Nano-Encapsulation of Mithramycin in Transfersomes and Polymeric Micelles for the Treatment of Sarcomas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PLGA Polymeric Micelles
2.3. Synthesis of Transfersomes
2.4. Nanocolloids Characterization
2.4.1. Nanocolloids Size
2.4.2. Nanocolloids Morphology
2.4.3. Encapsulation Efficiency (EE)
2.5. Cell Culture, Drugs and Ethics Statement
2.6. Cell Viability Assays
2.7. Tumorsphere Culture
2.8. Western Blotting
2.9. Nanocolloids/MTM Confocal Imaging
3. Results
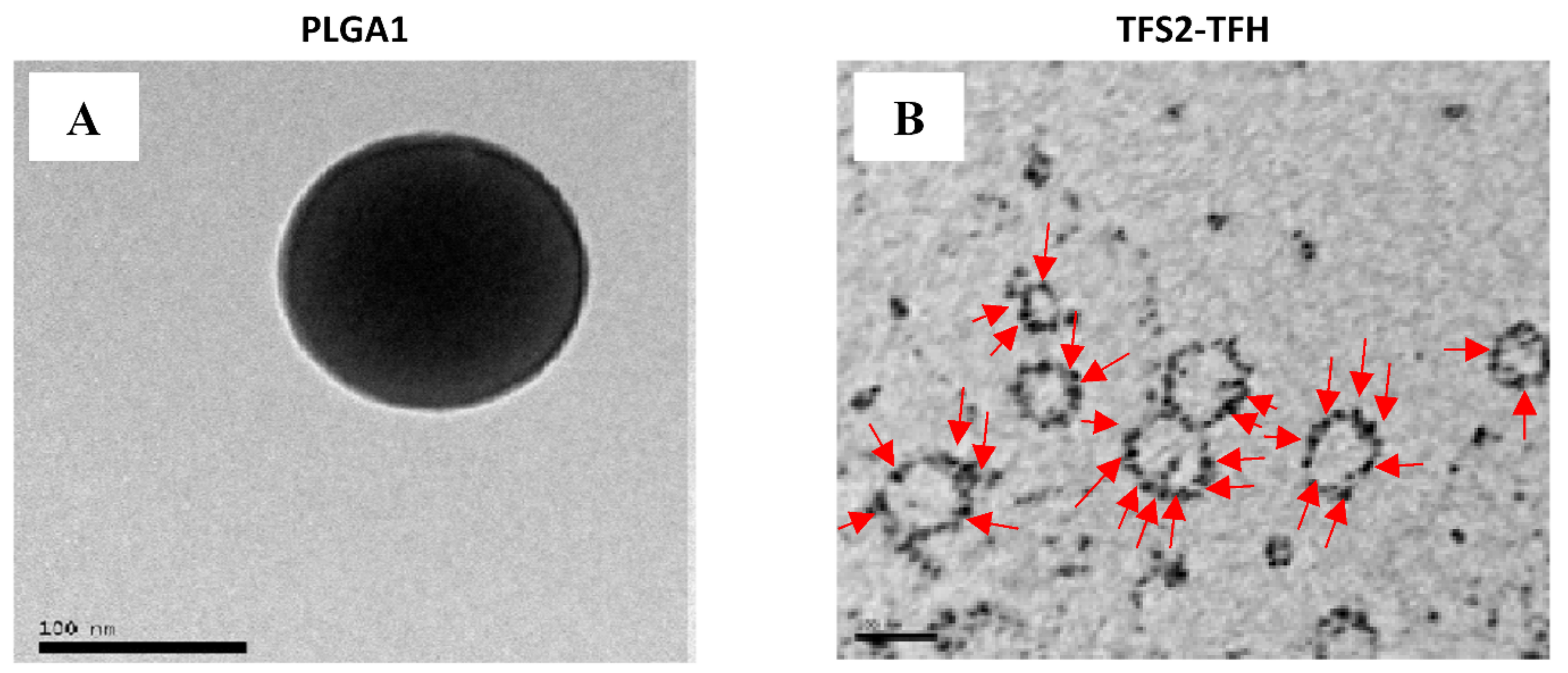
3.1. Nanocolloids Characterization
3.2. Free and Encapsulated MTM Shows Similar Cytotoxic Effect in Sarcoma Cells
3.3. Nano-Encapsulated MTM Targets Cancer Stem Cells (CSCs) Subpopulations in Sarcoma.
3.4. Free and MTM-Loaded Nanoparticles Inhibit SP1 Pathway Similarly
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grunewald, T.G.; Alonso, M.; Avnet, S.; Banito, A.; Burdach, S.; Cidre-Aranaz, F.; Di Pompo, G.; Distel, M.; Dorado-Garcia, H.; Garcia-Castro, J.; et al. Sarcoma treatment in the era of molecular medicine. EMBO Mol. Med. 2020, 12, e11131. [Google Scholar] [CrossRef]
- Casali, P.G.; Abecassis, N.; Aro, H.T.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.; Brodowicz, T.; et al. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv51–iv67. [Google Scholar] [CrossRef] [PubMed]
- Casali, P.G.; Bielack, S.; Abecassis, N.; Aro, H.T.; Bauer, S.; Biagini, R.; Bonvalot, S.; Boukovinas, I.; Bovee, J.; Brennan, B.; et al. Bone sarcomas: ESMO-PaedCan-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv79–iv95. [Google Scholar] [CrossRef]
- Lombó, F.; Menéndez, N.; Salas, J.A.; Méndez, C. The aureolic acid family of antitumor compounds: Structure, mode of action, biosynthesis, and novel derivatives. Appl. Microbiol. Biotechnol. 2006, 73, 1–14. [Google Scholar] [CrossRef]
- Miller, D.M.; Polansky, D.A.; Thomas, S.D.; Ray, R.; Campbell, V.W.; Sanchez, J.; Koller, C.A. Mithramycin selectively inhibits transcription of G-C containing DNA. Am. J. Med. Sci. 1987, 294, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Grohar, P.J.; Woldemichael, G.M.; Griffin, L.B.; Mendoza, A.; Chen, Q.R.; Yeung, C.; Currier, D.G.; Davis, S.; Khanna, C.; Khan, J.; et al. Identification of an inhibitor of the EWS-FLI1 oncogenic transcription factor by high-throughput screening. J. Natl. Cancer Inst. 2011, 103, 962–978. [Google Scholar] [CrossRef]
- Green, L.; Donehower, R.C. Hepatic toxicity of low doses of mithramycin in hypercalcemia. Cancer Treat. Rep. 1984, 68, 1379–1381. [Google Scholar] [PubMed]
