Longitudinal Patterns in Antithrombotic Therapy in Patients with Atrial Fibrillation after Percutaneous Coronary Intervention in the Non-Vitamin K Oral Anticoagulant Era: A Nationwide Population-Based Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Antithrombotic Therapies
2.3. Clinical Risk Factors
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
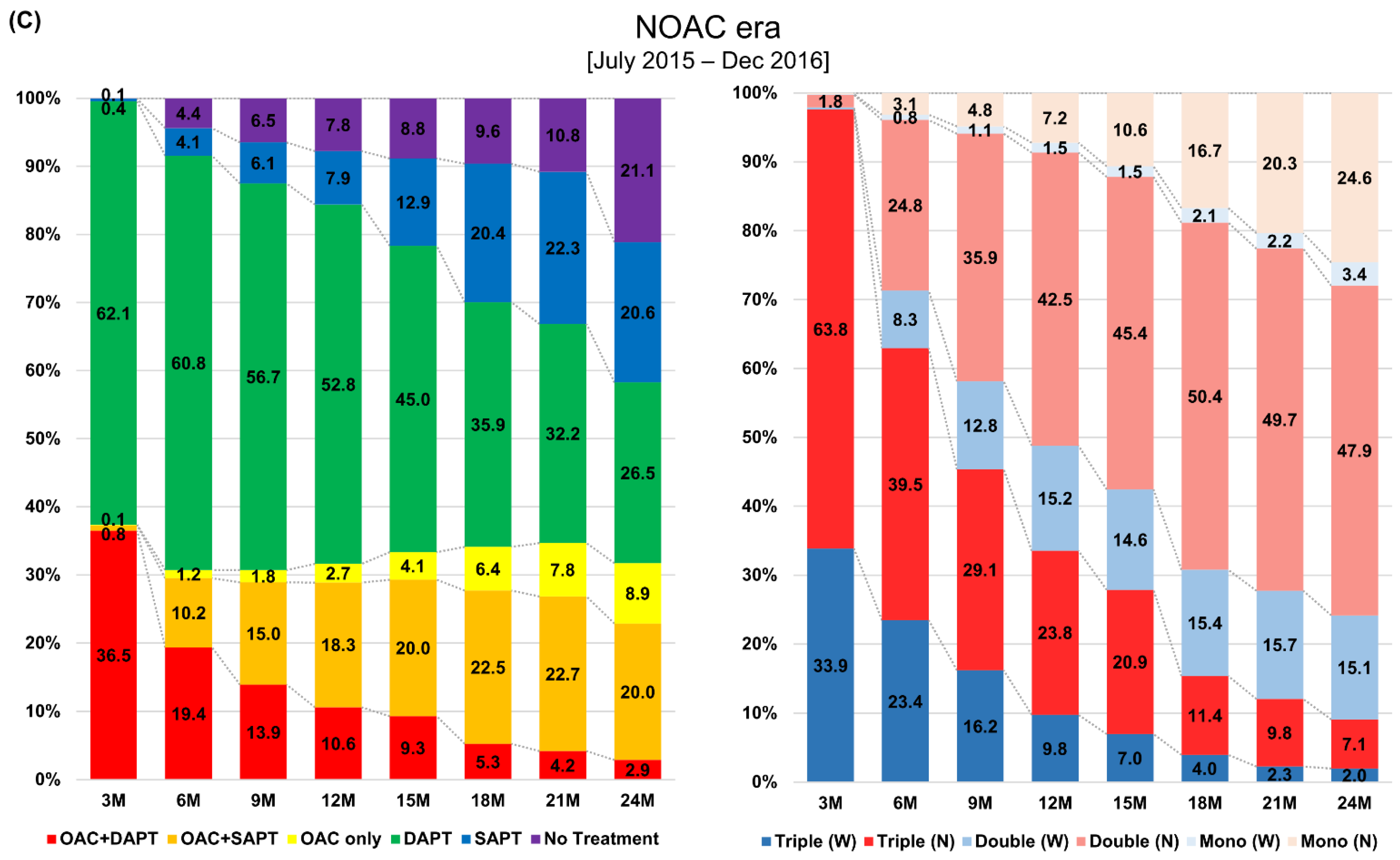
3.2. Two-Year Prescription Patterns in Antithrombotic Therapy after Percutaneous Coronary Intervention
3.3. Clinical Factors Associated with Oral Anticoagulants Use 1 Year after Percutaneous Coronary Intervention
3.4. Clinical Factors Associated with a Preference for Oral Anticoagulant Monotherapy 1 Year after Percutaneous Coronary Intervention
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lip, G.Y.; Banerjee, A.; Boriani, G.; Chiang, C.E.; Fargo, R.; Freedman, B.; Lane, D.A.; Ruff, C.T.; Turakhia, M.; Werring, D.; et al. Antithrombotic Therapy for Atrial Fibrillation. Chest 2018, 154, 1121–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lip, G.Y.H.; Collet, J.P.; Haude, M.; Byrne, R.; Chung, E.H.; Fauchier, L.; Halvorsen, S.; Lau, D.; Lopez-Cabanillas, N.; Lettino, M.; et al. 2018 Joint European consensus document on the management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous cardiovascular interventions: A joint consensus document of the European Heart Rhythm Associ-ation (EHRA), European Society of Cardiology Working Group on Thrombosis, European Association of Percutaneous Cardiovascular Interventions (EAPCI), and European Association of Acute Cardiac Care (AC-CA) endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), Latin America Heart Rhythm Society (LAHRS), and Cardiac Arrhythmia Society of Southern Africa (CASSA). Europace 2019, 21, 192–193. [Google Scholar]
- Lamberts, M.; Olesen, J.B.; Ruwald, M.H.; Hansen, C.M.; Karasoy, D.; Kristensen, S.L.; Køber, L.; Torp-Pedersen, C.; Gislason, G.H.; Hansen, M.L. Response to letter regarding article, “Bleeding after initiation of multiple antithrombotic drugs, including triple therapy, in atrial fibrillation patients following myocardial infarction and coronary intervention: A nationwide cohort study”. Circulation 2013, 127, e585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Choi, E.-K.; Han, K.-D.; Choi, Y.-J.; Lee, E.; Choe, W.; Lee, S.-R.; Cha, M.-J.; Lim, W.-H.; Kang, J.; et al. Temporal trends in prevalence and antithrombotic treatment among Asians with atrial fibrillation undergoing percutaneous coronary intervention: A nationwide Korean population-based study. PLoS ONE 2019, 14, e0209593. [Google Scholar] [CrossRef]
- Park, J.; Choi, E.-K.; Han, K.-D.; Choi, Y.-J.; Lee, S.-R.; Cha, M.-J.; Kang, J.; Park, K.W.; Oh, S.; Lip, G.Y. Antithrombotic Therapy in Patients with Atrial Fibrillation After Percutaneous Coronary Intervention During 2-Year Follow-Up, from a Nationwide Population Study. Am. J. Cardiol. 2019, 123, 1921–1926. [Google Scholar] [CrossRef]
- Choi, E.-K. Cardiovascular Research Using the Korean National Health Information Database. Korean Circ. J. 2020, 50, 754–772. [Google Scholar] [CrossRef]
- Gibson, C.M.; Mehran, R.; Bode, C.; Halperin, J.; Verheugt, F.W.; Wildgoose, P.; Birmingham, M.; Ianus, J.; Burton, P.; van Eickels, M.; et al. Prevention of Bleeding in Patients with Atrial Fibrillation Undergoing PCI. N. Engl. J. Med. 2016, 375, 2423–2434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannon, C.P.; Bhatt, D.L.; Oldgren, J.; Lip, G.Y.; Ellis, S.G.; Kimura, T.; Maeng, M.; Merkely, B.; Zeymer, U.; Gropper, S.; et al. Dual Antithrombotic Therapy with Dabigatran after PCI in Atrial Fibrillation. N. Engl. J. Med. 2017, 377, 1513–1524. [Google Scholar] [CrossRef]
- Lopes, R.D.; Heizer, G.; Aronson, R.; Vora, A.N.; Massaro, T.; Mehran, R.; Goodman, S.G.; Windecker, S.; Darius, H.; Li, J.; et al. Antithrombotic Therapy after Acute Coronary Syndrome or PCI in Atrial Fibrillation. N. Engl. J. Med. 2019, 380, 1509–1524. [Google Scholar] [CrossRef] [Green Version]
- Vranckx, P.; Valgimigli, M.; Eckardt, L.; Tijssen, J.; Lewalter, T.; Gargiulo, G.; Batushkin, V.; Campo, G.; Lysak, Z.; Vakaliuk, I.; et al. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): A randomised, open-label, phase 3b trial. Lancet 2019, 394, 1335–1343. [Google Scholar] [CrossRef]
- Kim, H.K.; Tantry, U.S.; Smith, S.C., Jr.; Jeong, M.H.; Park, S.J.; Kim, M.H.; Lim, D.S.; Shin, E.S.; Park, D.W.; Huo, Y.; et al. The East Asian Paradox: An Updated Position Statement on the Challenges to the Current Antithrombotic Strategy in Patients with Cardiovascular Disease. Thromb. Haemost. 2020, 10. in press. [Google Scholar] [CrossRef]
- Lee, S.-R.; Choi, E.-K.; Han, K.-D.; Cha, M.-J.; Oh, S.; Lip, G.Y.H. Temporal trends of antithrombotic therapy for stroke prevention in Korean patients with non-valvular atrial fibrillation in the era of non-vitamin K antagonist oral anticoagulants: A nationwide population-based study. PLoS ONE 2017, 12, e0189495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.-L.; Giugliano, R.P.; Goto, S.; Chiu, C.-C.; Lin, C.-Y.; Lai, E.-Y.; Chiang, C.-E. Standard dose versus low dose non–vitamin K antagonist oral anticoagulants in Asian patients with atrial fibrillation: A meta-analysis of contemporary randomized controlled trials. Hear. Rhythm. 2016, 13, 2340–2347. [Google Scholar] [CrossRef] [PubMed]
- Rohla, M.; Weiss, T.W.; Pecen, L.; Patti, G.; Siller-Matula, J.M.; Schnabel, R.B.; Schilling, R.; Kotecha, D.; Lucerna, M.; Huber, K.; et al. Risk factors for thromboembolic and bleeding events in anticoagulated patients with atrial fibrillation: The prospective, multicentre observational PREvention oF thromboembolic events—European Registry in Atrial Fibrillation (PREFER in AF). BMJ Open 2019, 9, e022478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisters, R.; Lane, D.A.; Nieuwlaat, R.; de Vos, C.B.; Crijns, H.J.; Lip, G.Y. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The euro heart survey. Chest 2010, 138, 1093–1100. [Google Scholar] [CrossRef] [Green Version]
- Limbruno, U.; De Sensi, F.; Cresti, A.; Picchi, A.; Lena, F.; De Caterina, R. Optimal antithrombotic treatment of patients with atrial fibrillation early after an acute coronary syndrome-triple therapy, dual antithrombotic therapy with an anticoagulant. Or, rather, temporary dual antiplatelet therapy? J. Clin. Med. 2020, 9, 2673. [Google Scholar] [CrossRef]
- Stone, G.W.; Généreux, P.; Harrington, R.A.; White, H.D.; Gibson, C.M.; Steg, P.G.; Hamm, C.W.; Mahaffey, K.W.; Price, M.J.; Prats, J.; et al. Impact of lesion complexity on peri-procedural adverse events and the benefit of potent intravenous platelet adenosine diphosphate receptor inhibition after percutaneous coronary intervention: Core laboratory analysis from 10 854 patients from the CHAMPION PHOENIX trial. Eur. Heart J. 2018, 39, 4112–4121. [Google Scholar] [CrossRef] [Green Version]
- Kerneis, M.; Gibson, C.M.; Chi, G.; Mehran, R.; AlKhalfan, F.; Talib, U.; Pahlavani, S.; Mir, M.; Bode, C.; Halperin, J.L.; et al. Effect of Procedure and Coronary Lesion Characteristics on Clinical Outcomes Among Atrial Fibrillation Patients Undergoing Percutaneous Coronary Intervention: Insights From the PIO-NEER AF-PCI Trial. JACC Cardiovasc. Interv. 2018, 11, 626–634. [Google Scholar] [CrossRef]
- Dewilde, W.J.; Oirbans, T.; Verheugt, F.W.; Kelder, J.C.; De Smet, B.J.; Herrman, J.P.; Adriaenssens, T.; Vrolix, M.; Heestermans, A.A.; Vis, M.M.; et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: An open-label, randomised, controlled trial. Lancet 2013, 381, 1107–1115. [Google Scholar] [CrossRef]
- Potter, B.J.; Ando, G.; Cimmino, G.; Ladeiras-Lopes, R.; Frikah, Z.; Chen, X.Y.; Virga, V.; Goncalves-Almeida, J.; Camm, A.J.; Fox, K.A.A. Time trends in antithrombotic management of patients with atrial fibrillation treated with coronary stents: Results from TALENT-AF (The internAtionaL stENT—Atrial Fibrilla-tion study) multicenter registry. Clin. Cardiol. 2018, 41, 470–475. [Google Scholar] [CrossRef] [Green Version]
- Boivin-Proulx, L.; Deneault-Marchand, A.; Matteau, A.; Mansour, S.; Gobeil, F.; Camm, J.A.; Fox, K.A.A.; Potter, B.J. Time-trends and treatment gaps in the antithrombotic management of patients with atrial fibrillation after percutaneous coronary intervention: Insights from the CHUM AF-STENT Registry. Clin. Cardiol. 2019, 43, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Choi, E.K.; Han, K.D.; Kim, B.; Choi, Y.J.; Lee, S.R.; Kang, J.; Cha, M.J.; Park, K.W.; Oh, S.; et al. Outcomes in relation to antithrombotic therapy among patients with atrial fibrillation after percutaneous coronary intervention. PLoS ONE 2020, 15, e0240161. [Google Scholar] [CrossRef]
- Yasuda, S.; Kaikita, K.; Akao, M.; Ako, J.; Matoba, T.; Nakamura, M.; Miyauchi, K.; Hagiwara, N.; Kimura, K.; Hirayama, A.; et al. Antithrombotic Therapy for Atrial Fibrillation with Stable Coronary Disease. N. Engl. J. Med. 2019, 381, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Matsumura-Nakano, Y.; Shizuta, S.; Komasa, A.; Morimoto, T.; Masuda, H.; Shiomi, H.; Goto, K.; Nakai, K.; Ogawa, H.; Kobori, A.; et al. Open-Label Randomized Trial Comparing Oral Anticoagulation with and Without Single Antiplatelet Therapy in Patients with Atrial Fibrillation and Stable Coronary Artery Dis-ease Beyond 1 Year After Coronary Stent Implantation. Circulation 2019, 139, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-I.; Ahn, J.-M.; Kang, S.H.; Lee, P.H.; Kang, S.-J.; Lee, S.-W.; Kim, Y.-H.; Lee, C.W.; Park, S.-W.; Park, D.-W.; et al. Prevalence, Management, and Long-Term (6-Year) Outcomes of Atrial Fibrillation Among Patients Receiving Drug-Eluting Coronary Stents. JACC Cardiovasc. Interv. 2017, 10, 1075–1085. [Google Scholar] [CrossRef] [PubMed]




| (A) Pre-NOAC Era (n = 5044) | (B) Transition Era (n = 7209) | (C) NOAC Era (n = 6438) | A vs. B | A vs. C | B vs. C | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, years | 71 (63–77) | 72 (63–78) | 72 (63–78) | 0.007 | <0.001 | 0.434 |
| Age groups | <0.001 | <0.001 | 0.105 | |||
| <65 years | 1420 (28.2) | 2056 (28.5) | 1846 (28.7) | |||
| 65–74 years | 1885 (37.4) | 2444 (33.9) | 2080 (32.3) | |||
| ≥75 years | 1739 (34.5) | 2709 (37.6) | 2512 (39.0) | |||
| Women | 1775 (35.2) | 2449 (34.0) | 2167 (33.7) | 0.487 | 0.259 | 0.999 |
| Comorbidities | ||||||
| Diabetes Mellitus | 2041 (40.5) | 2867 (39.8) | 2516 (39.1) | 0.999 | 0.398 | 0.999 |
| Hypertension | 4500 (89.2) | 6503 (90.2) | 5770 (89.6) | 0.223 | 0.999 | 0.777 |
| Dyslipidemia | 4023 (79.8) | 6173 (85.6) | 5626 (87.4) | <0.001 | <0.001 | 0.008 |
| Congestive Heart Failure | 1891 (37.5) | 3064 (42.5) | 3053 (47.4) | <0.001 | <0.001 | <0.001 |
| Myocardial Infarction | 1876 (37.2) | 2895 (40.2) | 2746 (42.7) | 0.003 | <0.001 | 0.009 |
| Peripheral Arterial Disease | 1360 (27.0) | 1946 (27.0) | 1824 (28.3) | 0.999 | 0.312 | 0.243 |
| Stroke/TIA/Systemic Thromboembolism | 897 (17.8) | 1196 (16.6) | 1043 (16.2) | 0.253 | 0.074 | 0.999 |
| Intracranial Hemorrhage | 43 (0.9) | 52 (0.7) | 39 (0.6) | 0.999 | 0.358 | 0.999 |
| Gastrointestinal Bleeding | 443 (8.8) | 580 (8.0) | 458 (7.1) | 0.440 | 0.003 | 0.121 |
| Renal Disease | 863 (17.1) | 1407 (19.5) | 1323 (20.5) | 0.002 | <0.001 | 0.397 |
