Prevalence of QTc Prolongation in Patients with Parkinson’s Disease. Assessment of the Effects of Drugs, Clinical Risk Factors and Used Correction Formula
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population and QTc Assessment
- QTcB = QT/RR1/2
- QTcF = QT + 0.154(1−RR).
2.2. Statistical Analysis
2.3. Ethical Considerations
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef]
- Cersosimo, M.G.; Benarroch, E.E. Autonomic involvement in Parkinson’s disease: Pathology, pathophysiology, clinical features and possible peripheral biomarkers. J. Neurol. Sci. 2012, 313, 57–63. [Google Scholar] [CrossRef]
- Scorza, F.A.; Fiorini, A.C.; Scorza, C.A.; Finsterer, J. Cardiac abnormalities in Parkinson’s disease and Parkinsonism. J. Clin. Neurosci. 2018, 53, 1–5. [Google Scholar] [CrossRef]
- Piqueras-Flores, J.; López-García, A.; Moreno-Reig, Á.; González-Martínez, A.; Hernández-González, A.; Vaamonde-Gamo, J.; Jurado-Román, A. Structural and functional alterations of the heart in Parkinson’s disease. Neurol. Res. 2017, 40, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, K.; Sasaki, I.; Tsukaguchi, M.; Kamoda, M.; Touge, T.; Takeuchi, H.; Kuriyama, S. Abnormalities of rate-corrected QT intervals in Parkinson’s disease—a comparison with multiple system atrophy and progressive supranuclear palsy. J. Neurol. Sci. 2002, 199, 31–37. [Google Scholar] [CrossRef]
- Oka, H.; Mochio, S.; Sato, H.; Katayama, K. Prolongation of QTc Interval in Patients with Parkinson’s Disease. Eur. Neurol. 1997, 37, 186–189. [Google Scholar] [CrossRef]
- Türk, A.Ş.; Köksal, A.; Altiokka, Ö.; Karademir, F.; Dirican, A.C.; Altunkaynak, Y.; Yazar, T.; Baybaş, S. Assessment of autonomic dysfunction in Parkinson patients by electrocardiogram. Dusunen Adam. 2012, 25, 147–150. [Google Scholar] [CrossRef] [Green Version]
- Cunnington, A.-L.; Hood, K.; White, L. Outcomes of screening Parkinson’s patients for QTc prolongation. Park. Relat. Disord. 2013, 19, 1000–1003. [Google Scholar] [CrossRef]
- Malek, N.M.; Grosset, K.A.; Stewart, D.; Macphee, G.J.; Grosset, D.G. Prescription of drugs with potential adverse effects on cardiac conduction in Parkinson’s disease. Park. Relat. Disord. 2013, 19, 586–589. [Google Scholar] [CrossRef]
- Heranval, A.; Lefaucheur, R.; Fetter, D.; Rouillé, A.; Le Goff, F.; Maltête, D. Drugs with potential cardiac adverse effects: Retrospective study in a large cohort of parkinsonian patients. Rev. Neurol. 2016, 172, 318–323. [Google Scholar] [CrossRef]
- Gibbons, C.H.; Simon, D.K.; Huang, M.; Tilley, B.; Aminoff, M.J.; Bainbridge, J.L.; Brodsky, M.; Freeman, R.; Goudreau, J.; Hamill, R.W.; et al. Autonomic and electrocardiographic findings in Parkinson’s disease. Auton. Neurosci. 2017, 205, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Rautaharju, P.M.; Surawicz, B.; Gettes, L.S. AHA/ACCF/HRS Recommendations for the Standardization and Interpretation of the Electrocardiogram. Part IV: The ST Segment, T and U Waves, and the QT Interval A Scientific Statement From the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J. Am. Coll. Cardiol. 2009, 53, 982–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenberk, B.; Vandael, E.; Robyns, T.; Vandenberghe, J.; Garweg, C.; Foulon, V.; Ector, J.; Willems, R. Which QT Correction Formulae to Use for QT Monitoring? J. Am. Hear. Assoc. 2016, 5, e003264. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D.; Stern, M.B.; Poewe, W.; Olanow, C.W.; Oertel, W.H.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- Woosley, R.L.; Heise, C.W.; Romero, K.A. QTdrugs List; AZCERT, Inc.: Valley, AZ, USA, 2020; Available online: www.Crediblemeds.org (accessed on 29 March 2020).
- Baranowski, R.; Wojciechowski, D.; Kozłowski, D.; Kukla, P.; Kurpesa, M.; Lelakowski, J.; Maciejewska, M.; Sredniawa, B.; Wranicz, J.K. Electrocardiographic criteria for diagnosis of the heart chamber enlargement, necrosis and repolarisation abnormalities including acute coronary syndromes. Experts’ group statement of the Working Group on Noninvasive Electrocardiology and Telemedicine of. Kardiologia Polska 2016, 74, 812–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [Green Version]
- Trinkley, K.E.; Page, R.L.; Lien, H.; Yamanouye, K.; Tisdale, J.E. QT interval prolongation and the risk of torsades de pointes: Essentials for clinicians. Curr. Med Res. Opin. 2013, 29, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Malik, M. Problems of heart rate correction in assessment of drug-induced QT interval prolongation. J. Cardiovasc. Electrophysiol. 2001, 12, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Michler, K.; Johnston, P.; Macfarlane, P.W. A comparison of commonly used QT correction formulae: The effect of heart rate on the QTc of normal ECGs. J. Electrocardiol. 2004, 37, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Montanez, A.; Ruskin, J.N.; Hebert, P.R.; Lamas, G.A.; Hennekens, C.H. Prolonged QTc Interval and Risks of Total and Cardiovascular Mortality and Sudden Death in the General Population. Arch. Intern. Med. 2004, 164, 943–948. [Google Scholar] [CrossRef] [Green Version]
- García, P.A.; Martín, J.J.A.; Abad, C.G.; Hernández, R.M.J.; Ruigómez, A.C.; Calle, P.T.; Varela, C.C.; Antolín, J.S.; Muñiz, J.; Doblas, J.J.G.; et al. Prevalencia de patrones electrocardiográficos asociados a muerte súbita en la población española de 40 años o más. Resultados del estudio OFRECE. Revista Española de Cardiología 2017, 70, 801–807. [Google Scholar] [CrossRef]
- Ma, Q.; Li, Z.; Guo, X.; Guo, L.; Yu, S.; Yang, H.; Zou, L.; Zheng, L.; Pan, G.; Zhang, Y.; et al. Prevalence and risk factors of prolonged corrected QT interval in general Chinese population. BMC Cardiovasc. Disord. 2019, 19, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Benoit, S.R.; Mendelsohn, A.B.; Nourjah, P.; Staffa, J.A.; Graham, D.J. Risk factors for prolonged QTc among US adults: Third National Health and Nutrition Examination Survey. Eur. J. Cardiovasc. Prev. Rehabilitation 2005, 12, 363–368. [Google Scholar] [CrossRef]
- Rabkin, S.W.; Cheng, X.-B.J.; Thompson, D.J. Detailed analysis of the impact of age on the QT interval. J. Geriatr. Cardiol. 2016, 13, 740–748. [Google Scholar]
- Heemskerk, C.P.M.; Pereboom, M.; Van Stralen, K.; Berger, F.A.; Bemt, P.M.L.A.V.D.; Kuijper, A.F.M.; Van Der Hoeven, R.T.M.; Mantel-Teeuwisse, A.K.; Becker, M.L. Risk factors for QTc interval prolongation. Eur. J. Clin. Pharmacol. 2018, 74, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Mochizuki, H.; Ebihara, Y.; Shiomi, K.; Nakazato, M. Body mass index and severity of parkinsonism in multiple system atrophy. Neurology International 2017, 9, 7276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Pablo-Fernandez, E.; Tur, C.; Revesz, T.; Lees, A.J.; Holton, J.L.; Warner, T.T. Association of Autonomic Dysfunction With Disease Progression and Survival in Parkinson Disease. JAMA Neurol. 2017, 74, 970–976. [Google Scholar] [CrossRef]
- Szewczyk-Krolikowski, K.; Tomlinson, P.; Nithi, K.; Wade-Martins, R.; Talbot, K.; Ben-Shlomo, Y.; Hu, M.T. The influence of age and gender on motor and non-motor features of early Parkinson’s disease: Initial findings from the Oxford Parkinson Disease Center (OPDC) discovery cohort. Park. Relat. Disord. 2014, 20, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, H.; Ebihara, Y.; Ugawa, Y.; Ishii, N.; Taniguchi, A.; Nagamachi, S.; Shiomi, K.; Nakazato, M. PR prolongation and cardiac 123I-MIBG uptake reduction in Parkinson’s disease. Eur. Neurol. 2015, 74, 107–111. [Google Scholar] [CrossRef]
- Renoux, C.; Dell’Aniello, S.; Khairy, P.; Marras, C.; Bugden, S.; Turin, T.C.; Blais, L.; Tamim, H.; Evans, C.; Steele, R. Ventricular tachyarrhythmia and sudden cardiac death with domperidone use in Parkinson’s disease. Br. J. Clin. Pharmacol. 2016, 461–472. [Google Scholar] [CrossRef] [Green Version]
- Klietz, M.; Tulke, A.; Müschen, L.H.; Paracka, L.; Schrader, C.; Dressler, D.W.; Wegner, F. Impaired Quality of Life and Need for Palliative Care in a German Cohort of Advanced Parkinson’s Disease Patients. Front. Neurol. 2018, 9, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klietz, M.; Greten, S.; Wegner, F.; Höglinger, G.U. Safety and Tolerability of Pharmacotherapies for Parkinson’s Disease in Geriatric Patients. Drugs Aging 2019, 36, 511–530. [Google Scholar] [CrossRef] [PubMed]
- Greten, S.; Müller-Funogea, J.I.; Wegner, F.; Höglinger, G.U.; Simon, N.; Junius-Walker, U.; Gerbel, S.; Krause, O.; Klietz, M. Drug safety profiles in geriatric patients with Parkinson’s disease using the FORTA (Fit fOR The Aged) classification: Results from a mono-centric retrospective analysis. J. Neural Transm. 2021, 128, 49–60. [Google Scholar] [CrossRef] [PubMed]
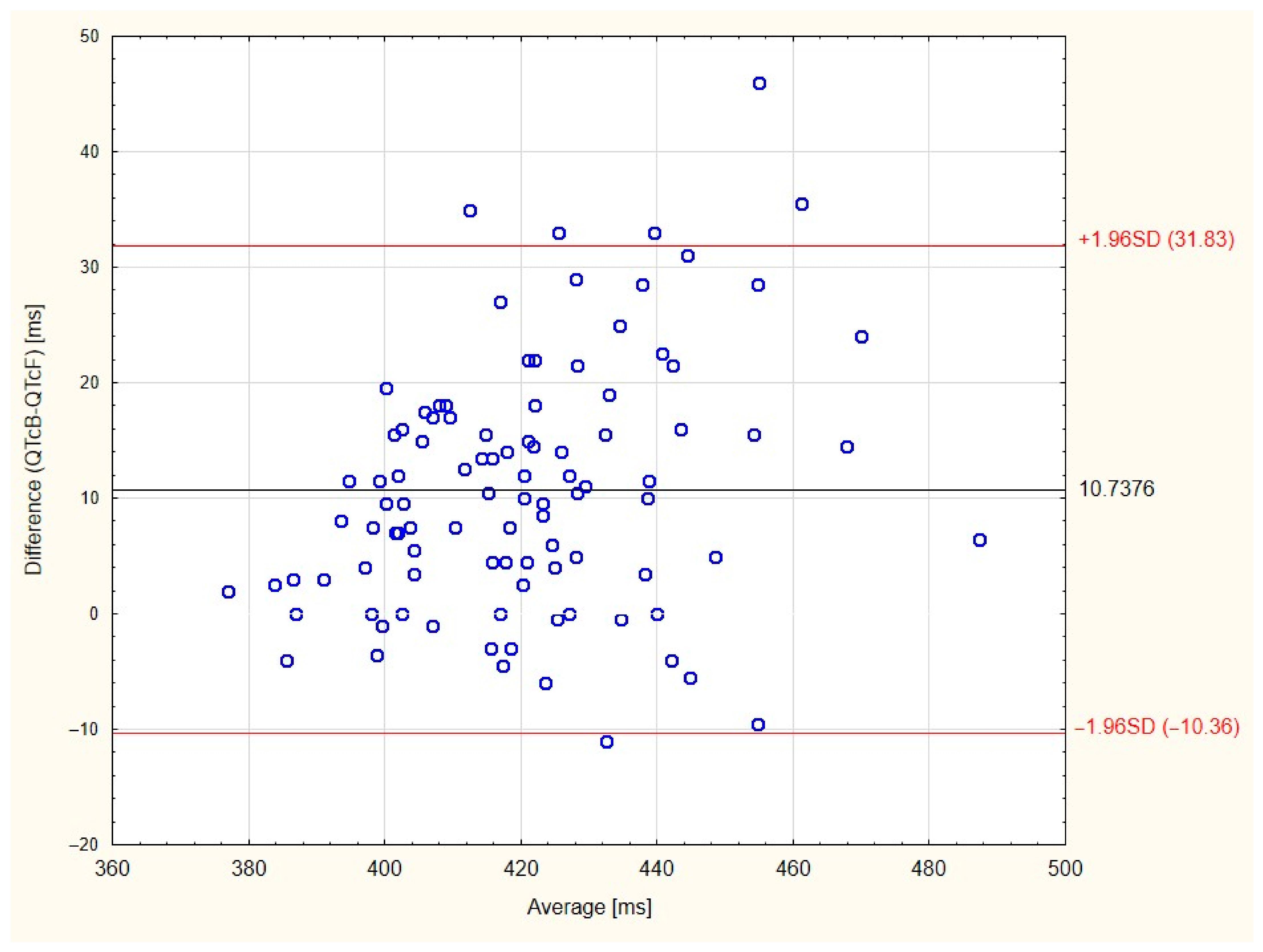
| Patients’ Characteristics | Patients n = 101 |
| Males | 65 (64%) |
| Age | 64.5 ± 8.1 years |
| PD duration | 9.3 ± 4.8 years |
| UPDRS ON | 13 (8–19) |
| UPDRS OFF | 37.9 ± 14.8 |
| Hoehn-Yahr scale ≥3 | 62 (61%) |
| Hypertension | 45 (45%) |
| Heart diseases * | 16 (16%) |
| Diabetes mellitus | 8 (8%) |
| Heart rate | 71 ± 10.7 |
| On QTc-prolonging drugs | 56 (55%) |
| Long QTcB | 13 (13%) |
| Long QTcF | 4 (4%) |
| Drugs According to Category 1 | ||||
|---|---|---|---|---|
| Known | Possible | Conditional | Mean Dose | |
| Psychiatric | 7 (8%) | 8 (10%) | 18 (22%) | |
| Escitalopram | 6 (7%) | 21.6 mg | ||
| Quetiapine | 5 (6%) | 35 mg | ||
| Paroxetine | 4 (5%) | 20 mg | ||
| Trazodone | 4 (5%) | 100 mg | ||
| Sertraline | 4 (5%) | 50 mg | ||
| Mianserin | 4 (5%) | 15 mg | ||
| Venlafaxine | 2 (2%) | 112.5 mg | ||
| Citalopram | 1 (1%) | 5 mg | ||
| Mirtazapine | 1 (1%) | 30 mg | ||
| Clozapine | 1 (1%) | 25 mg | ||
| Olanzapine | 1 (1%) | 5 mg | ||
| Neurological | 3 (4%) | 1 (1%) | 20 (24%) | |
| Donepzeil | 3 (4%) | 10 mg | ||
| Memantine | 1 (1%) | 10 mg | ||
| Amantadine | 20 (24%) | 235 mg | ||
| Cardiac | 1 (1%) | 2 (2%) | 6 (7%) | |
| Sotalol | 1 (1%) | 160 mg | ||
| Hydrochlorothiazide | 2 (2%) | 18.8 mg | ||
| Indapamide | 6 (7%) | 1.7 mg | ||
| Urological | 0 | 2 (2%) | 2 (2%) | |
| Tolterodine | 2 (2%) | 2 mg | ||
| Solifenacin | 2 (2%) | 5 mg | ||
| Gastrointestinal | 0 | 0 | 13 (16%) | |
| Pantoprazole | 10 | 22 mg | ||
| Omeprazole | 2 (2%) | 20 mg | ||
| Lansoprazole | 1 (1%) | 30 mg | ||
| Sum = 83 (100%) | 11 (13%) | 13 (16%) | 59 (71%) | |
| Sex, Male = 1 | Age (Years) | Drugs (mg/d) | Diabetes | Hypertension | Heart Disease | HYs | UPDRS ON | UPDRS OFF | PD Duration (Years) | QTcB (ms) | QTcF (ms) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 70 | Sotalol 160 Indapamide 1.5 | 1 | 1 | 0 | 3 | 19 | 49 | 10 | 450 | 460 |
| 1 | 73 | Quetiapine 25 | 0 | 0 | 0 | 2 | 5 | 27 | 7 | 482 | 458 |
| 1 | 70 | Sertraline 50 | 0 | 0 | 1 | 3 | 5 | 24 | 11 | 491 | 484 |
| 1 | 68 | Pantoprazole 20 Trazodone 100 | 0 | 1 | 1 | 4 | 24 | 56 | 13 | 475 | 461 |
| 1 | 59 | Pantoprazole 20 Paroxetine 20 | 0 | 1 | 0 | 3 | 16 | 43 | 18 | 479 | 444 |
| 1 | 69 | Sertraline 50 | 0 | 1 | 1 | 5 | 37 | 58 | 8 | 478 | 432 |
| 1 | 66 | Tolteradine 2 Amantadine 200 | 0 | 1 | 0 | 3 | 12 | 47 | 11 | 453 | 432 |
| 1 | 64 | Paroxetine 20 Trazodone 150 | 0 | 1 | 0 | 3 | 19 | 34 | 17 | 462 | 447 |
| 1 | 60 | Tolteradine 2 | 0 | 0 | 0 | 5 | 31 | 68 | 6 | 452 | 436 |
| 1 | 55 | - | 0 | 1 | 0 | 3 | 15 | 54 | 10 | 452 | 430 |
| 1 | 79 | Memantine 10 Pantoprazole 20 | 0 | 0 | 1 | 3 | 16 | 30 | 10 | 456 | 423 |
| 1 | 66 | - | 0 | 0 | 0 | 2 | 4 | 12 | 2 | 460 | 429 |
| 0 | 71 | - | 0 | 1 | 0 | 4 | 25 | 57 | 15 | 469 | 441 |
| QTcB | QTcF | |||
|---|---|---|---|---|
