1. Introduction
Hemorrhoidal disease (HD) is the most frequent benign proctologic condition, which affects a large portion of the world population [
1], with greater incidences in higher socioeconomic status, middle age (45–65 years) [
2,
3] and during pregnancy and postpartum [
4], and with a prevalence ranging between 4.4% in the general population and 36.4% in general practice [
5].
Different symptoms can characterize HD, such as bleeding, prolapse, discomfort, itching, sometimes pain and, more rarely, soiling with hygiene problems.
Conservative approaches are indicated for low-grade HD, which are based on dietary and lifestyle recommendations [
6]. Moreover, a pharmacological therapy with phlebotonics or vasoactive medications is indicated to prevent, relieve, minimize or control acute HD symptoms [
7,
8,
9]. Avoiding or postponing invasive procedures of acute HD could be convenient to plan the most appropriate therapeutic approach [
10].
Many different medications are available to treat and control HD, including analgesics and corticosteroid creams with a local action, or flavonoids and mesoglycan with a systemic action. The latter ones, also called phlebotonics, which act on the capillary and venous complex, are heterogeneous and widely investigated categories of drugs extracted from plants, initially used to treat chronic venous insufficiency and edema [
11].
The role of flavonoids is still debated; however, they have an anti-inflammatory action in addition to affecting the venous tone, capillary permeability and lymphatic drainage [
12,
13,
14,
15].
Recent studies show that flavonoids represent a valid therapeutic alternative both in acute symptomatic HD and in “long-term” hemorrhoid treatment, to bridge patients to surgical treatment in a better condition [
16,
17,
18]. In addition, a potential benefit has been observed in reducing post-hemorrhoidectomy symptoms [
19,
20]. However, the role of flavonoids to improve the clinical condition of patients with III- and IV-degree hemorrhoids (according to the Goligher classification [
21]) awaiting surgery has not been fully understood.
Primary aim of this study was to evaluate the effects of two different phlebotonic preparations based on symptoms reported by patients and local inflammation sign caused by III- and IV-degree HD. The secondary aim was to evaluate patient satisfaction.
2. Materials and Methods
2.1. Study Setting and Approval
This was a prospective, monocentric observational study. The study was conducted between December 2018 and December 2019 and promoted by the Proctology Unit of the Fondazione Policlinico Universitario A. Gemelli IRCCS of Rome, Italy. The local Ethics Committee approved the study (Protocol n°1268–1301). All patients signed a written informed consent.
2.2. Inclusion and Exclusion Criteria
Patients were evaluated according to common clinical practice at our center: assessment of the medical history, including that specifically related to HD (physical examination performed by digital rectal examination and anoscopy). Moreover, all patients received hygienic-behavioral suggestions, and a diet rich in fiber and water was prescribed.
Inclusion criteria were as follows: age between 18 and 70 years, advanced HD (III and IV-degree hemorrhoids according to the Goligher classification [
21]) with indication for surgery, consent to participate in the study and consent to attend all scheduled FU visits. Exclusion criteria were as follows: colorectal or anal cancer; obstructive defecation syndrome; irritable bowel syndrome; inflammatory bowel disease; coagulation disorders; other proctologic diseases, such as anal abscess or fistula; anal fissure or acute hemorrhoidal thrombosis; pregnancy; and anticoagulant or anti-aggregant intake for another disease.
2.3. Data Collection and Treatment
The overall study period was 12 weeks, which was divided as follows: baseline visit (T0), first follow-up visit after 4 weeks of therapy (T1), second follow-up visit 8 weeks after therapy discontinuation (T2) and preoperative.
During each visit, local signs of hemorrhoidal inflammation, such as mucosal dystrophy, dysepithelialization and edema, were assessed by digital rectal examination and anoscopy, and were classified by a 11-point Numeric Rating Scale (NRS—from 0 = perfect condition to 10 = the worst condition). In addition, at each visit, the most typical HD symptoms (e.g., bleeding, prolapse, itching/discomfort and anal pain) were assessed by means of a self-reported patient questionnaire, and each symptom was graded with a 10-point Visual Analogue Scale (VAS) scale (except for pain, which was evaluated with a 100-point VAS scale).
Patient satisfaction was evaluated at T1 and T2, using a 4-point Likert scale, ranging from 0 to 3 (0 = very dissatisfied, 1 = dissatisfied, 2 = satisfied, 3 = very satisfied). A score of 2 or 3 was considered as a “good” degree of satisfaction.
