Hypoparathyroidism in Pregnancy and Lactation: Current Approach to Diagnosis and Management
Abstract
:1. Introduction
2. Materials and Methods
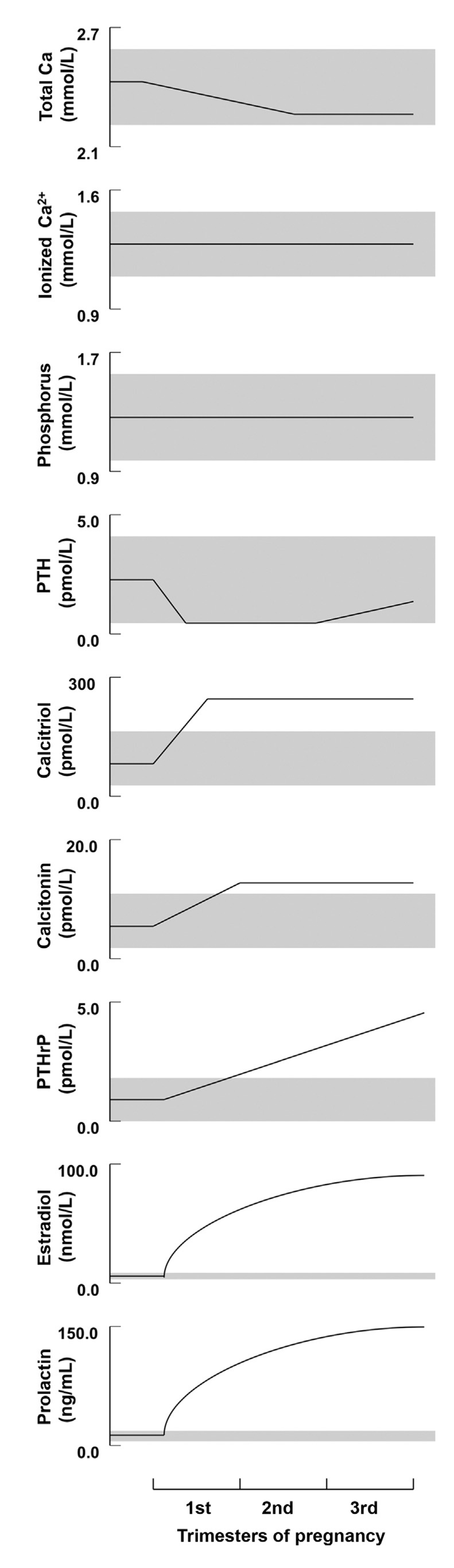
3. Calcium and Mineral Homeostasis in Euparathyroid Pregnancy and Lactation
3.1. Calcium and Phosphorus
3.1.1. Pregnancy
3.1.2. Lactation
3.2. Parathyroid Hormone
3.2.1. Pregnancy
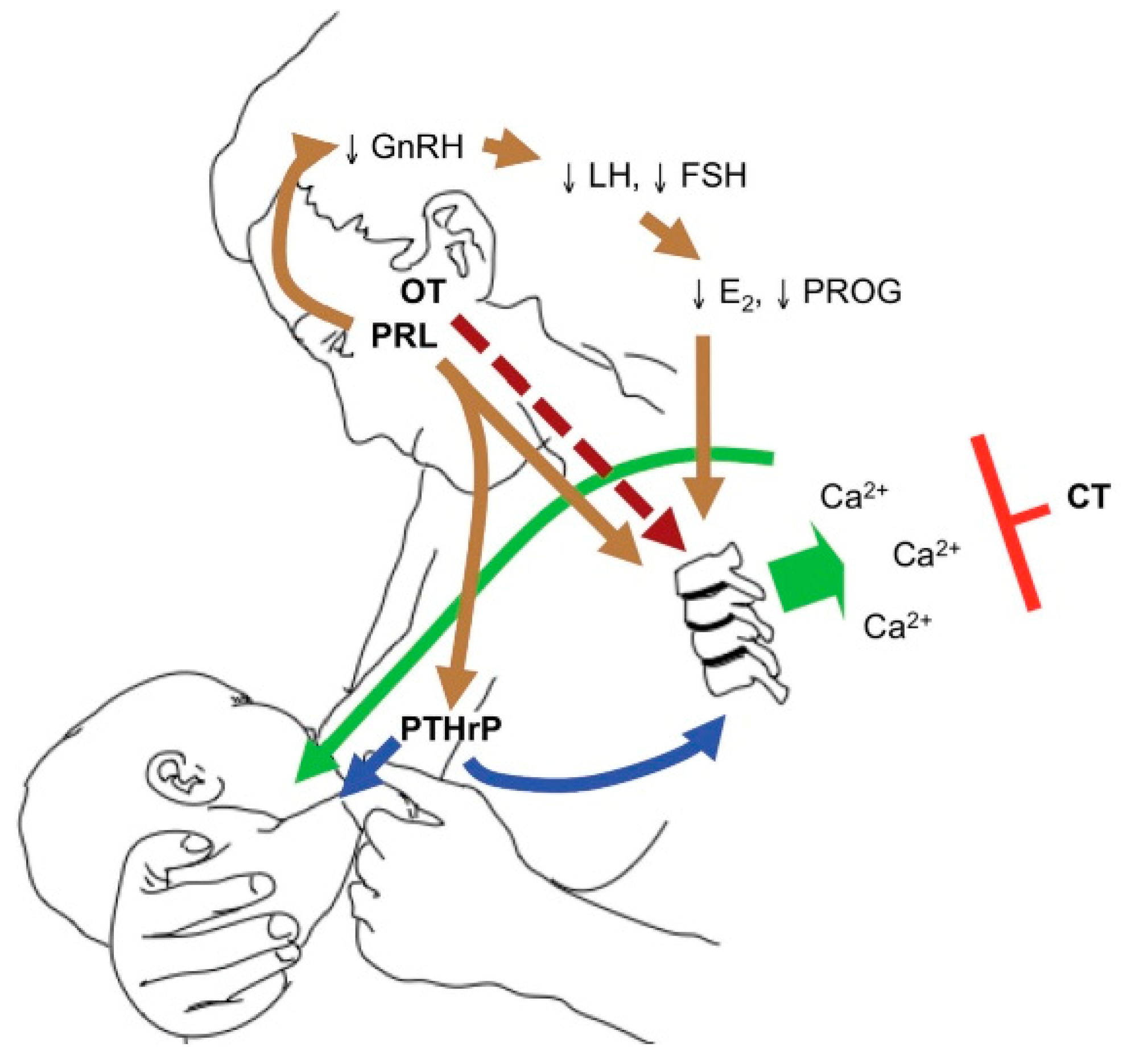
3.2.2. Lactation
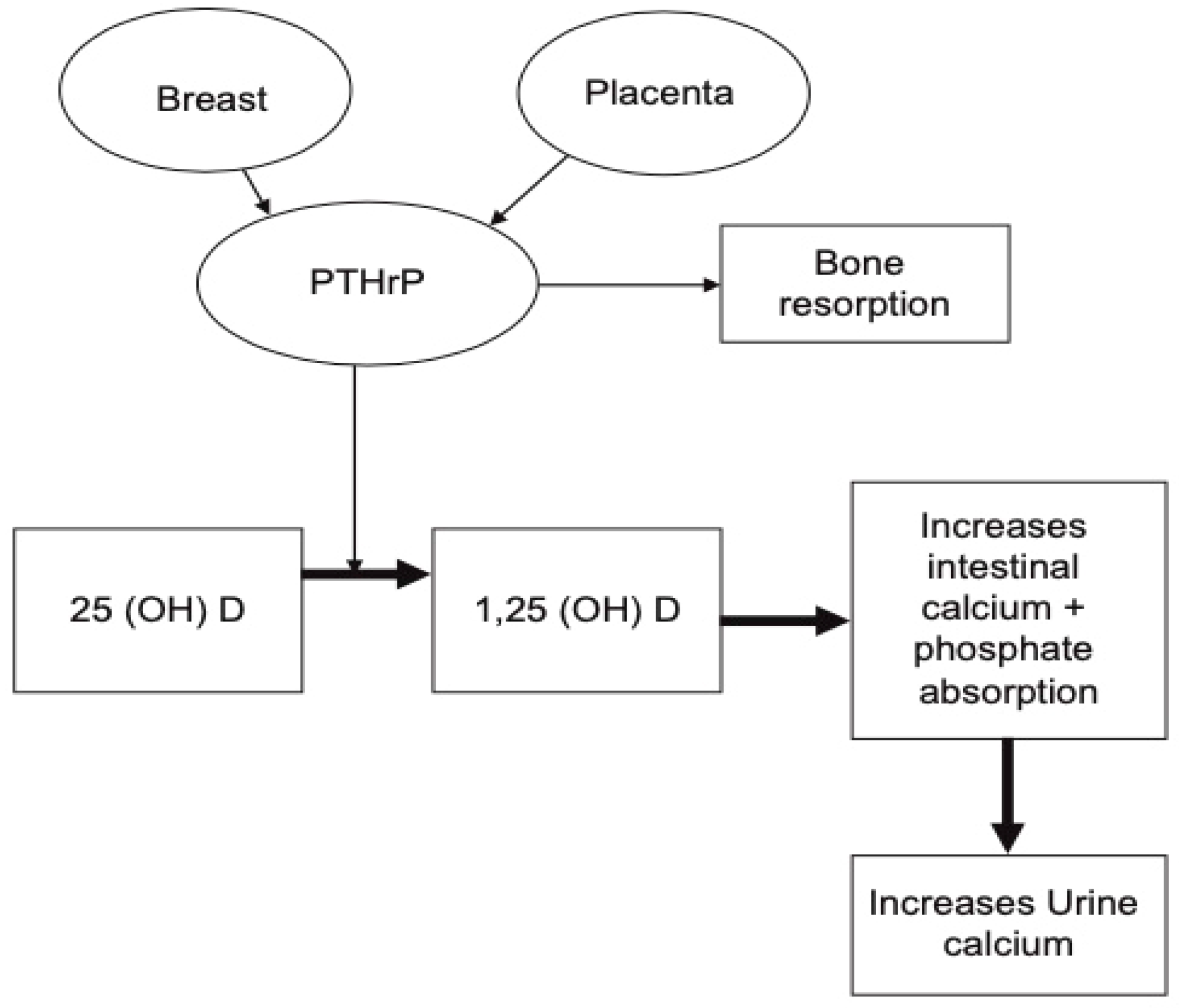
3.3. Parathyroid-Related Peptide (PTHrP)
3.3.1. Pregnancy
3.3.2. Lactation
3.4. 1,25-dihydroxyvitamin D (1,25-(OH)2-D3)
3.4.1. Pregnancy
3.4.2. Lactation
3.5. Calcitonin
3.5.1. Pregnancy
3.5.2. Lactation
4. Maternal and Fetal Calcium Requirements during Euparathyroid Pregnancy and Lactation
5. Diagnosis of HypoPT in Pregnancy
5.1. History
5.2. Physical Examination
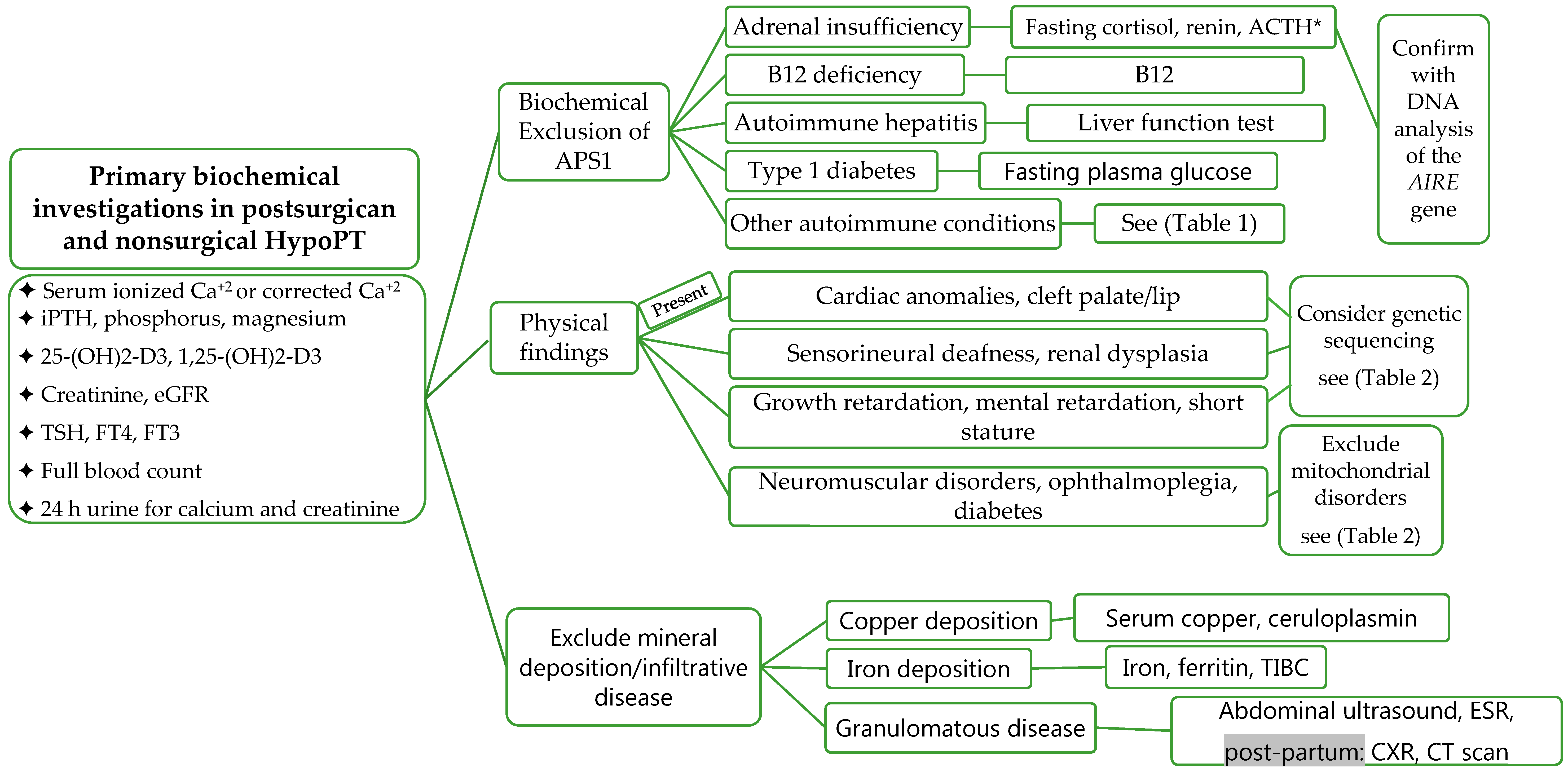
5.3. Laboratory Investigations
5.4. Assessment of Long-Term Complications
6. Impact of HypoPT on Mother and Fetus during Pregnancy
7. Clinical Management of HypoPT in Pregnancy
8. Clinical Management of HypoPT during Lactation
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, A.A.; Koch, C.A.; Van Uum, S.; Baillargeon, J.P.; Bollerslev, J.; Brandi, M.L.; Marcocci, C.; Rejnmark, L.; Rizzoli, R.; Shrayyef, M.Z.; et al. Standards of care for hypoparathyroidism in adults: A Canadian and International Consensus. Eur. J. Endocrinol. 2019, 180, P1–P22. [Google Scholar] [CrossRef] [PubMed]
- Cusano, N.E.; Rubin, M.R.; Bilezikian, J.P. Parathyroid hormone therapy for hypoparathyroidism. Best Pr. Res. Clin. Endocrinol. Metab. 2015, 29, 47–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rejnmark, L.; Underbjerg, L.; Sikjaer, T. Therapy of Hypoparathyroidism by Replacement with Parathyroid Hormone. Science 2014, 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Puzziello, A.; Rosato, L.; Innaro, N.; Orlando, G.; Avenia, N.; Perigli, G.; Calò, P.G.; De Palma, M. Hypocalcemia following thyroid surgery: Incidence and risk factors. A longitudinal multicenter study comprising 2,631 patients. Endocrine 2014, 47, 537–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cocchiara, G.; Cajozzo, M.; Amato, G.; Mularo, A.; Agrusa, A.; Romano, G. Terminal ligature of inferior thyroid artery branches during total thyroidectomy for multinodular goiter is associated with higher postoperative calcium and PTH levels. J. Visc. Surg. 2010, 147, e329–e332. [Google Scholar] [CrossRef] [PubMed]
- Betterle, C.; Garelli, S.; Presotto, F. Diagnosis and classification of autoimmune parathyroid disease. Autoimmun. Rev. 2014, 13, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Thakker, R.; Thakker, R. Genetic developments in Hypoparathyroidism. Lancet 2001, 357, 974–976. [Google Scholar] [CrossRef]
- Hakami, Y.; Khan, A. Hypoparathyroidism. Front. Horm. Res. 2019, 51, 109–126. [Google Scholar] [PubMed]
- Krysiak, R.; Kobielusz-Gembala, I.; Okopień, B. Hypoparathyroidism in pregnancy. Gynecol. Endocrinol. 2010, 27, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, C.S.; Fuleihan, G.E.-H. Calcium and Bone Disorders During Pregnancy and Lactation. Endocrinol. Metab. Clin. N. Am. 2006, 35, 21–51. [Google Scholar] [CrossRef]
- Mitchell, D.M.; Jüppner, H. Regulation of calcium homeostasis and bone metabolism in the fetus and neonate. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Aceto, T.; Batt, R.E.; Bruck, E.; Schultz, R.B.; Perez, Y.R.; Jablonski, E. Intrauterine Hyperparathyroidism: A Complication of Untreated Maternal Hypoparathyroidism1. J. Clin. Endocrinol. Metab. 1966, 26, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Alikasifoglu, A.; Gonc, E.N.; Yalcin, E.; Dogru, D.; Yordam, N. Neonatal Hyperparathyroidism Due to Maternal Hypoparathyroidism and Vitamin D Deficiency: A Cause of Multiple Bone Fractures. Clin. Pediatr. 2005, 44, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Demirel, N.; Aydin, M.; Zenciroglu, A.; Okumus, N.; Çetinkaya, S.; Yıldız, Y.T.; Ipek, M.S.; Yildiz, Y.T. Hyperparathyroidism secondary to maternal hypoparathyroidism and vitamin D deficiency: An uncommon cause of neonatal respiratory distress. Ann. Trop. Paediatr. 2009, 29, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Richa, C.G.; Issa, A.I.; Echtay, A.S.; El Rawas, M.S. Idiopathic Hypoparathyroidism and Severe Hypocalcemia in Pregnancy. Case Rep. Endocrinol. 2018, 2018, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.; Miller, S. Successful pregnancies and reduced treatment requirement while breast feeding in a patient with congenital hypoparathyroidism due to homozygous c.68C>A null parathyroid hormone gene mutation. BMJ Case Rep. 2018, 2018, 223811. [Google Scholar] [CrossRef] [PubMed]
- Hatswell, B.; Allan, C.; Teng, J.; Wong, P.; Ebeling, P.; Wallace, E.; Fuller, P.; Milat, F. Management of hypoparathyroidism in pregnancy and lactation—A report of 10 cases. Bone Rep. 2015, 3, 15–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartogsohn, E.A.R.; Khan, A.A.; Kjærsulf, L.U.; Sikjaer, T.; Hussain, S.; Rejnmark, L. Changes in treatment needs of hypoparathyroidism during pregnancy and lactation: A case series. Clin. Endocrinol. 2020, 93, 261–268. [Google Scholar] [CrossRef]
- Khan, A.A.; Clarke, B.; Rejnmark, L.; Brandi, M.L. Management of endocrine disease: Hypoparathyroidism in pregnancy: Review and evidence-based recommendations for management. Eur. J. Endocrinol. 2019, 180, R37–R44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovacs, C.S. Maternal Mineral and Bone Metabolism During Pregnancy, Lactation, and Post-Weaning Recovery. Physiol. Rev. 2016, 96, 449–547. [Google Scholar] [CrossRef] [Green Version]
- Ardawi, M.S.; Nasrat, H.A.; Ba’Aqueel, H.S. Calcium-regulating hormones and parathyroid hormone-related peptide in normal human pregnancy and postpartum: A longitudinal study. Eur. J. Endocrinol. 1997, 137, 402–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovacs, C.S. Physiology of Calcium, Phosphorus, and Bone Metabolism During Pregnancy, Lactation, and Postweaning; Elsevier: Amsterdam, The Netherlands, 2020; pp. 61–73. [Google Scholar]
- Cross, N.A.; Hillman, L.S.; Allen, S.H.; Krause, G.F.; Vieira, N.E. Calcium homeostasis and bone metabolism during pregnancy, lactation, and postweaning: A longitudinal study. Am. J. Clin. Nutr. 1995, 61, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Black, A.J.; Topping, J.; Durham, B.; Farquharson, R.G.; Fraser, W.D. A Detailed Assessment of Alterations in Bone Turnover, Calcium Homeostasis, and Bone Density in Normal Pregnancy. J. Bone Miner. Res. 2010, 15, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Møller, U.K.; Streym, S.; Mosekilde, L.; Heickendorff, L.; Flyvbjerg, A.; Frystyk, J.; Jensen, L.T.; Rejnmark, L. Changes in calcitropic hormones, bone markers and insulin-like growth factor I (IGF-I) during pregnancy and postpartum: A controlled cohort study. Osteoporos. Int. 2013, 24, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, L.D.; Fung, E.B.; Halloran, B.P.; Turnlund, J.R.; Van Loan, M.D.; Cann, C.E.; King, J.C. A longitudinal study of calcium homeostasis during human pregnancy and lactation and after resumption of menses. Am. J. Clin. Nutr. 1998, 67, 693–701. [Google Scholar] [CrossRef] [Green Version]
- Carneiro, R.M.; Prebehalla, L.; Tedesco, M.B.; Sereika, S.M.; Gundberg, C.M.; Stewart, A.F.; Horwitz, M.J. Evaluation of Markers of Bone Turnover During Lactation in African-Americans: A Comparison With Caucasian Lactation. J. Clin. Endocrinol. Metab. 2013, 98, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Bertelloni, S.; Baroncelli, G.I.; Pelletti, A.; Battini, R.; Saggese, G. Parathyroid hormone-related protein in healthy pregnant women. Calcif. Tissue Int. 1994, 54, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Yadav, Y.S.; Goel, M.M.; Singh, U.; Natu, S.M.; Negi, M.P.S. Calcitonin gene- and parathyroid hormone-related peptides in normotensive and preeclamptic pregnancies: A nested case–control study. Arch. Gynecol. Obstet. 2014, 290, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Glerean, M.; Furci, A.; Galich, A.M.; Fama, B.; Plantalech, L. Bone and mineral metabolism in primiparous women and its relationship with breastfeeding: A longitudinal study. Medicina 2010, 70, 227–232. [Google Scholar] [PubMed]
- Al Nozha, O.M.; Malakzadeh-Shirvani, P. Calcium homeostasis in a patient with hypoparathyroidism during pregnancy, lactation and menstruation. J. Taibah Univ. Med. Sci. 2013, 8, 50–53. [Google Scholar] [CrossRef] [Green Version]
- Alalawi, Y.; M’Hiri, I.; Alrob, H.A.; Khan, A. Hypoparathyroidism in Pregnancy, in Hypoparathyroidism: A Clinical Casebook; Cusano, N.E., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 143–153. [Google Scholar]
- Seki, K.; Makimura, N.; Mitsui, C.; Hirata, J.; Nagata, I. Calcium-regulating hormones and osteocalcin levels during pregnancy: A longitudinal study. Am. J. Obstet. Gynecol. 1991, 164, 1248–1252. [Google Scholar] [CrossRef]
- Seely, E.W.; Brown, E.M.; DeMaggio, D.M.; Weldon, D.K.; Graves, S.W. A prospective study of calciotropic hormones in pregnancy and post partum: Reciprocal changes in serum intact parathyroid hormone and 1,25-dihydroxyvitamin D. Am. J. Obstet. Gynecol. 1997, 176, 214–217. [Google Scholar] [CrossRef]
- Kirby, B.J.; Ma, Y.; Martin, H.M.; Favaro, K.L.B.; Karaplis, A.C.; Kovacs, C.S. Upregulation of calcitriol during pregnancy and skeletal recovery after lactation do not require parathyroid hormone. J. Bone Miner. Res. 2013, 28, 1987–2000. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.C.; Levine, M.A. Perinatal calcium metabolism: Physiology and pathophysiology. Semin. Neonatol. 2004, 9, 23–36. [Google Scholar] [CrossRef]
- Silva, O.L.; Titus-Dillon, P.; Becker, K.L.; Snider, R.H.; Moore, C.F. Increased Serum Calcitonin in Pregnancy. J. Natl. Med. Assoc. 1981, 73, 649–652. [Google Scholar] [PubMed]
- Stevenson, J.; Hillyard, C.; MacIntyre, I.; Cooper, H.; Whitehead, M. A physiological role for calcitonin: Protection of the maternal skeleton. Lancet 1979, 314, 769–770. [Google Scholar] [CrossRef]
- DeLellis, R.A. Pathology and genetics of thyroid carcinoma. J. Surg. Oncol. 2006, 94, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Bucht, E.; Telenius-Berg, M.; Lundell, G.; Sjöberg, H.-E. Immunoextracted calcitonin in milk and plasma from totally thyroidectomized women. Evidence of monomeric calcitonin in plasma during pregnancy and lactation. Eur. J. Endocrinol. 1986, 113, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Sweeney, L.L.; Malabanan, A.O.; Rosen, H. Decreased Calcitriol Requirement During Pregnancy and Lactation with a Window of Increased Requirement Immediately Post Partum. Endocr. Pract. 2010, 16, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, C.S.; Kronenberg, H.M. Maternal-Fetal Calcium and Bone Metabolism During Pregnancy, Puerperium, and Lactation. Endocr. Rev. 1997, 18, 832–872. [Google Scholar] [CrossRef] [Green Version]
- Fomon, S.; Nelson, S. Calcium, phosphorus, magnesium, and sulfur. In Nutrition of Normal Infants; Fomon, S.J., Ed.; Mosby-Year Book: St. Louis, MI, USA, 1993; pp. 192–216. [Google Scholar]
- Ziegler, E.E.; O’Donnell, A.M.; Nelson, S.E.; Fomon, S.J. Body composition of the reference fetus. Growth 1976, 40, 329–341. [Google Scholar]
- Specker, B.L.; Binkley, T. High parity is associated with increased bone size and strength. Osteoporos. Int. 2005, 16, 1969–1974. [Google Scholar] [CrossRef] [PubMed]
- More, C.; Bettembuk, P.; Bhattoa, H.P.; Balogh, A. The Effects of Pregnancy and Lactation on Bone Mineral Density. Osteoporos. Int. 2001, 12, 732–737. [Google Scholar] [CrossRef]
- Carneiro, R.M.; Prebehalla, L.; Tedesco, M.B.; Sereika, S.M.; Hugo, M.; Hollis, B.W.; Gundberg, C.M.; Stewart, A.F.; Horwitz, M.J. Lactation and Bone Turnover: A Conundrum of Marked Bone Loss in the Setting of Coupled Bone Turnover. J. Clin. Endocrinol. Metab. 2010, 95, 1767–1776. [Google Scholar] [CrossRef]
- Brembeck, P.; Lorentzon, M.; Ohlsson, C.; Winkvist, A.; Augustin, H. Changes in Cortical Volumetric Bone Mineral Density and Thickness, and Trabecular Thickness in Lactating Women Postpartum. J. Clin. Endocrinol. Metab. 2015, 100, 535–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjørnerem, A.; Ghasem-Zadeh, A.; Wang, X.; Bui, M.; Walker, S.P.; Zebaze, R.; Seeman, E. Irreversible Deterioration of Cortical and Trabecular Microstructure Associated with Breastfeeding. J. Bone Miner. Res. 2017, 32, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, C.S.; Chakhtoura, M.; Fuleihan, G.E.-H. Disorders of Mineral and Bone Metabolism During Pregnancy and Lactation; Elsevier: Amsterdam, The Netherlands, 2020; pp. 329–370. [Google Scholar]
- Khan, A.A.; Kenshole, A.; Ezzat, S.; Goguen, J.; Gomez-Hernandez, K.; Hegele, R.A.; Houlden, R.; Joy, T.; Keely, E.; Killinger, D.; et al. Tools for Enhancement and Quality Improvement of Peer Assessment and Clinical Care in Endocrinology and Metabolism. J. Clin. Densitom. 2019, 22, 125–149. [Google Scholar] [CrossRef] [PubMed]
- Stremmel, W.; Merle, U.; Weiskirchen, R. Clinical features of Wilson disease. Ann. Transl. Med. 2019, 7, S61. [Google Scholar] [CrossRef]
- Maggadottir, S.M.; Sullivan, K.E. The Diverse Clinical Features of Chromosome 22q11.2 Deletion Syndrome (DiGeorge Syndrome). J. Allergy Clin. Immunol. Pract. 2013, 1, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, G.; Spada, A. Mutations in the Gs alpha gene causing hormone resistance. Best Pract. Res. Clin. Endocrinol. Metab. 2006, 20, 501–513. [Google Scholar] [CrossRef]
- Malfatti, E.; Laforêt, P.; Jardel, C.; Stojkovic, T.; Behin, A.; Eymard, B.; Lombes, A.; Benmalek, A.; Becane, H.-M.; Berber, N.; et al. High risk of severe cardiac adverse events in patients with mitochondrial m.3243A>G mutation. Neurology 2013, 80, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Morten, K.; Cooper, J.; Brown, G.; Lake, B.; Pike, D.; Poulton, J. A new point mutation associated with mitochondrial encephalomyopathy. Hum. Mol. Genet. 1993, 2, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.J.; Raygada, M.; Rennert, O.M. Barakat syndrome revisited. Am. J. Med. Genet. Part A 2018, 176, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Hujoel, I.A. The association between serum calcium levels and Chvostek sign: A population-based study. Neurol. Clin. Pract. 2016, 6, 321–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jesus, J.E.; Landry, A. Chvostek’s and Trousseau’s Signs. N. Engl. J. Med. 2012, 367, e15. [Google Scholar] [CrossRef] [PubMed]
- Shoback, D. Hypoparathyroidism. N. Engl. J. Med. 2008, 359, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Karaca, Z.; Tanriverdi, F.; Unluhizarci, K.; Kelestimur, F. Pregnancy and pituitary disorders. Eur. J. Endocrinol. 2010, 162, 453–475. [Google Scholar] [CrossRef] [Green Version]
- Nolten, W.E.; Lindheimer, M.D.; Rueckert, P.A.; Oparil, S.; Ehrlich, E.N. Diurnal Patterns and Regulation of Cortisol Secretion in Pregnancy. J. Clin. Endocrinol. Metab. 1980, 51, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.M.; McGarrigle, H.H.G.; Lachelin, G.C.L. The Increase in Plasma and Saliva Cortisol Levels in Pregnancy is not due to the Increase in Corticosteroid-Binding Globulin Levels. J. Clin. Endocrinol. Metab. 1990, 71, 639–644. [Google Scholar] [CrossRef] [Green Version]
- Foyouzi, N.; Frisbaek, Y.; Norwitz, E.R. Pituitary gland and pregnancy. Obstet. Gynecol. Clin. N. Am. 2004, 31, 873–892. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeier, L.; Marcus, R. Calcium Disorders of Pregnancy. Endocrinol. Metab. Clin. N. Am. 1995, 24, 15–39. [Google Scholar] [CrossRef]
- Glass, E.J.; Barr, D.G. Transient neonatal hyperparathyroidism secondary to maternal pseudohypoparathyroidism. Arch. Dis. Child. 1981, 56, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Loughead, J.L.; Mughal, Z.; Mimouni, F.; Tsang, R.C.; Oestreich, A.E. Spectrum and Natural History of Congenital Hyperparathyroidism Secondary to Maternal Hypocalcemia. Am. J. Perinatol. 1990, 7, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Stuart, C.; Aceto, T.; Kuhn, J.P.; Terplan, K. Intrauterine Hyperparathyroidism. Am. J. Dis. Child. 1979, 133, 67. [Google Scholar] [CrossRef] [PubMed]
- Bronsky, D.; Kiamko, R.T.; Moncada, R.; Rosenthal, I.M. Intra-uterine hyperparathyroidism secondary to maternal hypoparathyroidism. Pediatry 1968, 42, 606–613. [Google Scholar]
- Borkenhagen, J.F.; Connor, E.L.; Stafstrom, C.E. Neonatal Hypocalcemic Seizures Due to Excessive Maternal Calcium Ingestion. Pediatr. Neurol. 2013, 48, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Shani, H.; Sivan, E.; Cassif, E.; Simchen, M.J. Maternal hypercalcemia as a possible cause of unexplained fetal polyhydramnion: A case series. Am. J. Obstet. Gynecol. 2008, 199, 410.e1–410.e5. [Google Scholar] [CrossRef] [PubMed]
- Mestman, J.H. Parathyroid Disorders of Pregnancy. Semin. Perinatol. 1998, 22, 485–496. [Google Scholar] [CrossRef]
- Eastell, R.; Edmonds, C.J.; De Chayal, R.C.; McFadyen, I.R. Prolonged hypoparathyroidism presenting eventually as second trimester abortion. BMJ 1985, 291, 955–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almas, T.; Ullah, I.; Kaneez, M.; Ehtesham, M.; Rauf, S. Idiopathic Primary Hypoparathyroidism Presenting as Focal Seizures in a Neonate: A Rare Occurrence. Cureus 2020, 12, 10348. [Google Scholar] [CrossRef]
- Kaneko, N.; Kaneko, S.; Yamada, S.; Suzuki, K.; Ohyama, Y.; Nakagawa, O.; Tani, N.; Aizawa, Y. Untreated Idiopathic Hypoparathyroidism Associated with Infant Congenital Perinatal Abnormalities. Intern. Med. 1999, 38, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callies, F.; Arlt, W.; Scholz, H.J.; Reincke, M.; Allolio, B. Management of hypoparathyroidism during pregnancy--report of twelve cases. Eur. J. Endocrinol. 1998, 139, 284–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilany, J.; Vered, I.; Cohen, O. The effect of continuous subcutaneous recombinant PTH (1–34) infusion during pregnancy on calcium homeostasis—A case report. Gynecol. Endocrinol. 2013, 29, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Mather, J.K.; Chik, C.L.; Corenblum, B. Maintenance of Serum Calcium by Parathyroid Hormone-Related Peptide During Lactation in a Hypoparathyroid Patient. J. Clin. Endocrinol. Metab. 1999, 84, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Shomali, M.E.; Ross, D.S. Hypercalcemia in a Woman with Hypoparathyroidism Associated with Increased Parathyroid Hormone-Related Protein During Lactation. Endocr. Pract. 1999, 5, 198–200. [Google Scholar] [CrossRef]
- Shah, K.H.; Bhat, S.; Shetty, S.M.; Umakanth, S. Hypoparathyroidism in pregnancy. BMJ Case Rep. 2015, 2015, 2015210228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Postsurgical | Autoimmune Disorders | Genetic Disorders | Radiation Exposure | Mineral Deposition | Infiltration | Magnesium Abnormality | Drugs |
|---|---|---|---|---|---|---|---|
| Most common 75% of cases post-neck surgery for thyroid cancer, laryngeal cancer, multinodular goitre, Grave’s disease, etc. Features: Presence of neck scar | ♦APS1 Major features Mucocutaneous candidiasis HypoPT Adrenal insufficiency Minor features keratitis, AIH, POF, enamel hypoplasia, pneumonitis, nephritis, pancreatitis, enteropathy with chronic diarrhea or constipation, photophobia, periodic fever with rash, functional asplenia, celiac disease, type 1 diabetes, thyroiditis, retinitis, pure red cell aplasia, polyarthritis, and B12 deficiency ♦Isolated | ♦DiGeorge syndrome ♦Barakat syndrome: HypoPT, deafness, renal anomaly (HDR) syndrome ♦Autosomal dominant HypoPT ♦Isolated HypoPT ♦Kenny–Caffey syndrome - Type 1 (Sanjad Sakati syndrome) - Type 2 ♦Mitochondrial disorders - Kearns Sayre syndrome - MTPD - MELAS syndrome | Exposure to ionized radiation (RAI) Features: Post-RAI therapy for thyroid cancer | ♦Copper deposition (e.g., Wilson’s disease) → destruction of parathyroid gland Features: Tremors, ataxia, jaundice, psychosis, depression, and Kayser–Fleischer corneal rings ♦Iron deposition (e.g., hemochromatosis, beta-thalassemia) Features: Chronic transfusion history | Rare ♦Granulomatous disease (e.g., sarcoidosis and amyloidosis) ♦Metastasis to parathyroid gland Features: Constitutional symptoms, weight loss, and night sweats | ♦Magnesium deficiency low Mg+2 → block cAMP in parathyroid cell, causing impaired PTH secretion Mg+2 activates CaSR → low Mg+2 → impair PTH synthesis and release ♦Hypermagnesemia high Mg+2 → inhibit PTH release | Chemotherapy (e.g., L asparaginase) → parathyroid necrosis Features: History of malignancy |
| Genetic Condition | Mode of Inheritance | Clinical Features | Genetic Sequencing |
|---|---|---|---|
| DiGeorge syndrome | AD | Cardiac anomalies: VSD, TOF; neurocognitive abnormalities; immune deficiency: Recurrent infections; palatal defects: Cleft palat; renal anomalies; ocular; skeletal anomalies; hearing loss | Fluorescence in situ hybridization (FISH), TBX1 gene sequencing |
| AD HypoPT ♦ADH type 1 and 2 ♦ADH type 1 with Barter’s syndrome type 5 | AD | ♦Type 1 and 2: Asymptomatic, mild hypocalcemia +/− hypocalciuria ♦Type1 with Barter’s: ↓Ca+2, ↓Mg+2, ↓K+, hypercalciuria | ♦ADH type 1—CaSR gene sequencing ♦ADH type 2—GNA11 gene sequencing ♦ADH 1/Bartter’s type 5—CaSR gene |
| Barakat syndrome HypoPT, deafness, renal anomaly (HDR) syndrome | AD | Sensorineural deafness Renal dysplasia, renal failure, renal agenesis Uterine agenesis (rare) | GATA3 gene sequencing |
| Isolated HypoPT | AR AD XLR | Clinical and biochemical features of HypoPT | ♦AR/AD: PTH or GCM2 gene sequencing ♦ XLR: SOX3 gene sequencing in (males) |
| Kenny–Caffey syndrome ♦Type 1 (Sanjad Sakati syndrome) ♦Type 2 | AR AD | ♦Type 1: Short stature, dysmorphic features, growth retardation, cortical thickening of long bones, small hands and feet ♦Type2: Short stature, cortical thickening of the tubular bones, gracile bone dysplasia | ♦Type 1: TBCE sequencing ♦Type 2: FAM111A sequencing |
| Mitochondrial disorders ♦Kearns Sayre syndrome ♦MTPD ♦MELAS syndrome | AR | ♦Kearns Sayre: Ophthalmoplegia, retinal pigmentation, cardiac conduction defects, bulbar weakness. ♦MTPD: Neuropathy, retinopathy, fatty liver. ♦MELAS: Lactic acidosis, stroke-like symptoms, external ophthalmoplegia, diabetes, hearing loss, early-onset stroke symptoms | ♦Kearns Sayre: MTTL1 gene mutation ♦MTPD: HADHA/HADHB gene mutation ♦MELAS: MTTL1 gene mutation (commonest) |
| Pseudohypoparathyroidism | AD | Short stature, brachydactyly Obesity, round facies +/– mental retardation | GNAS gene sequencing |
| Maternal Serum Calcium | Fetus | Mother |
|---|---|---|
| High Maternal Ca+2 | Hypoparathyroidism, polyhydramnios, and neonatal seizures | Hypercalciuria and kidney stones |
| Low Maternal Ca+2 | Hyperparathyroidism, increased bone resorption, intrauterine fragility fractures, subperiosteal bone resorption, osteitis fibrosa cystica, and respiratory distress | Miscarriage, preterm labor, seizure, and arrhythmia |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, D.S.; Dandurand, K.; Khan, A.A. Hypoparathyroidism in Pregnancy and Lactation: Current Approach to Diagnosis and Management. J. Clin. Med. 2021, 10, 1378. https://doi.org/10.3390/jcm10071378
Ali DS, Dandurand K, Khan AA. Hypoparathyroidism in Pregnancy and Lactation: Current Approach to Diagnosis and Management. Journal of Clinical Medicine. 2021; 10(7):1378. https://doi.org/10.3390/jcm10071378
Chicago/Turabian StyleAli, Dalal S., Karel Dandurand, and Aliya A. Khan. 2021. "Hypoparathyroidism in Pregnancy and Lactation: Current Approach to Diagnosis and Management" Journal of Clinical Medicine 10, no. 7: 1378. https://doi.org/10.3390/jcm10071378






