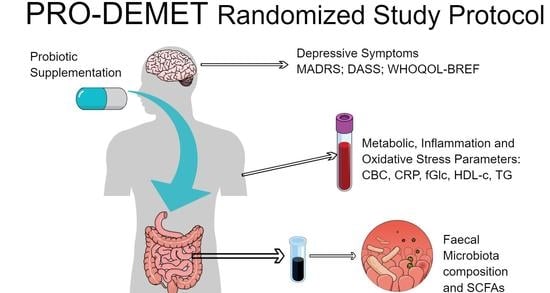
The Influence of Probiotic Supplementation on Depressive Symptoms, Inflammation, and Oxidative Stress Parameters and Fecal Microbiota in Patients with Depression Depending on Metabolic Syndrome Comorbidity—PRO-DEMET Randomized Study Protocol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Patients
- PRO-DMS: probiotic + depression + MetS
- PLC-DMS: placebo + depression + MetS
- PRO-D: probiotic + depression
- PLC-D: placebo + depression
- V0, “recruitment visit”, preferably an online formula: assessment of the inclusion and exclusion criteria, the study questionnaire (SQ) and the MADRS completion, informed consent, full psychiatric examination
- V1 (no longer that five days after V0), “randomization visit”: Depression, Anxiety, Stress Scale (DASS), The World Health Organization quality of life-BREF (WHOQOL-BREF) completion, blood pressure (BP), body mass index (BMI), waist circumference (WC) measurements, blood and stool collection
- t1–t3: personal, telephone, or e-mail monitoring every two weeks according to the monitoring questionnaire (MQ)
- V2, “the end of the study visit”, eight weeks after V1: MQ, MADRS, DASS, WHOQOL-BREF completion, BP, BMI, WC measurements, blood and stool collection
- V3, “a follow-up visit”, 12 weeks after V2: SQ, MADRS, DASS, WHOQOL-BREF completion, BP, BMI, WC measurements
2.3. Intervention
2.4. Outcome Measures
2.4.1. Questionnaires and Scales
2.4.2. Biological Samples
Venous Blood
- Interleukin-6 (IL-6) is a multi-functional cytokine that regulates immune responses, acute phase reactions and hematopoiesis and may play a central role in host defense mechanisms [63]. It acts on a wide range of tissues, exerting growth-induction, growth-inhibition, and differentiation, respectively, depending on the nature of the target cells [46,47,48]. We plan to use the Human IL-6 ELISA Kit (Diaclone, Besançon, France, 950.030.192, minimum detectable dose of 2 pg/mL) or Interleukin-6 Human ELISA Kit (Biovendor, Brno, Czech Republic, RD194015200R). A highly specific capture antibody against IL-6 is coated to the wells of the microtiter strip plate provided in the kit.
- Tumor necrosis factor alpha (TNFα) is a polypeptide cytokine produced by monocytes and macrophages. It functions as a multipotent modulator of immune response and further acts as a potent pyrogen. TNFα circulates throughout the body responding to stimuli (infectious agents or tissue injury), activating neutrophils, altering the properties of vascular endothelial cells, regulating metabolic activities of other tissues, as well as exhibiting tumoricidal activity by inducing localized blood clotting [64,65,66].We plan to use the Human TNF alpha ELISA Kit (Diaclone, 950.090.192, minimum detectable dose of 8 pg/mL) or TNF-alpha Human ELISA, High Sensitivity Kit (Biovendor, RAF145R). A highly specific capture antibody against TNFα is coated to the wells of the microtiter strip plate provided in the kit.
- Malondialdehyde (MDA) is a naturally occurring product resulting from lipid peroxidation of polyunsaturated fatty acids. It is also produced in the prominent product in thromboxane A2 biosynthesis wherein cyclooxygenase 1 or cycloxygenase 2 metabolizes arachidonic acid to prostaglandin H2. MDA has a mutagenic and carcinogenic effect [67]. We plan to use the Highly Sensitive ELISA Kit for Malondialdehyde (Cloud Clone Corp, Houston, TX, USA. HEA597Ge, minimum detectable dose of 4.94 ng/mL). A highly specific capture antibody against MDA is coated to the wells of the microtiter strip plate provided in the kit.