2.4. Phlebotonic Therapies: Composition and Mechanism of Action
Patients were treated by two different phlebotonic therapies. In group A, oral intake of two doses two times per day for 4 weeks of micronized purified flavonoid fraction with diosmin (450 mg) and hesperidin (50 mg) was administered. This medication has documented effects on the microcirculation and bleeding due to their antioxidant, anti-inflammatory and phlebotonic action [
15,
16,
17]. In group B, oral intake of one sublingual sachet two times per day for 4 weeks of a nano-emulsion combining micronized diosmin (67.5 mg) with hesperidin (7.9 mg), boswellia (75 mg), lysine (37.5 mg), cysteine (21 mg), ruscus (25 mg), vitamin E (9 mg), copper (0.65 mg) and arginine (2.1 mg) was administered.
2.5. Statistical Analysis
Sample size calculation: In a pilot study conducted at our institution (unpublished data), we found that the mean values of the “bleeding score” assessed with the VAS scale before and after treatment with a flavonoids preparation were 6.5 and 4.5, respectively, with a standard deviation of 2; based on these data, and considering an alpha confidence level = 0.05 and a unilateral test, the number of patients needed to reach a 95% power was calculated to be 48 individuals for each group; after establishing a 5% drop-off rate, we determined that the total number needed for the present study was finally about 100 patients.
Continuous data were analyzed as mean (with SD) and compared, using the paired or unpaired samples t-test or Wilcoxon Mann-Whitney test or Friedman test. Categorical data were analyzed as frequencies and percentages and compared by using either chi-square or marginal homogeneity test, as necessary. A p < 0.05 was considered statistically significant. The analysis of variance (ANOVA) models for repeated measures were adopted to analyze the differences between more than 2 groups over the study period. All data recorded were collected with Excel spreadsheet and analyzed with SPSS statistical version 21.0 for Windows software (SPSS, Chicago, IL, USA).
3. Results
3.1. Descriptive Data
One hundred patients fulfilled the inclusion criteria: In group A, 50 patients were enrolled, 22 of whom were males, with a mean age of 46.9 ± 11.2 years, while group B consisted of 50 patients, 24 of whom were males, with a mean age of 47.0 ± 11.5 years. Patient baseline characteristics of the two groups are detailed in
Table 1; they were similar, expect for dysepithelialization (group A 6.2 ± 2.4 vs. group B 5.3 ± 2.4
p = 0.045). No patient was lost to follow-up. No adverse events were registered in either group, due to the medication administered.
3.2. Group A: Effect of the Therapy
In group A, at T0, 17 (34%) patients had bleedings, and 28 (56%) IV-degree hemorrhoidal prolapse; at T1, 5 (10%) patients referred bleeding, and 24 (48%) IV-degree hemorrhoidal prolapse; at T2 visit, 12 (24%) patients had bleeding, and 23 (46%) IV-degree hemorrhoidal prolapse. A significant improvement in bleeding (
p = 0.006) and a significant reduction in the degree of hemorrhoidal prolapse, from IV to III degrees (
p = 0.050), were registered (
Figure 1).
All symptoms reported by patients significantly improved after therapy (T0 vs. T1) (
Figure 2). Similarly, also, all signs of inflammation significantly improved (
Figure 3). At the T2 visit, a significant reduction of therapeutic efficacy was observed for all symptoms and local signs of inflammation, except for itching (
Figure 2) and edema (
Figure 3). When comparing data of T1 with T2, a worsening of all domains was observed. It was statistically significant for bleeding and itching (
Figure 2) and for dystrophy and edema (
Figure 3).
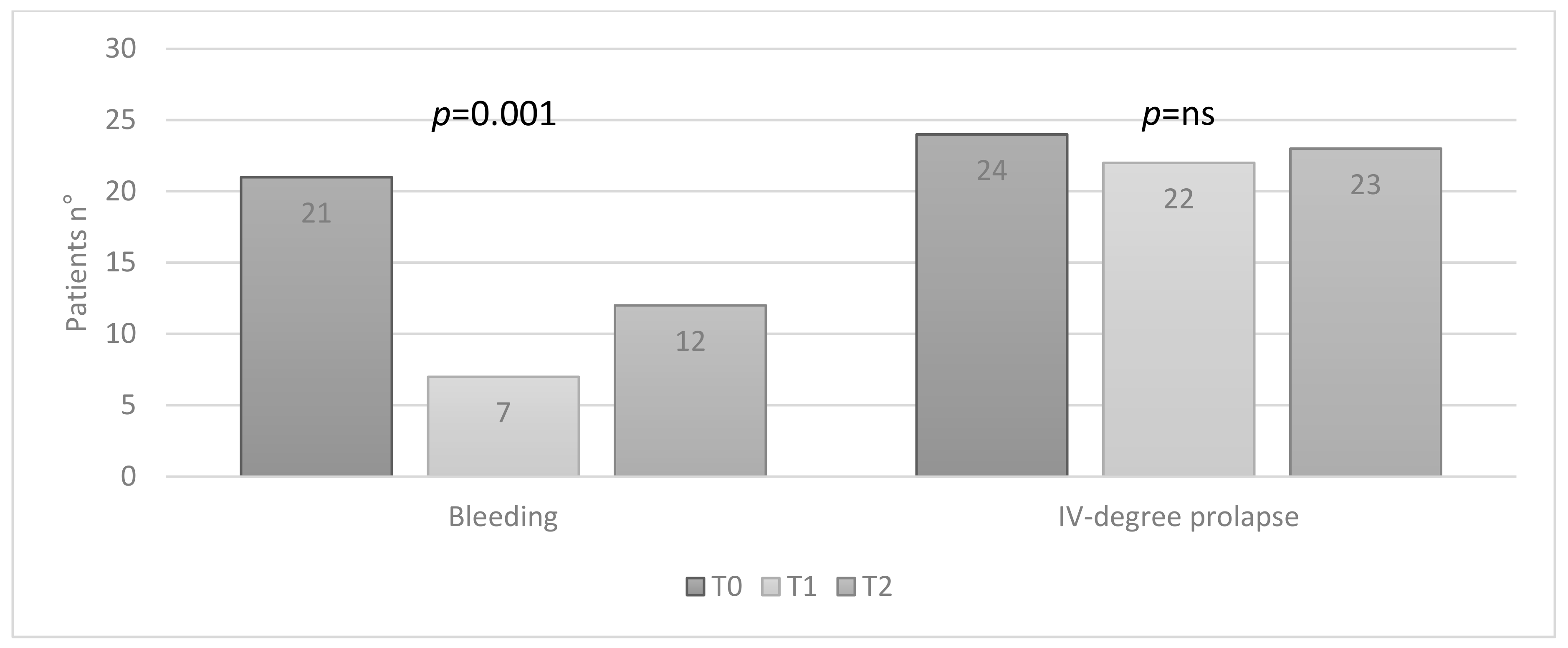
3.3. Group B: Effect of the Therapy
In group B, at T0, 21 (42%) patients had bleeding and 24 (48%) IV-degree hemorrhoidal prolapse; at T1, 7 (14%) patients still had bleeding, and 22 (44%) IV-degree prolapse; at T2, 12 (24%) patients had bleeding and 23 (46%) IV-degree prolapse. A statistically significant improvement in bleeding was observed (
p = 0.001), while no statistically significant reduction in the degree of prolapse was found (
Figure 4).
For group B, data analysis showed a statistically significant improvement after therapy (T0 vs. T1) for all symptoms reported by patients (
Figure 5) and for all signs of inflammation (
Figure 6). Unlike group A, the efficacy of the therapy was still evident for all symptoms referred by patients (
Figure 5) and edema (
Figure 6), even after the discontinuation of therapy (T2). When comparing T1 and T2, the discontinuation of therapy showed a reduction in beneficial effects for all domains analyzed with a statistically significant worsening only for edema (
Figure 6).
3.4. Patients’ Satisfaction
In both groups, the satisfaction score was “good” in 30 out of 50 (60%) patients, and patients were statistically significant more satisfied at T1, compared with T2 (mean value in group A, 1.8 ± 1.0 vs. 1.4 ± 0.9,
p = 0.0001; in group B, 1.7 ± 1.0 vs. 1.4 ± 1.0,
p = 0.0001) (
Figure 7).
When comparing group A to group B, no statistically significant difference emerged regarding all clinical outcomes examined.
4. Discussion
The efficacy of phlebotonic therapy in HD treatment, widely studied and used in chronic vascular insufficiency, remains, among scientists and physicians, debatable [
9,
22]. This approach is usually indicated for low-grade HD [
23,
24]. Although the mechanism of action is not completely understood, several studies have demonstrated the efficacy of flavonoids in patients affected by HD. In 2006, Alonso-Coello et al. [
16] conducted a meta-analysis, comparing the use of flavonoids versus placebo or no treatment in HD: 14 randomized trials were included, with 1514 participants, demonstrating a statistically significant improvement in bleeding, pain and itching using flavonoids. Other studies reported a significant benefit from using flavonoid preparations, particularly for bleeding [
18,
19,
20,
25]. Unfortunately, only a few studies evaluated the non-operative treatment with phlebotonic preparations in patients with advanced HD, confirming their usefulness and safety [
26].
This study confirms and supports the beneficial effects of flavonoid-based therapy in patients affected by advanced HD candidate for surgery: Significant benefits on both symptoms and local inflammation signs were documented. In particular, we observed, at the end of the pharmacological treatment (T1), but also, in some regards, after up to an 8-week washout period from drug intake (T2), improvements. Reduction of treatment efficacy was observed especially for group A, when comparing the outcome at T1 and T2; however, also group B showed, at T2, a statistically significant improvement for all symptoms, including edema due to flavonoid therapy. This could be hypothetically attributed to the combination of flavonoids with other components (hesperidin, boswellia, lysine, cysteine, ruscus, vitamin E, copper and arginine). Therefore, both types of flavonoid-based therapies could be useful to control the disease in patients waiting for surgery, thus optimizing the hemorrhoidal condition for surgery.
More detailed, both medication regimens produced a significant benefit on hemorrhoids hemodynamic (reducing bleeding) and inflammation (reducing itching and pain and specific signs), but they did not impact significantly the hemorrhoidal prolapse, which was expected; in fact, no pharmacological treatment is nowadays able to significantly reduce and/or to restore the degradation of the extracellular matrix. This is the reason why, in the vast majority of patients complaining about hemorrhoidal prolapse, surgery represents a pivotal treatment. This gives a more significant value to patient satisfaction observed in this study, in which both medication regimens resulted as being “good” for the majority of patients with an advanced HD who required surgery anyway.
Limitations of this study are both the relatively small sample size and no evaluation of patients’ quality of life. Increasing the sample of the study could give even more solidity to the results obtained, highlighting the importance of preoperative treatment in patients with advanced disease, and this could be guaranteed by a multicenter study. We also believe that the quality of life of patients can be both a very useful element to evaluate patients with HD but also a useful tool to evaluate the benefit of a conservative or surgical treatment.
5. Conclusions
This study confirms that flavonoids and preparations including flavonoids with phlebotonic action could play a crucial role to treat patients with advanced HD. Their adoption was safe and well tolerated by patients in this study. Although, flavonoids usually cannot replace surgery in most advanced HD degrees, both therapies represented a valid “bridge-therapy” for patients waiting for surgery. They improved the hemorrhoidal condition during the overall study period (particularly for patients treated with sublingual nano-emulsion flavonoids), reducing severity and symptom occurrence, as well as local inflammation.
Author Contributions
Conceptualization, R.O., F.L. and C.R.; data curation, R.O., F.L., A.P., V.D.S., P.C. and A.A.M.; formal analysis, F.L.; investigation, V.D.S., P.C. and A.A.M.; methodology, A.A.M.; supervision, A.P. and C.R.; writing—original draft, R.O., F.L. and C.R.; A.P. is the corresponding author. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Fondazione Policlinico Universitario “A. Gemelli” IRCCS of Rome (protocol n°1268–1301, 15 September 2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author upon reasonable request.
Conflicts of Interest
R.O., F.L., A.P., V.D.S. and P.C. have no conflict of interest to declare. C.R. received travel reimbursement by THD company, to attend conferences, and was proctor in courses on SphinKeeper implant.
References
- Altomare, D.F.; Giannini, I. Pharmacological treatment of hemorrhoids: A narrative review. Expert Opin. Pharmacother. 2013, 14, 2343–2349. [Google Scholar] [CrossRef] [PubMed]
- Johanson, J.F.; Sonnenberg, A. The prevalence of hemorrhoids and chronic constipation. An epidemiologic study. Gastroenterology 1990, 98, 380–386. [Google Scholar] [CrossRef]
- Gazet, J.C.; Redding, W.; Rickett, J.W. The prevalence of haemorrhoids. A preliminary survey. Proc. R Soc. Med. 1970, 63, 78–80. [Google Scholar]
- Quijano, C.E.; Abalos, E. Conservative management of symptomatic and/or complicated haemorrhoids in pregnancy and the puerperium. Cochrane Database Syst. Rev. 2005, 3, CD004077. [Google Scholar] [CrossRef]
- Perera, N.; Liolitsa, D.; Iype, S. Phlebotonics for haemorrhoids. Cochrane Database Syst. Rev. 2012, 8, CD004322. [Google Scholar] [CrossRef]
- Lohsiriwat, V. Treatment of hemorrhoids: A coloproctologist’s view. World J. Gastroenterol. 2015, 31, 9245–9252. [Google Scholar] [CrossRef]
- Altomare, D.F.; Roveran, A.; Pecorella, G.; Gaj, F.; Stortini, E. The treatment of hemorrhoids: Guidelines of the Italian society of colorectal surgery. Tech. Coloproctol. 2006, 10, 181–186. [Google Scholar] [CrossRef]
- Rivadeneira, D.E.; Steele, S.R.; Ternent, C.; Chalasani, S.; Buie, W.D.; Rafferty, J.L. