; Kellenberger, C.J.; Kaiser, T.; Beerbaum, P.; Boersma, E.; Helbing, W.A. Multicentre referencevalues for cardiacmagnetic reso-nance imaging derived ventricular size and function for childrenaged 0–18 years. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 102–113. [Google Scholar] [CrossRef] [Green Version]
- Friedrich, M.G.; Sechtem, U.; Schulz-Menger, J.; Holmvang, G.; Alakija, P.; Cooper, L.T.; White, J.A.; Abdel-Aty, H.; Gutberlet, M.; Prasad, S.; et al. Cardiovascular Magnetic Resonance in Myocarditis: A JACC White Paper. J. Am. Coll. Cardiol. 2009, 53, 1475–1487. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef] [PubMed]
- Marcus, F.I.; McKenna, W.J.; Sherrill, D.L.; Basso, C.; Bauce, B.; Bluemke, D.A.; Calkins, H.; Corrado, D.; Cox, M.G.; Daubert, J.P.; et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: Proposed Modification of the Task Force Criteria. Eur. Heart J. 2010, 31, 806–814. [Google Scholar] [CrossRef] [Green Version]
- Małek, Ł.A.; Kamińska, H.; Barczuk-Falęcka, M.; Ferreira, V.M.; Wójcicka, J.; Brzewski, M.; Werner, B. Children With Acute Myocarditis Often Have Persistent Subclinical Changes as Revealed by Cardiac Magnetic Resonance. J. Magn. Reson. Imaging 2020, 52, 488–496. [Google Scholar] [CrossRef]
- Shanbhag, S.M.; Greve, A.M.; Aspelund, T.; Schelbert, E.B.; Cao, J.J.; Danielsen, R.; Þorgeirsson, G.; Sigurðsson, S.; Eiríksdóttir, G.; Harris, T.B.; et al. Prevalence and prognosis of ischaemic and non-ischaemic myocardial fibrosis in older adults. Eur. Heart J. 2018, 40, 529–538. [Google Scholar] [CrossRef]
- Corrado, D.; Van Tintelen, P.J.; McKenna, W.J.; Hauer, R.N.W.; Anastastakis, A.; Asimaki, A.; Basso, C.; Bauce, B.; Brunckhorst, C.; Bucciarelli-Ducci, C.; et al. Arrhythmogenic right ventricular cardiomyopathy: Evaluation of the current diagnostic criteria and differential diagnosis. Eur. Heart J. 2019, 41, 1414–1429. [Google Scholar] [CrossRef] [Green Version]
- Mast, T.P.; James, C.A.; Calkins, H.; Teske, A.J.; Tichnell, C.; Murray, B.; Loh, P.; Russell, S.D.; Velthuis, B.K.; Judge, D.P.; et al. Evaluation of Structural Progression in Arrhythmogenic Right Ventricular Dys-plasia/Cardiomyopathy. JAMA Cardiol. 2017, 2, 293–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mast, T.P.; Taha, K.; Cramer, M.J.; Lumens, J.; van der Heijden, J.F.; Bouma, B.J.; van den Berg, M.P.; Asselbergs, F.W.; Doevendans, P.A.; Teske, A.J. The Prognostic Value of Right Ventricular Deformation Imaging in Early Ar-rhythmogenic Right Ventricular Cardiomyopathy. JACC Cardiovasc. Imaging 2018, 12, 446–455. [Google Scholar] [CrossRef]
- Cresti, A.; Cannarile, P.; Aldi, E.; Solari, M.; Sposato, B.; Franci, L.; Limbruno, U. Multimodality Imaging and Clinical Sig-nificance of Congenital Ventricular Outpouchings: Recesses, Diverticula, Aneurysms, Clefts, and Crypts. J. Cardiovasc. Echogr. 2018, 28, 9–17. [Google Scholar] [CrossRef]
- Brouwer, W.P.; Germans, T.; Head, M.C.; Van Der Velden, J.; Heymans, M.W.; Christiaans, I.; Houweling, A.C.; Wilde, A.A.; Van Rossum, A.C. Multiple myocardial crypts on modified long-axis view are a specific finding in pre-hypertrophic HCM mutation carriers. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 292–297. [Google Scholar] [CrossRef]
- Cappai, A.; Basciu, A.; Monti, L.; Malvindi, P.G.; Raffa, G.M. Left ventricular cleft. Eur. Heart J. Cardiovasc. Imaging 2012, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Seraphim, A.; Papachristidis, A.; Bratt, N.; Shiu, M.F. Significance of left ventricular clefts—A case report. J. Cardiol. Cases 2014, 9, 138–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dostálová, G.; Paleček, T.; Kuchynka, P.; Havránek, Š.; Mašek, M.; Hlubocká, Z.; Karetová, D.; Wichterle, D.; Dušková, J.; Lindner, J.; et al. A congenital diverticulum of the left ventricular apex manifested by stroke and recurrent ventricular tachycardia. Cardiovasc. Pathol. 2017, 28, 3–6. [Google Scholar] [CrossRef]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2017, 19, 1–24. [Google Scholar] [CrossRef] [Green Version]