- Monto, R.W.; Talley, R.W.; Caldwell, M.J.; Levin, W.C.; Guest, M.M. Observations on the mechanism of hemorrhagic toxicity in mithramycin (NSC 24559) therapy. Cancer Res. 1969, 29, 697–704. [Google Scholar] [PubMed]
- Bailey, K.; Cost, C.; Davis, I.; Glade-Bender, J.; Grohar, P.; Houghton, P.; Isakoff, M.; Stewart, E.; Laack, N.; Yustein, J.; et al. Emerging novel agents for patients with advanced Ewing sarcoma: A report from the Children’s Oncology Group (COG) New Agents for Ewing Sarcoma Task Force. F1000Research 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Flores, G.; Everett, J.H.; Boguslawski, E.A.; Oswald, B.M.; Madaj, Z.B.; Beddows, I.; Dikalov, S.; Adams, M.; Klumpp-Thomas, C.A.; Kitchen-Goosen, S.M.; et al. CDK9 Blockade Exploits Context-dependent Transcriptional Changes to Improve Activity and Limit Toxicity of Mithramycin for Ewing Sarcoma. Mol. Cancer Ther. 2020, 19, 1183–1196. [Google Scholar] [CrossRef]
- Nair, R.R.; Piktel, D.; Geldenhuys, W.J.; Gibson, L.F. Combination of cabazitaxel and plicamycin induces cell death in drug resistant B-cell acute lymphoblastic leukemia. Leuk Res. 2018, 72, 59–66. [Google Scholar] [CrossRef]
- Dong, P.; Xiong, Y.; Hanley, S.J.B.; Yue, J.; Watari, H. Musashi-2, a novel oncoprotein promoting cervical cancer cell growth and invasion, is negatively regulated by p53-induced miR-143 and miR-107 activation. J. Exp. Clin. Cancer Res. 2017, 36, 150. [Google Scholar] [CrossRef] [PubMed]
- Menendez, S.T.; Rey, V.; Martinez-Cruzado, L.; Gonzalez, M.V.; Morales-Molina, A.; Santos, L.; Blanco, V.; Alvarez, C.; Estupinan, O.; Allonca, E.; et al. SOX2 Expression and Transcriptional Activity Identifies a Subpopulation of Cancer Stem Cells in Sarcoma with Prognostic Implications. Cancers 2020, 12, 964. [Google Scholar] [CrossRef]
- Quarni, W.; Dutta, R.; Green, R.; Katiri, S.; Patel, B.; Mohapatra, S.S.; Mohapatra, S. Mithramycin A Inhibits Colorectal Cancer Growth by Targeting Cancer Stem Cells. Sci. Rep. 2019, 9, 15202. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Kollipara, R.K.; Vemireddy, V.; Yang, X.L.; Sun, Y.; Regmi, N.; Klingler, S.; Hatanpaa, K.J.; Raisanen, J.; Cho, S.K.; et al. Oncogenes Activate an Autonomous Transcriptional Regulatory Circuit That Drives Glioblastoma. Cell Rep. 2017, 18, 961–976. [Google Scholar] [CrossRef]
- Tornin, J.; Martinez-Cruzado, L.; Santos, L.; Rodriguez, A.; Nunez, L.E.; Oro, P.; Hermosilla, M.A.; Allonca, E.; Fernandez-Garcia, M.T.; Astudillo, A.; et al. Inhibition of SP1 by the mithramycin analog EC-8042 efficiently targets tumor initiating cells in sarcoma. Oncotarget 2016, 7, 30935–30950. [Google Scholar] [CrossRef] [PubMed]
- Vanner, R.J.; Remke, M.; Gallo, M.; Selvadurai, H.J.; Coutinho, F.; Lee, L.; Kushida, M.; Head, R.; Morrissy, S.; Zhu, X.; et al. Quiescent sox2(+) cells drive hierarchical growth and relapse in sonic hedgehog subgroup medulloblastoma. Cancer Cell 2014, 26, 33–47. [Google Scholar] [CrossRef]
- Banik, B.L.; Fattahi, P.; Brown, J.L. Polymeric nanoparticles: The future of nanomedicine. Wiley Interdiscip Rev. Nanomed. Nanobiotechnol. 2016, 8, 271–299. [Google Scholar] [CrossRef]
- Awasthi, R.; Roseblade, A.; Hansbro, P.M.; Rathbone, M.J.; Dua, K.; Bebawy, M. Nanoparticles in Cancer Treatment: Opportunities and Obstacles. Curr. Drug Targets 2018, 19, 1696–1709. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, B.; Hojjat-Farsangi, M.; Mohammadi, H.; Anvari, E.; Ghalamfarsa, G.; Yousefi, M.; Jadidi-Niaragh, F. Nanoparticles and targeted drug delivery in cancer therapy. Immunol. Lett. 2017, 190, 64–83. [Google Scholar] [CrossRef] [PubMed]
- Golombek, S.K.; May, J.N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor targeting via EPR: Strategies to enhance patient responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Asghari, F.; Khademi, R.; Esmaeili Ranjbar, F.; Veisi Malekshahi, Z.; Faridi Majidi, R. Application of Nanotechnology in Targeting of Cancer Stem Cells: A Review. Int. J. stem. Cells 2019, 12, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.H.; Wang, J.; Makena, M.R.; Lee, J.S.; Paz, N.; Hall, C.P.; Song, M.M.; Calderon, R.I.; Cruz, R.E.; Hindle, A.; et al. Activity of MM-398, nanoliposomal irinotecan (nal-IRI), in Ewing’s family tumor xenografts is associated with high exposure of tumor to drug and high SLFN11 expression. Clin. Cancer Res. 2015, 21, 1139–1150. [Google Scholar] [CrossRef]
- Porche, D.J. Liposomal doxorubicin (Doxil). J. Assoc. Nurses AIDS Care 1996, 7, 55–59. [Google Scholar] [CrossRef]
- Cohen-Sela, E.; Teitlboim, S.; Chorny, M.; Koroukhov, N.; Danenberg, H.D.; Gao, J.; Golomb, G. Single and double emulsion manufacturing techniques of an amphiphilic drug in PLGA nanoparticles: Formulations of mithramycin and bioactivity. J. Pharm. Sci. 2009, 98, 1452–1462. [Google Scholar] [CrossRef]
- Liu, X.J.; Li, L.; Liu, X.J.; Li, Y.; Zhao, C.Y.; Wang, R.Q.; Zhen, Y.S. Mithramycin-loaded mPEG-PLGA nanoparticles exert potent antitumor efficacy against pancreatic carcinoma. Int. J. Nanomed. 2017, 12, 5255–5269. [Google Scholar] [CrossRef] [PubMed]
- Capretto, L.; Mazzitelli, S.; Brognara, E.; Lampronti, I.; Carugo, D.; Hill, M.; Zhang, X.; Gambari, R.; Nastruzzi, C. Mithramycin encapsulated in polymeric micelles by microfluidic technology as novel therapeutic protocol for beta-thalassemia. Int. J. Nanomed. 2012, 7, 307–324. [Google Scholar] [CrossRef]
- Frézard, F.; Garnier-Suillerot, A.; Demicheli, C. Encapsulation of Mithramycin in Liposomes in Response to a Transmembrane Gradient of Calcium Ions. J. Incl. Phenom. Mol. Recognit. Chem. 1997, 28, 51–62. [Google Scholar] [CrossRef]
- El-Hammadi, M.M.; Arias, J.L. An update on liposomes in drug delivery: A patent review (2014–2018). Expert. Opin. Ther. Pat. 2019, 29, 891–907. [Google Scholar] [CrossRef] [PubMed]
- Garg, V.; Singh, H.; Bimbrawh, S.; Singh, S.K.; Gulati, M.; Vaidya, Y.; Kaur, P. Ethosomes and Transfersomes: Principles, Perspectives and Practices. Curr. Drug Deliv. 2017, 14, 613–633. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, G.N.; Parakh, S.R.; Devraj, R.; Apte, S.S.; Rao, B.R.; Rambhau, D. Release Studies on Niosomes Containing Fatty Alcohols as Bilayer Stabilizers Instead of Cholesterol. J. Colloid Interface Sci. 2002, 251, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.; Rosu-Myles, M.; Aráuzo-Bravo, M.; Horrillo, A.; Pan, Q.; Gonzalez-Rey, E.; Delgado, M.; Menendez, P. Human bone marrow stromal cells lose immunosuppressive and anti-inflammatory properties upon oncogenic transformation. Stem. Cell Reports 2014, 3, 606–619. [Google Scholar] [CrossRef]
- Rodriguez, R.; Tornin, J.; Suarez, C.; Astudillo, A.; Rubio, R.; Yauk, C.; Williams, A.; Rosu-Myles, M.; Funes, J.M.; Boshoff, C.; et al. Expression of FUS-CHOP fusion protein in immortalized/transformed human mesenchymal stem cells drives mixoid liposarcoma formation. Stem. Cells 2013, 31, 2061–2072. [Google Scholar] [CrossRef]
- Martinez-Cruzado, L.; Tornin, J.; Santos, L.; Rodriguez, A.; Garcia-Castro, J.; Moris, F.; Rodriguez, R. Aldh1 Expression and Activity Increase During Tumor Evolution in Sarcoma Cancer Stem Cell Populations. Sci. Rep. 2016, 6, 27878. [Google Scholar] [CrossRef] [PubMed]
- Rey, V.; Menendez, S.T.; Estupinan, O.; Rodriguez, A.; Santos, L.; Tornin, J.; Martinez-Cruzado, L.; Castillo, D.; Ordonez, G.R.; Costilla, S.; et al. New Chondrosarcoma Cell Lines with Preserved Stem Cell Properties to Study the Genomic Drift During In Vitro/In Vivo Growth. J. Clin. Med. 2019, 8, 455. [Google Scholar] [CrossRef] [PubMed]
- Novakova, R.; Nunez, L.E.; Homerova, D.; Knirschova, R.; Feckova, L.; Rezuchova, B.; Sevcikova, B.; Menendez, N.; Moris, F.; Cortes, J.; et al. Increased heterologous production of the antitumoral polyketide mithramycin A by engineered Streptomyces lividans TK24 strains. Appl. Microbiol. Biotechnol. 2018, 102, 857–869. [Google Scholar] [CrossRef]
- Tornin, J.; Hermida-Prado, F.; Padda, R.S.; Gonzalez, M.V.; Alvarez-Fernandez, C.; Rey, V.; Martinez-Cruzado, L.; Estupinan, O.; Menendez, S.T.; Fernandez-Nevado, L.; et al. FUS-CHOP Promotes Invasion in Myxoid Liposarcoma through a SRC/FAK/RHO/ROCK-Dependent Pathway. Neoplasia 2018, 20, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Cruzado, L.; Tornin, J.; Rodriguez, A.; Santos, L.; Allonca, E.; Fernandez-Garcia, M.T.; Astudillo, A.; Garcia-Pedrero, J.M.; Rodriguez, R. Trabectedin and Campthotecin Synergistically Eliminate Cancer Stem Cells in Cell-of-Origin Sarcoma Models. Neoplasia 2017, 19, 460–470. [Google Scholar] [CrossRef]
- Zuazua-Villar, P.; Rodriguez, R.; Gagou, M.E.; Eyers, P.A.; Meuth, M. DNA replication stress in CHK1-depleted tumour cells triggers premature (S-phase) mitosis through inappropriate activation of Aurora kinase B. Cell Death Dis. 2014, 5, e1253. [Google Scholar] [CrossRef] [PubMed]
- Mali, N.; Darandale, S.; Vavia, P. Niosomes as a vesicular carrier for topical administration of minoxidil: Formulation and in vitro assessment. Drug Deliv. Transl. Res. 2013, 3, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Estupiñan, O.R.; Garcia-Manrique, P.; Blanco-Lopez, M.d.C.; Matos, M.; Gutiérrez, G. Vitamin D3 Loaded Niosomes and Transfersomes Produced by Ethanol Injection Method: Identification of the Critical Preparation Step for Size Control. Foods 2020, 9, 1367. [Google Scholar] [CrossRef]
- Garcia-Manrique, P.; Gutierrez, G.; Matos, M.; Cristaldi, A.; Mosayyebi, A.; Carugo, D.; Zhang, X.; Blanco-Lopez, M.C. Continuous flow production of size-controllable niosomes using a thermostatic microreactor. Colloids Surf. B Biointerfaces 2019, 182, 110378. [Google Scholar] [CrossRef]
- Pando, D.; Matos, M.; Gutierrez, G.; Pazos, C. Formulation of resveratrol entrapped niosomes for topical use. Colloids Surf. B Biointerfaces 2015, 128, 398–404. [Google Scholar] [CrossRef]
- García-Manrique, P.; Machado, N.D.; Fernández, M.A.; Blanco-López, M.C.; Matos, M.; Gutiérrez, G. Effect of drug molecular weight on niosomes size and encapsulation efficiency. Colloids Surf. B Biointerfaces 2020, 186, 110711. [Google Scholar] [CrossRef]
- Abarrategi, A.; Tornin, J.; Martinez-Cruzado, L.; Hamilton, A.; Martinez-Campos, E.; Rodrigo, J.P.; Gonzalez, M.V.; Baldini, N.; Garcia-Castro, J.; Rodriguez, R. Osteosarcoma: Cells-of-Origin, Cancer Stem Cells, and Targeted Therapies. Stem. Cells Int. 2016, 2016, 3631764. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.S.; Nam, J.S.; Jung, J.Y.; Cho, N.P.; Cho, S.D. Modulation of specificity protein 1 by mithramycin A as a novel therapeutic strategy for cervical cancer. Sci. Rep. 2014, 4, 7162. [Google Scholar] [CrossRef] [PubMed]
- Vizcaino, C.; Mansilla, S.; Portugal, J. Sp1 transcription factor: A long-standing target in cancer chemotherapy. Pharmacol. Ther. 2015, 152, 111–124. [Google Scholar] [CrossRef]
- Beishline, K.; Azizkhan-Clifford, J. Sp1 and the ‘hallmarks of cancer’. FEBS J. 2015, 282, 224–258. [Google Scholar] [CrossRef] [PubMed]
- Perlia, C.P.; Gubisch, N.J.; Wolter, J.; Edelberg, D.; Dederick, M.M.; Taylor, S.G., 3rd. Mithramycin treatment of hypercalcemia. Cancer 1970, 25, 389–394. [Google Scholar] [CrossRef]
- Kennedy, B.J.; Torkelson, J.L. Long-term follow-up of stage III testicular carcinoma treated with mithramycin (plicamycin). Med. Pediatr. Oncol. 1995, 24, 327–328. [Google Scholar] [CrossRef]
- Kofman, S.; Perlia, C.P.; Economou, S.G. Mithramycin in the treatment of metastatic Ewing’s sarcoma. Cancer 1973, 31, 889–893. [Google Scholar] [CrossRef]
- Grohar, P.J.; Glod, J.; Peer, C.J.; Sissung, T.M.; Arnaldez, F.I.; Long, L.; Figg, W.D.; Whitcomb, P.; Helman, L.J.; Widemann, B.C. A phase I/II trial and pharmacokinetic study of mithramycin in children and adults with refractory Ewing sarcoma and EWS-FLI1 fusion transcript. Cancer Chemother. Pharmacol. 2017, 80, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Chasse, M.H.; Johnson, B.K.; Boguslawski, E.A.; Sorensen, K.M.; Rosien, J.E.; Kang, M.H.; Reynolds, C.P.; Heo, L.; Madaj, Z.B.; Beddows, I.; et al. Mithramycin induces promoter reprogramming and differentiation of rhabdoid tumor. EMBO Mol. Med. 2020, e12640. [Google Scholar] [CrossRef]
- Osgood, C.L.; Maloney, N.; Kidd, C.G.; Kitchen-Goosen, S.; Segars, L.; Gebregiorgis, M.; Woldemichael, G.M.; He, M.; Sankar, S.; Lessnick, S.L.; et al. Identification of Mithramycin Analogues with Improved Targeting of the EWS-FLI1 Transcription Factor. Clin. Cancer Res. 2016, 22, 4105–4118. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; Rohr, J.; Bae, Y. Nanoparticulate formulations of mithramycin analogs for enhanced cytotoxicity. Int. J. Nanomed. 2011, 6, 2757–2767. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, C.; Liu, P.; Wang, Z.; Ding, J.; Zhou, W. Facile construction of dual-targeting delivery system by using lipid capped polymer nanoparticles for anti-glioma therapy. RSC Adv. 2018, 8, 444–453. [Google Scholar] [CrossRef]
- García-Manrique, P.; Matos, M.; Gutiérrez, G.; Estupiñán, O.R.; Blanco-López, M.C.; Pazos, C. Using Factorial Experimental Design To Prepare Size-Tuned Nanovesicles. Ind. Eng. Chem. Res. 2016, 55, 9164–9175. [Google Scholar] [CrossRef]
- Niza, E.; Castro-Osma, J.A.; Posadas, I.; Alonso-Moreno, C.; Bravo, I.; Garzon, A.; Canales-Vazquez, J.; Cena, V.; Lara-Sanchez, A.; Albaladejo, J.; et al. Assessment of doxorubicin delivery devices based on tailored bare polycaprolactone against glioblastoma. Int. J. Pharm. 2019, 558, 110–119. [Google Scholar] [CrossRef]
- Chadalapaka, G.; Jutooru, I.; Sreevalsan, S.; Pathi, S.; Kim, K.; Chen, C.; Crose, L.; Linardic, C.; Safe, S. Inhibition of rhabdomyosarcoma cell and tumor growth by targeting specificity protein (Sp) transcription factors. Int. J. Cancer 2013, 132, 795–806. [Google Scholar] [CrossRef]
- Lou, Z.; O’Reilly, S.; Liang, H.; Maher, V.M.; Sleight, S.D.; McCormick, J.J. Down-regulation of overexpressed sp1 protein in human fibrosarcoma cell lines inhibits tumor formation. Cancer Res. 2005, 65, 1007–1017. [Google Scholar]
- Meng, Y.; Hao, D.; Huang, Y.; Jia, S.; Zhang, J.; He, X.; Sun, L.; Liu, D. Positive feedback loop SP1/MIR17HG/miR-130a-3p promotes osteosarcoma proliferation and cisplatin resistance. Biochem. Biophys. Res. Commun. 2020, 521, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Alfranca, A.; Martinez-Cruzado, L.; Tornin, J.; Abarrategi, A.; Amaral, T.; de Alava, E.; Menendez, P.; Garcia-Castro, J.; Rodriguez, R. Bone microenvironment signals in osteosarcoma development. Cell Mol. Life Sci. 2015, 72, 3097–3113. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Electronic address, e.d.s.c.; Cancer Genome Atlas Research, N. Comprehensive and Integrated Genomic Characterization of Adult Soft Tissue Sarcomas. Cell 2017, 171, 950–965. [Google Scholar] [CrossRef] [PubMed]
- Kovac, M.; Blattmann, C.; Ribi, S.; Smida, J.; Mueller, N.S.; Engert, F.; Castro-Giner, F.; Weischenfeldt, J.; Kovacova, M.; Krieg, A.; et al. Exome sequencing of osteosarcoma reveals mutation signatures reminiscent of BRCA deficiency. Nat. Commun. 2015, 6, 8940. [Google Scholar] [CrossRef]
- Sayles, L.C.; Breese, M.R.; Koehne, A.L.; Leung, S.G.; Lee, A.G.; Liu, H.Y.; Spillinger, A.; Shah, A.T.; Tanasa, B.; Straessler, K.; et al. Genome-Informed Targeted Therapy for Osteosarcoma. Cancer Discov. 2019, 9, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Harwood, J.L.; Alexander, J.H.; Mayerson, J.L.; Scharschmidt, T.J. Targeted Chemotherapy in Bone and Soft-Tissue Sarcoma. Orthop. Clin. N. Am. 2015, 46, 587–608. [Google Scholar] [CrossRef] [PubMed]



| Formulation | Z-Average Size (nm) | PDI | EE (%) | |
|---|---|---|---|---|
| PLGA polymeric micelles | PLGA1 S60:Cho (1:0.5) | 216 ± 26 | 0.101 ± 0.039 | 87 ± 15 |
| PLGA2 S60 | 267 ± 57 | 0.137 ± 0.02 | 82.5 ± 4.0 | |
| PLGA3 PC | 210 ± 5 | 0.084 ± 0.02 | 63.8 ± 12.3 | |
| PLGA4 S60:PC (1:1) | 221 ± 2 | 0.083 ± 0.02 | 76.4 ± 14.5 | |
| TFS–EIM | TFS1–EIM PC:S60:20:Cho (1:0.5:0.5:0.5) | 115 ± 9 | 0.240 ± 0.018 | 58 ± 15 |
| TFS2–EIM PC:S60:20:Cho (1:1.5:1.5:1) | 126 ± 4 | 0.246 ± 0.004 | 52 ± 18 | |
| TFS3–EIM PC:S60:T20:Cho (3:3:3:1) | 117 ± 5 | 0.235 ± 0.021 | 51 ± 10 | |
| TFS4–EIM PC:S60:T20:Cho (1.5:1:0.5:1) | 126 ± 3 | 0.244 ± 0.005 | 50 ± 11 | |
| TFS–TFH | TFS1–TFH PC:S60:20:Cho (1:0.5:0.5:0.5) | 107 ± 4 | 0.400 ± 0.008 | 52 ± 14 |
| TFS2–TFH PC:S60:20:Cho (1:1,5:1,5:1) | 127 ± 29 | 0.382 ± 0.074 | 50 ± 16 | |
| TFS3–TFH PC:S60:T20:Cho (3:3:3:1) | 97 ± 7 | 0.388 ± 0.053 | 56 ± 4 | |
| TFS4–TFH PC:S60:20:Cho (1.5:1:0.5:1) | 133 ± 39 | 0.366 ± 0.074 | 52 ± 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estupiñán, Ó.; Rendueles, C.; Suárez, P.; Rey, V.; Murillo, D.; Morís, F.; Gutiérrez, G.; Blanco-López, M.d.C.; Matos, M.; Rodríguez, R. Nano-Encapsulation of Mithramycin in Transfersomes and Polymeric Micelles for the Treatment of Sarcomas. J. Clin. Med. 2021, 10, 1358. https://doi.org/10.3390/jcm10071358
Estupiñán Ó, Rendueles C, Suárez P, Rey V, Murillo D, Morís F, Gutiérrez G, Blanco-López MdC, Matos M, Rodríguez R. Nano-Encapsulation of Mithramycin in Transfersomes and Polymeric Micelles for the Treatment of Sarcomas. Journal of Clinical Medicine. 2021; 10(7):1358. https://doi.org/10.3390/jcm10071358
Chicago/Turabian StyleEstupiñán, Óscar, Claudia Rendueles, Paula Suárez, Verónica Rey, Dzohara Murillo, Francisco Morís, Gemma Gutiérrez, María del Carmen Blanco-López, María Matos, and René Rodríguez. 2021. "Nano-Encapsulation of Mithramycin in Transfersomes and Polymeric Micelles for the Treatment of Sarcomas" Journal of Clinical Medicine 10, no. 7: 1358. https://doi.org/10.3390/jcm10071358
APA StyleEstupiñán, Ó., Rendueles, C., Suárez, P., Rey, V., Murillo, D., Morís, F., Gutiérrez, G., Blanco-López, M. d. C., Matos, M., & Rodríguez, R. (2021). Nano-Encapsulation of Mithramycin in Transfersomes and Polymeric Micelles for the Treatment of Sarcomas. Journal of Clinical Medicine, 10(7), 1358. https://doi.org/10.3390/jcm10071358