| Liver Disease | 1816 (36.0) | 2656 (36.8) | 2600 (40.4) | 0.999 | <0.001 | <0.001 |
| CHA2DS2-VAS score | 3 (2–5) | 4 (2–5) | 4 (2–5) | 0.018 | <0.001 | 0.012 |
| 0 | 83 (1.6) | 108 (1.5) | 84 (1.3) | |||
| 1 | 541 (10.7) | 671 (9.3) | 587 (9.1) | |||
| 2 | 946 (18.8) | 1310 (18.2) | 1121 (17.4) | |||
| 3 | 998 (19.8) | 1465 (20.3) | 1206 (18.7) | |||
| 4 | 874 (17.3) | 1244 (17.3) | 1141 (17.7) | |||
| 5 or higher | 1602 (31.8) | 2411 (33.4) | 2299 (35.7) | |||
| Modified HAS-BLED score | 3 (3–4) | 3 (3–4) | 3 (3–4) | 0.645 | 0.024 | 0.363 |
| 1 | 163 (3.2) | 200 (2.8) | 179 (2.8) | |||
| 2 | 827 (16.4) | 1183 (16.4) | 969 (15.1) | |||
| 3 or higher | 4054 (80.4) | 5826 (80.8) | 5290 (82.2) | |||
| Baseline Antithrombotic Therapy after PCI | ||||||
| OACs overall | 1256 (24.9) | 1941 (26.9) | 2263 (35.2) | 0.036 | <0.001 | <0.001 |
| VKAs | 1235 (24.5) | 1872 (26.0) | 826 (12.8) | 0.005 | <0.001 | <0.001 |
| NOACs | 21 (0.4) | 69 (1.0) | 1437 (22.3) | 0.005 | <0.001 | <0.001 |
| Triple therapy | 1228 (24.3) | 1886 (26.2) | 2202 (34.2) | 0.070 | <0.001 | <0.001 |
| VKA-based | 1207 (23.9) | 1819 (25.2) | 817 (12.7) | 0.007 | <0.001 | <0.001 |
| NOAC-based | 21 (0.4) | 67 (0.9) | 1385 (21.5) | 0.007 | <0.001 | <0.001 |
| Double therapy (OACs + SAPT) | 26 (0.5) | 53 (0.7) | 56 (0.9) | 0.404 | 0.076 | 0.999 |
| VKA-based | 26 (0.5) | 52 (0.7) | 8 (0.1) | 0.999 | <0.001 | <0.001 |
| NOAC-based | 0 (0.0) | 1 (0.0) | 48 (0.7) | 0.999 | <0.001 | <0.001 |
| DAPT | 3697 (73.3) | 5147 (71.4) | 4093 (63.6) | 0.063 | <0.001 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Jung, J.-H.; Choi, E.-K.; Lee, S.-W.; Kwon, S.; Lee, S.-R.; Kang, J.; Han, K.-D.; Park, K.-W.; Oh, S.; et al. Longitudinal Patterns in Antithrombotic Therapy in Patients with Atrial Fibrillation after Percutaneous Coronary Intervention in the Non-Vitamin K Oral Anticoagulant Era: A Nationwide Population-Based Study. J. Clin. Med. 2021, 10, 1505. https://doi.org/10.3390/jcm10071505
Park J, Jung J-H, Choi E-K, Lee S-W, Kwon S, Lee S-R, Kang J, Han K-D, Park K-W, Oh S, et al. Longitudinal Patterns in Antithrombotic Therapy in Patients with Atrial Fibrillation after Percutaneous Coronary Intervention in the Non-Vitamin K Oral Anticoagulant Era: A Nationwide Population-Based Study. Journal of Clinical Medicine. 2021; 10(7):1505. https://doi.org/10.3390/jcm10071505
Chicago/Turabian StylePark, Jiesuck, Jin-Hyung Jung, Eue-Keun Choi, Seung-Woo Lee, Soonil Kwon, So-Ryoung Lee, Jeehoon Kang, Kyung-Do Han, Kyung-Woo Park, Seil Oh, and et al. 2021. "Longitudinal Patterns in Antithrombotic Therapy in Patients with Atrial Fibrillation after Percutaneous Coronary Intervention in the Non-Vitamin K Oral Anticoagulant Era: A Nationwide Population-Based Study" Journal of Clinical Medicine 10, no. 7: 1505. https://doi.org/10.3390/jcm10071505