| Feature | Study Group n = 80 |
|---|---|
| Mean age, SD [years] | 13.1 ± 3.6 |
| Male sex | 48 (60%) |
| PVCs morphology | LBBB 65 (81%) RBBB 10 (12.5%) Undetermined 5 (6.5%) |
| >10% PVCs/24 h | 48 (60%) |
| Complex PVCs | 27 (34%) |
| Polymorphic PVCs | 13 (16%) |
| Severe VA (>10%PVCs/24 h OR complex forms) | 55 (69%) |
| Symptoms | 9 (11%) |
| Palpitations | 4 |
| Poor exercise tolerance | 2 |
| Chest pain | 2 |
| Cardiogenic shock | 1 |
| Pharmacotherapy | 24 (30%) |
| Metoprolol | 12 |
| Sotalol | 5 |
| Amiodarone | 4 |
| Flecainide + metoprolol | 4 |
| Propaphenone | 2 |
| Clinical Characteristic | Abnormal CMR Result (n = 21) | Normal CMR Result (n = 59) | p-Value | |
|---|---|---|---|---|
| Mean age (years) | 14.4 | 13.2 | 0.165 | |
| BSA | 1.58 ± 0.23 | 1.49 ± 0.33 | 0.215 | |
| Male sex | 12 (57%) | 36 (61%) | 0.799 | |
| Sport participant | 3 (14%) | 8 (14%) | 1 | |
| Symptoms | 4 (19%) | 5 (8%) | 0.232 | |
| Abnormal echocardiography | 5 (23%) | 0 (0%) | 0.001 | |
| Arrhythmia | ||||
| Total PVCs burden | PVCs < 10%/24 h | 4 (19%) | 29 (49%) | 0.020 |
| PVCs 10–20%/24 h | 9 (43%) | 8 (14%) | 0.011 | |
| PVCs > 20%/24 h | 8 (38%) | 22 (37%) | 1 | |
| Complex forms (nsVT or VT) | 10 (48%) | 18 (30%) | 0.188 | |
| Polymorphic arrhythmia | 4 (19%) | 9 (15%) | 0.735 | |
| Pharmacotherapy | 8 (38%) | 16 (27%) | 0.409 | |
| CMR Results | All Patients (n = 80) | Abnormal CMR Result (n = 21) | Normal CMR Result (n = 59) | p-Value |
|---|---|---|---|---|
| LVEDVi [mL/m2] | 84.85 ± 26.59 | 83.40 ± 20.35 | 85.34 ± 28.57 | 0.743 |
| LVESVi [mL/m2] | 34.21 ± 24.27 | 34.20 ± 9.51 | 34.21 ± 26.86 | 0.998 |
| LVSVi [mL/m2] | 50.59 ± 8.31 | 49.75 ± 9.51 | 50.89 ± 7.92 | 0.634 |
| LVEF [%] | 61.28 ± 7.64 | 60.10 ± 7.99 | 61.71 ± 7.54 | 0.440 |
| LV mass index [mL/m2] | 57.68 ± 12.72 | 58.05 ± 13.86 | 57.55 ± 12.43 | 0.889 |
| RVEDVi [mL/m2] | 89.39 ± 15.77 | 87.65 ± 12.39 | 89.96 ± 16.79 | 0.547 |
| RVESVi [mL/m2] | 38.30 ± 10.54 | 37.35 ± 9.01 | 38.62 ± 11.07 | 0.641 |
| RVSVi [mL/m2] | 51.13 ± 9.32 | 50.00 ± 8.88 | 51.52 ± 9.52 | 0.554 |
| RVEF [%] | 57.54 ± 6.13 | 57.47 ± 6.98 | 57.56 ± 5.89 | 0.963 |
| LGE | 12 | 12 | 0 | <0.0001 |
| CMR Result | <10% PVCs/24 h and Nocomplex Forms (n = 25) | >10% PVCs/24 h OR Complex Forms (n = 55) | p-Value |
|---|---|---|---|
| Abnormal CMR findings | 2 (8%) | 19 (35%) | 0.014 |
| Detailed analysis | |||
| All cases with LGE | 1 (4%) | 11 (20%) | 0.092 |
| Isolated non-ischemic scar | 1 (4%) | 7 (13%) | 0.424 |
| Criteria for ARVC | 1 (4%) | 5 (9%) | 0.660 |
| LV clefts | 0 (0%) | 4 (7%) | 0.304 |
| Active myocarditis | 0 (0%) | 3 (5%) | 0.548 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamińska, H.; Małek, Ł.A.; Barczuk-Falęcka, M.; Bartoszek, M.; Strzałkowska-Kominiak, E.; Marszałek, M.; Brzezik, E.; Brzewski, M.; Werner, B. The Role of Cardiac Magnetic Resonance in Evaluation of Idiopathic Ventricular Arrhythmia in Children. J. Clin. Med. 2021, 10, 1335. https://doi.org/10.3390/jcm10071335
Kamińska H, Małek ŁA, Barczuk-Falęcka M, Bartoszek M, Strzałkowska-Kominiak E, Marszałek M, Brzezik E, Brzewski M, Werner B. The Role of Cardiac Magnetic Resonance in Evaluation of Idiopathic Ventricular Arrhythmia in Children. Journal of Clinical Medicine. 2021; 10(7):1335. https://doi.org/10.3390/jcm10071335
Chicago/Turabian StyleKamińska, Halszka, Łukasz A. Małek, Marzena Barczuk-Falęcka, Marta Bartoszek, Ewa Strzałkowska-Kominiak, Mikołaj Marszałek, Ewa Brzezik, Michał Brzewski, and Bożena Werner. 2021. "The Role of Cardiac Magnetic Resonance in Evaluation of Idiopathic Ventricular Arrhythmia in Children" Journal of Clinical Medicine 10, no. 7: 1335. https://doi.org/10.3390/jcm10071335