- Total antioxidant capacity (TAC) (Total antioxidative status—TAS) is an analyte frequently used to assess the antioxidant status of biological samples and can evaluate the antioxidant response against the free radicals produced in the body in a given disease or analyzed condition. Overproductions of radical oxygen species (ROS) or insufficient defense mechanisms lead to a dangerous disbalance in the organism observed in lipid peroxidation, a mutagenic effect on DNA. The elevated level of ROS is associated with pathomechanisms implicated in aging and over 100 human diseases, e.g., cardiovascular disease, cancer, diabetes mellitus, inflammatory disease [68,69]. TAC measurements provide a tool for establishing links between antioxidant capacity and the risk of disease, as well as for monitoring of antioxidant therapy.
- low antioxidative capacity < 280 μmol/L
- middle antioxidative capacity 280–320 μmol/L
- high antioxidative capacity > 320 μmol/L
Feces
2.5. Data Management
2.6. Ethics
2.7. Analyses
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
- I.
- MADRS score
- II.
- SCFAs levels
- III.
- α-diversity
Appendix B
| Inflammation and Oxidative Stress Parameters | Primary Antibody | Secondary Antibody | Volume of Serum Per Patient/Timepoint | |
|---|---|---|---|---|
| IL-6 | cytokine | Biotinylated Anti-IL-6 | Streptavidin-HRP **, colorimetric reaction with TNM * substrate | 100 μL × 2 repetitions |
| TNFα | polypeptide cytokine | Biotinylated Anti-TNF alpha | Streptavidin-HRP, colorimetric reaction with TNM substrate | 100 μL × 2 repetitions |
| MDA | Prostaglandin, enol | Biotinylated Anti-MDA MDA in the sample or standard competes with a fixed amount of MDA on the solid phase supporter for sites on the Biotinylated Detection Ab specific to MDA. | Avidin-HRP, colorimetric reaction with TNM substrate | 50 μL × 2 repetitions |
| TAC | Total count of | Photometrically by an enzymatic reaction that involves the conversion of TMB to a colored product, compared with the calibrator. | 50 μL × 2 cond. × 2 repetitions | |
References
- Marazziti, D.; Rutigliano, G.; Baroni, S.; Landi, P.; Dell’Osso, L. Metabolic syndrome and major depression. CNS Spectr. 2014, 19, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Shinkov, A.; Borissova, A.M.; Kovatcheva, R.; Vlahov, J.; Dakovska, L.; Atanassova, I.; Petkova, P. Increased prevalence of depression and anxiety among subjects with metabolic syndrome and known type 2 diabetes mellitus—A population-based study. Postgrad. Med. 2018, 130, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Penninx, B.W.J.H.; Lange, S.M.M. Metabolic syndrome in psychiatric patients: Overview, mechanisms, and implications. Dialogues Clin. Neurosci. 2018, 20, 63–73. [Google Scholar]
- Vancampfort, D.; Correll, C.U.; Wampers, M.; Sienaert, P.; Mitchell, A.J.; De Herdt, A.; Probst, M.; Scheewe, T.W.; De Hert, M. Metabolic syndrome and metabolic abnormalities in patients with major depressive disorder: A meta-analysis of prevalences and moderating variables. Psychol. Med. 2014, 44, 2017–2028. [Google Scholar] [CrossRef]
- Lamers, F.; Milaneschi, Y.; De Jonge, P.; Giltay, E.J.; Penninx, B.W.J.H. Metabolic and inflammatory markers: Associations with individual depressive symptoms. Psychol. Med. 2018, 48, 1102–1110. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Soczynska, J.K.; Konarski, J.Z.; Woldeyohannes, H.O.; Law, C.W.Y.; Miranda, A.; Fulgosi, D.; Kennedy, S.H. Should Depressive Syndromes Be Reclassified as “Metabolic Syndrome Type II”? Ann. Clin. Psychiatry 2007, 19, 257–264. [Google Scholar] [CrossRef]
- Chan, K.L.; Cathomas, F.; Russo, S.J. Central and peripheral inflammation link metabolic syndrome and major depressive disorder. Physiology 2019, 34, 123–133. [Google Scholar] [CrossRef]
- Farzi, A.; Hassan, A.M.; Zenz, G.; Holzer, P. Diabesity and mood disorders: Multiple links through the microbiota-gut-brain axis. Mol. Aspects Med. 2019, 66, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Lamers, F.; Milaneschi, Y.; Vinkers, C.H.; Schoevers, R.A.; Giltay, E.J.; Penninx, B.W.J.H. Depression profilers and immuno-metabolic dysregulation: Longitudinal results from the NESDA study. Brain. Behav. Immun. 2020, 88, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [Green Version]
- Pasolli, E.; Asnicar, F.; Manara, S.; Zolfo, M.; Karcher, N.; Armanini, F.; Beghini, F.; Manghi, P.; Tett, A.; Ghensi, P.; et al. Extensive Unexplored Human Microbiome Diversity Revealed by Over 150,000 Genomes from Metagenomes Spanning Age, Geography, and Lifestyle. Cell 2019, 176, 649–662.e20. [Google Scholar] [CrossRef] [Green Version]
- Lach, G.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotherapeutics 2018, 15, 36–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skonieczna-Żydecka, K.; Marlicz, W.; Misera, A.; Koulaouzidis, A.; Łoniewski, I. Microbiome—The Missing Link in the Gut-Brain Axis: Focus on Its Role in Gastrointestinal and Mental Health. J. Clin. Med. 2018, 7, 521. [Google Scholar] [CrossRef] [Green Version]
- Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol. Clin. N. Am. 2017, 46, 77–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lkhagva, E.; Chung, H.-J.; Hong, J.; Tang, W.H.W.; Lee, S.-I.; Hong, S.-T.; Lee, S. The regional diversity of gut microbiome along the GI tract of male C57BL/6 mice. BMC Microbiol. 2021, 21, 44. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, E.; Tsuji, H.; Asahara, T.; Takahashi, T.; Teraishi, T.; Yoshida, S.; Ota, M.; Koga, N.; Hattori, K.; Kunugi, H. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 2016, 202, 254–257. [Google Scholar] [CrossRef]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef]
- Naseribafrouei, A.; Hestad, K.; Avershina, E.; Sekelja, M.; Linløkken, A.; Wilson, R.; Rudi, K. Correlation between the human fecal microbiota and depression. Neurogastroenterol. Motil. 2014, 26, 1155–1162. [Google Scholar] [CrossRef]
- Liśkiewicz, P.; Kaczmarczyk, M.; Misiak, B.; Wroński, M.; Bąba-Kubiś, A.; Skonieczna-Żydecka, K.; Marlicz, W.; Bieńkowski, P.; Misera, A.; Pełka-Wysiecka, J.; et al. Analysis of gut microbiota and intestinal integrity markers of inpatients with major depressive disorder. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2020, 110076. [Google Scholar] [CrossRef] [PubMed]
- Sanada, K.; Nakajima, S.; Kurokawa, S.; Barceló-Soler, A.; Ikuse, D.; Hirata, A.; Yoshizawa, A.; Tomizawa, Y.; Salas-Valero, M.; Noda, Y.; et al. Gut microbiota and majore depressive disorder: A systematic review and meta-analysis. J. Affect. Disord. 2020, 266, 1–13. [Google Scholar] [CrossRef]
- Mason, B.L.; Li, Q.; Minhajuddin, A.; Czysz, A.H.; Coughlin, L.A.; Hussain, S.K.; Koh, A.Y.; Trivedi, M.H. Reduced anti-inflammatory gut microbiota are associated with depression and anhedonia. J. Affect. Disord. 2020, 266, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Madan, A.; Thompson, D.; Fowler, J.C.; Ajami, N.J.; Salas, R.; Frueh, B.C.; Bradshaw, M.R.; Weinstein, B.L.; Oldham, J.M.; Petrosino, J.F. The gut microbiota is associated with psychiatric symptom severity and treatment outcome among individuals with serious mental illness. J. Affect. Disord. 2020, 264, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Dabke, K.; Hendrick, G.; Devkota, S. The gut microbiome and metabolic syndrome. J. Clin. Investig. 2019, 129, 4050–4057. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [Green Version]
- Vallianou, N.; Stratigou, T.; Christodoulatos, G.S.; Dalamaga, M. Understanding the Role of the Gut Microbiome and Microbial Metabolites in Obesity and Obesity-Associated Metabolic Disorders: Current Evidence and Perspectives. Curr. Obes. Rep. 2019, 8, 317–332. [Google Scholar] [CrossRef]
- Gawlik-Kotelnicka, O.; Strzelecki, D. Adiposity in Depression or Depression in Adiposity? The Role of Immune-Inflammatory-Microbial Overlap. Life 2021, 11, 117. [Google Scholar] [CrossRef]
- Dash, S.; Clarke, G.; Berk, M.; Jacka, F.N. The gut microbiome and diet in psychiatry: Focus on depression. Curr. Opin. Psychiatry 2015, 28, 1–6. [Google Scholar] [CrossRef]
- Karaduta, O.; Glazko, G.; Dvanajscak, Z.; Arthur, J.; Mackintosh, S.; Orr, L.; Rahmatallah, Y.; Yeruva, L.; Tackett, A.; Zybailov, B. Resistant starch slows the progression of CKD in the 5/6 nephrectomy mouse model. Physiol. Rep. 2020, 8, e14610. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, E.; Egea-Zorrilla, A.; Plaza-Díaz, J.; Aragón-Vela, J.; Muñoz-Quezada, S.; Tercedor-Sánchez, L.; Abadia-Molina, F. The Gut Microbiota and Its Implication in the Development of Atherosclerosis and Related Cardiovascular Diseases. Nutrients 2020, 12, 605. [Google Scholar] [CrossRef] [Green Version]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.; Wang, K.; Hu, J. Effect of probiotics on depression: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2016, 8, 483. [Google Scholar] [CrossRef]
- Goh, K.K.; Liu, Y.W.; Kuo, P.H.; Chung, Y.C.E.; Lu, M.L.; Chen, C.H. Effect of probiotics on depressive symptoms: A meta-analysis of human studies. Psychiatry Res. 2019, 282, 112568. [Google Scholar] [CrossRef]
- Liu, R.T.; Walsh, R.F.L.; Sheehan, A.E. Prebiotics and probiotics for depression and anxiety: A systematic review and meta-analysis of controlled clinical trials. Neurosci. Biobehav. Rev. 2019, 102, 13–23. [Google Scholar] [CrossRef]
- Ng, Q.X.; Peters, C.; Ho, C.Y.X.; Lim, D.Y.; Yeo, W.S. A meta-analysis of the use of probiotics to alleviate depressive symptoms. J. Affect. Disord. 2018, 228, 13–19. [Google Scholar] [CrossRef]
- Nikolova, V.; Zaidi, S.Y.; Young, A.H.; Cleare, A.J.; Stone, J.M. Gut feeling: Randomized controlled trials of probiotics for the treatment of clinical depression: Systematic review and meta-analysis. Ther. Adv. Psychopharmacol. 2019, 9, 204512531985996. [Google Scholar] [CrossRef] [Green Version]
- Nikolova, V.L.; Cleare, A.J.; Young, A.H.; Stone, J.M. Updated Review and Meta-Analysis of Probiotics for the Treatment of Clinical Depression: Adjunctive vs. Stand-Alone Treatment. J. Clin. Med. 2021, 10, 647. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, M.; Chen, L.; Bhochhibhoya, A. Probiotic Foods and Supplements Interventions for Metabolic Syndromes: A Systematic Review and Meta-Analysis of Recent Clinical Trials. Ann. Nutr. Metab. 2019, 74, 224–241. [Google Scholar] [CrossRef]
- Koutnikova, H.; Genser, B.; Monteiro-Sepulveda, M.; Faurie, J.M.; Rizkalla, S.; Schrezenmeir, J.; Clement, K. Impact of bacterial probiotics on obesity, diabetes and non-alcoholic fatty liver disease related variables: A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2019, 9, e017995. [Google Scholar] [CrossRef]
- Tenorio-Jiménez, C.; Martínez-Ramírez, M.J.; Gil, Á.; Gómez-Llorente, C. Effects of probiotics on metabolic syndrome: A systematic review of randomized clinical trials. Nutrients 2020, 12, 124. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Wu, H.; Yang, X.; Li, Y.; Zhang, Z.; Chen, F.; Zhao, L.; Zhang, C. Lactobacillus Mucosae Strain Promoted by a High-Fiber Diet in Genetic Obese Child Alleviates Lipid Metabolism and Modifies Gut Microbiota in ApoE-/- Mice on a Western Diet. Microorganisms 2020, 8, 1225. [Google Scholar] [CrossRef]
- Reed, G.M.; First, M.B.; Kogan, C.S.; Hyman, S.E.; Gureje, O.; Gaebel, W.; Maj, M.; Stein, D.J.; Maercker, A.; Tyrer, P.; et al. Innovations and changes in the ICD-11 classification of mental, behavioural and neurodevelopmental disorders. World Psychiatry 2019, 18, 3–19. [Google Scholar] [CrossRef] [Green Version]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Vich Vila, A.; Collij, V.; Sanna, S.; Sinha, T.; Imhann, F.; Bourgonje, A.R.; Mujagic, Z.; Jonkers, D.M.A.E.; Masclee, A.A.M.; Fu, J.; et al. Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat. Commun. 2020, 11, 362. [Google Scholar] [CrossRef] [PubMed]
- Salvucci, E. The disappearing microbiota: Diseases of the Western civilization. In How Fermented Foods Feed a Healthy Gut Microbiota: A Nutrition Continuum; Springer International Publishing: Cham, Switzerland, 2019; pp. 325–347. ISBN 9783030287375. [Google Scholar]
- Skonieczna-Żydecka, K.; Łoniewski, I.; Stachowska, E.; Marlicz, W.; Correll, C.U. Current and novel approaches to mitigate cardiometabolic adverse effects of second-generation antipsychotics. Int. J. Neuropsychopharmacol. 2020, 23, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Mir, R.A.; Schaut, R.G.; Allen, H.K.; Looft, T.; Loving, C.L.; Kudva, I.T.; Sharma, V.K. Cattle intestinal microbiota shifts following Escherichia coli O157:H7 vaccination and colonizationtravel. PLoS ONE 2019, 14, e0226099. [Google Scholar] [CrossRef]
- Elbere, I.; Kalnina, I.; Silamikelis, I.; Konrade, I.; Zaharenko, L.; Sekace, K.; Radovica-Spalvina, I.; Fridmanis, D.; Gudra, D.; Pirags, V.; et al. Association of metformin administration with gut microbiome dysbiosis in healthy volunteers. PLoS ONE 2018, 13, e0204317. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Yun, Y.; Kim, S.J.; Lee, E.-J.; Chang, Y.; Ryu, S.; Shin, H.; Kim, H.-L.; Kim, H.-N.; Lee, J.H. Association between Cigarette Smoking Status and Composition of Gut Microbiota: Population-Based Cross-Sectional Study. J. Clin. Med. 2018, 7, 282. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Liang, Q.; Balakrishnan, B.; Belobrajdic, D.P.; Feng, Q.J.; Zhang, W. Role of dietary nutrients in the modulation of gut microbiota: A narrative review. Nutrients 2020, 12, 381. [Google Scholar] [CrossRef] [Green Version]
- Cao, T.T.B.; Wu, K.-C.; Hsu, J.-L.; Chang, C.-S.; Chou, C.; Lin, C.-Y.; Liao, Y.-M.; Lin, P.-C.; Yang, L.-Y.; Lin, H.-W. Effects of Non-insulin Anti-hyperglycemic Agents on Gut Microbiota: A Systematic Review on Human and Animal Studies. Front. Endocrinol. (Lausanne) 2020, 11, 573891. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef]
- Requena, T.; Martínez-Cuesta, M.C.; Peláez, C. Diet and microbiota linked in health and disease. Food Funct. 2018, 9, 688–704. [Google Scholar] [CrossRef] [Green Version]
- Skonieczna-Żydecka, K.; Łoniewski, I.; Misera, A.; Stachowska, E.; Maciejewska, D.; Marlicz, W.; Galling, B. Second-generation antipsychotics and metabolism alterations: A systematic review of the role of the gut microbiome. Psychopharmacology 2019, 236, 1491–1512. [Google Scholar] [CrossRef] [Green Version]
- Bowyer, R.C.E.; Jackson, M.A.; Pallister, T.; Skinner, J.; Spector, T.D.; Welch, A.A.; Steves, C.J. Use of dietary indices to control for diet in human gut microbiota studies. Microbiome 2018, 6, 77. [Google Scholar] [CrossRef]
- Wądołowska, L. Validation of food frequency questionnaire (FFQ). Reproducibility assessment. Bromat. Chem. Toksykol. 2005, 38, 27–33. Available online: http://www.sciepub.com/reference/219880 (accessed on 20 November 2020).
- Montgomery, S.A.; Asberg, M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry 1979, 134, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Quilty, L.C.; Robinson, J.J.; Rolland, J.P.; De Fruyt, F.; Rouillon, F.; Bagby, R.M. The structure of the Montgomery-Åsberg depression rating scale over the course of treatment for depression. Int. J. Methods Psychiatr. Res. 2013, 22, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Guerra, L.S.; Gorenstein, C.; Paiva-Medeiros, P.F.; Santo, M.A.; Neto, F.L.; Wang, Y.P. Clinical utility of the Montgomery-Åsberg Depression Rating Scale for the detection of depression among bariatric surgery candidates. BMC Psychiatry 2016, 16, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, T.A.; Chorpita, B.F.; Korotitsch, W.; Barlow, D.H. Psychometric properties of the Depression Anxiety Stress Scales (DASS) in clinical samples. Behav. Res. Ther. 1997, 35, 79–89. [Google Scholar] [CrossRef]
- Skevington, S.M.; Lotfy, M.; O’Connell, K.A. The World Health Organization’s WHOQOL-BREF quality of life assessment: Psychometric properties and results of the international field trial a Report from the WHOQOL Group. Qual. Life Res. 2004, 13, 299–310. [Google Scholar] [CrossRef]
- Chehil, S.; Kutcher, S. Suicide Risk Management; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Agahi, A.; Hamidi, G.A.; Daneshvar, R.; Hamdieh, M.; Soheili, M.; Alinaghipour, A.; Taba, S.M.E.; Salami, M. Does severity of Alzheimer’s disease contribute to its responsiveness to modifying gut microbiota? A double blind clinical trial. Front. Neurol. 2018, 9, 662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jönsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity commissioned by the ILSI Europe Metabolic Syndrome and Diabetes Task Force. Br. J. Nutr. 2011, 106, S1–S78. [Google Scholar] [CrossRef]
- Margioris, A.N.; Dermitzaki, E.; Venihaki, M.; Tsatsanis, C. Chronic low-grade inflammation. In Diet, Immunity and Inflammation; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; pp. 105–120. ISBN 9780857090379. [Google Scholar]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Marnett, L.J. Lipid peroxidation-DNA damage by malondialdehyde. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1999, 424, 83–95. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [Green Version]
- Kampa, M.; Nistikaki, A.; Tsaousis, V.; Maliaraki, N.; Notas, G.; Castanas, E. A new automated method for the determination of the Total Antioxidant Capacity (TAC) of human plasma, based on the crocin bleaching assay. BMC Clin. Pathol. 2002, 2, 3. [Google Scholar] [CrossRef] [Green Version]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Ziebold, C.; Goldberg, D.P.; Reed, G.M.; Minhas, F.; Razzaque, B.; Fortes, S.; Robles, R.; Lam, T.P.; Bobes, J.; Iglesias, C.; et al. Dimensional analysis of depressive, anxious and somatic symptoms presented by primary care patients and their relationship with ICD-11 PHC proposed diagnoses. Psychol. Med. 2019, 49, 764–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandhu, K.V.; Sherwin, E.; Schellekens, H.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Feeding the microbiota-gut-brain axis: Diet, microbiome, and neuropsychiatry. Transl. Res. 2017, 179, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Bretler, T.; Weisberg, H.; Koren, O.; Neuman, H. The effects of antipsychotic medications on microbiome and weight gain in children and adolescents. BMC Med. 2019, 17, 112. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zareie, M.; Johnson-Henry, K.; Jury, J.; Yang, P.C.; Ngan, B.Y.; McKay, D.M.; Soderholm, J.D.; Perdue, M.H.; Sherman, P.M. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut 2006, 55, 1553–1560. [Google Scholar] [CrossRef] [Green Version]
- Girard, S.A.; Bah, T.M.; Kaloustian, S.; Lada-Moldovan, L.; Rondeau, I.; Tompkins, T.A.; Godbout, R.; Rousseau, G. Lactobacillus helveticus and Bifidobacterium longum taken in combination reduce the apoptosis propensity in the limbic system after myocardial infarction in a rat model. Br. J. Nutr. 2009, 102, 1420–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arseneault-Bréard, J.; Rondeau, I.; Gilbert, K.; Girard, S.A.; Tompkins, T.A.; Godbout, R.; Rousseau, G. Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduces post-myocardial infarction depression symptoms and restores intestinal permeability in a rat model. Br. J. Nutr. 2012, 107, 1793–1799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, K.; Arseneault-Bréard, J.; Monaco, F.F.; Beaudoin, A.; Bah, T.M.; Tompkins, T.A.; Godbout, R.; Rousseau, G. Attenuation of post-myocardial infarction depression in rats by n-3 fatty acids or probiotics starting after the onset of reperfusion. Br. J. Nutr. 2013, 109, 50–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ait-Belgnaoui, A.; Payard, I.; Rolland, C.; Harkat, C.; Braniste, V.; Théodorou, V.; Tompkins, T.A. Bifidobacterium longum and Lactobacillus helveticus synergistically suppress stress-related visceral hypersensitivity through hypothalamic-pituitary-adrenal axis modulation. J. Neurogastroenterol. Motil. 2018, 24, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.-F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messaoudi, M.; Violle, N.; Bisson, J.-F.; Desor, D.; Javelot, H.; Rougeot, C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes 2011, 2, 256–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diop, L.; Guillou, S.; Durand, H. Probiotic food supplement reduces stress-induced gastrointestinal symptoms in volunteers: A double-blind, placebo-controlled, randomized trial. Nutr. Res. 2008, 28, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Romijn, A.R.; Rucklidge, J.J.; Kuijer, R.G.; Frampton, C. A double-blind, randomized, placebo-controlled trial of Lactobacillus helveticus and Bifidobacterium longum for the symptoms of depression. Aust. N. Z. J. Psychiatry 2017, 51, 810–821. [Google Scholar] [CrossRef] [Green Version]
- Korkmaz, O.A.; Sumlu, E.; Koca, H.B.; Pektas, M.B.; Kocabas, A.; Sadi, G.; Akar, F. Effects of lactobacillus plantarum and Lactobacillus helveticus on renal insulin signaling, inflammatory markers, and glucose transporters in high-fructose-fed rats. Medicina 2019, 55, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.J.; Wang, R.; Li, X.F.; Wang, R.L. Bifidobacterium longum supplementation improved high-fat-fed-induced metabolic syndrome and promoted intestinal reg I gene expression. Exp. Biol. Med. 2011, 236, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Cheung, S.G.; Goldenthal, A.R.; Uhlemann, A.C.; Mann, J.J.; Miller, J.M.; Sublette, M.E. Systematic review of gut microbiota and major depression. Front. Psychiatry 2019, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar]
- Caspani, G.; Kennedy, S.; Foster, J.A.; Swann, J. Gut microbial metabolites in depression: Understanding the biochemical mechanisms. Microb. Cell 2019, 6, 454–481. [Google Scholar] [CrossRef] [PubMed]
- Skonieczna-żydecka, K.; Grochans, E.; Maciejewska, D.; Szkup, M.; Schneider-Matyka, D.; Jurczak, A.; Łoniewski, I.; Kaczmarczyk, M.; Marlicz, W.; Czerwińska-Rogowska, M.; et al. Faecal short chain fatty acids profile is changed in Polish depressive women. Nutrients 2018, 10, 1939. [Google Scholar] [CrossRef] [Green Version]
- Yamawaki, Y.; Yoshioka, N.; Nozaki, K.; Ito, H.; Oda, K.; Harada, K.; Shirawachi, S.; Asano, S.; Aizawa, H.; Yamawaki, S.; et al. Sodium butyrate abolishes lipopolysaccharide-induced depression-like behaviors and hippocampal microglial activation in mice. Brain Res. 2018, 1680, 13–38. [Google Scholar] [CrossRef]
- Bruschi, A.; Mazza, M.; Camardese, G.; Calò, S.; Palumbo, C.; Mandelli, L.; Callea, A.; Gori, A.; Di Nicola, M.; Marano, G.; et al. Psychopathological features of bipolar depression: Italian validation of the Bipolar Depression Rating Scale (I-BDRS). Front. Psychol. 2018, 9, 1047. [Google Scholar] [CrossRef] [Green Version]
- Farup, P.G.; Rudi, K.; Hestad, K. Faecal short-chain fatty acids—A diagnostic biomarker for irritable bowel syndrome? BMC Gastroenterol. 2016, 16, 51. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, A.; Navas-Molina, J.A.; Kosciolek, T.; McDonald, D.; Vázquez-Baeza, Y.; Ackermann, G.; DeReus, J.; Janssen, S.; Swafford, A.D.; Orchanian, S.B.; et al. Qiita: Rapid, web-enabled microbiome meta-analysis. Nat. Methods 2018, 15, 796–798. [Google Scholar] [CrossRef]


Inclusion criteria:
|
Exclusion criteria:
|
Reasons for discontinuation of the study by a participant:
|
| Psychometric Tools | Physical Examination | Biological Samples | ||||
|---|---|---|---|---|---|---|
| Self-Administered | Administered by an Interviewer | Blood | Feces | |||
| Metabolic Parameters | Inflammation Parameters | OxS Parameters | ||||
| SQ | MADRS | BP | HDL-C | WBC | TAC | MC |
| DASS | TASR | BMI | TG | LR | MDA | SCFAs |
| WHOQOL-BREF | WC | fGlc | CRP | |||
| FFQ | Il-6 | |||||
| MQ | TNFα | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gawlik-Kotelnicka, O.; Skowrońska, A.; Margulska, A.; Czarnecka-Chrebelska, K.H.; Łoniewski, I.; Skonieczna-Żydecka, K.; Strzelecki, D. The Influence of Probiotic Supplementation on Depressive Symptoms, Inflammation, and Oxidative Stress Parameters and Fecal Microbiota in Patients with Depression Depending on Metabolic Syndrome Comorbidity—PRO-DEMET Randomized Study Protocol. J. Clin. Med. 2021, 10, 1342. https://doi.org/10.3390/jcm10071342
Gawlik-Kotelnicka O, Skowrońska A, Margulska A, Czarnecka-Chrebelska KH, Łoniewski I, Skonieczna-Żydecka K, Strzelecki D. The Influence of Probiotic Supplementation on Depressive Symptoms, Inflammation, and Oxidative Stress Parameters and Fecal Microbiota in Patients with Depression Depending on Metabolic Syndrome Comorbidity—PRO-DEMET Randomized Study Protocol. Journal of Clinical Medicine. 2021; 10(7):1342. https://doi.org/10.3390/jcm10071342
Chicago/Turabian StyleGawlik-Kotelnicka, Oliwia, Anna Skowrońska, Aleksandra Margulska, Karolina H. Czarnecka-Chrebelska, Igor Łoniewski, Karolina Skonieczna-Żydecka, and Dominik Strzelecki. 2021. "The Influence of Probiotic Supplementation on Depressive Symptoms, Inflammation, and Oxidative Stress Parameters and Fecal Microbiota in Patients with Depression Depending on Metabolic Syndrome Comorbidity—PRO-DEMET Randomized Study Protocol" Journal of Clinical Medicine 10, no. 7: 1342. https://doi.org/10.3390/jcm10071342
APA StyleGawlik-Kotelnicka, O., Skowrońska, A., Margulska, A., Czarnecka-Chrebelska, K. H., Łoniewski, I., Skonieczna-Żydecka, K., & Strzelecki, D. (2021). The Influence of Probiotic Supplementation on Depressive Symptoms, Inflammation, and Oxidative Stress Parameters and Fecal Microbiota in Patients with Depression Depending on Metabolic Syndrome Comorbidity—PRO-DEMET Randomized Study Protocol. Journal of Clinical Medicine, 10(7), 1342. https://doi.org/10.3390/jcm10071342