| Assessed Factor | Comparisons and Correlations | p | Comparisons and Correlations | p |
| Male vs. female | 427.0 ± 24.5 vs. 422.7 ± 17.9 | 0.312 | 417.7 ± 20.6 vs. 409.5 ± 15.1 | 0.026 * |
| With vs. without QTc-prolonging drugs | 429.2 ± 24.3 vs. 420.9 ± 19.0 | 0.062 | 417.7 ± 21.3 vs. 411,1 ± 15.7 | 0.073 |
| With vs. without hypertension | 427.7 ± 23.9 vs. 423.8 ± 21.1 | 0.384 | 418.4 ± 19.3 vs. 411.9 ±18.8 | 0.092 |
| With vs. without heart diseases | 439.1 ± 24.2 vs. 423.0 ± 21.2 | 0.008 * | 426.3 ± 21.8 vs. 412.6 ± 18.0 | 0.008 * |
| With vs. without diabetes | 432 (414–434) vs. 423 (408–440) | 0.791 | 415 (400–425) vs. 407 (398–428) | 0.673 |
| HYs ≥ 3 vs. HYs < 3 | 428.3 ± 23.0 vs. 421.0 ± 20.8 | 0.109 | 416.0 ± 19.6 vs. 412.7 ± 18.6 | 0.396 |
| UPDRS ON | RS = 0.13 | 0.185 | RS = 0.10 | 0.312 |
| UPDRS OFF | RP = 0.05 | 0.615 | RP < 0.01 | 0.965 |
| Age | RP = 0.29 | 0.003 * | RP = 032 | 0.001 * |
| PD duration | RP = 0.07 | 0.512 | RP = 0.06 | 0.557 |
| HR | RP = 0.42 | <0.001 * | RP = -0.06 | 0.576 |
| QTcB | QTcF | |||||||
|---|---|---|---|---|---|---|---|---|
| β | β SE | p | rp2 | β | β SE | p | rp2 | |
| Male vs. female | 5.85 | 1.99 | 0.004 | 0.083 | 5.45 | 1.83 | 0.004 | 0.083 |
| QTc-prolonging drugs | 4.32 | 1.86 | 0.022 | 0.053 | 4.00 | 1.75 | 0.025 | 0.051 |
| Age | 0.88 | 0.23 | <0.001 | 0.132 | 0.84 | 0.21 | <0.001 | 0.134 |
| HR | 0.97 | 0.18 | <0.001 | 0.240 | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malkiewicz, J.J.; Malkiewicz, M.; Siuda, J. Prevalence of QTc Prolongation in Patients with Parkinson’s Disease. Assessment of the Effects of Drugs, Clinical Risk Factors and Used Correction Formula. J. Clin. Med. 2021, 10, 1396. https://doi.org/10.3390/jcm10071396
Malkiewicz JJ, Malkiewicz M, Siuda J. Prevalence of QTc Prolongation in Patients with Parkinson’s Disease. Assessment of the Effects of Drugs, Clinical Risk Factors and Used Correction Formula. Journal of Clinical Medicine. 2021; 10(7):1396. https://doi.org/10.3390/jcm10071396
Chicago/Turabian StyleMalkiewicz, Jakub J., Maciej Malkiewicz, and Joanna Siuda. 2021. "Prevalence of QTc Prolongation in Patients with Parkinson’s Disease. Assessment of the Effects of Drugs, Clinical Risk Factors and Used Correction Formula" Journal of Clinical Medicine 10, no. 7: 1396. https://doi.org/10.3390/jcm10071396
APA StyleMalkiewicz, J. J., Malkiewicz, M., & Siuda, J. (2021). Prevalence of QTc Prolongation in Patients with Parkinson’s Disease. Assessment of the Effects of Drugs, Clinical Risk Factors and Used Correction Formula. Journal of Clinical Medicine, 10(7), 1396. https://doi.org/10.3390/jcm10071396






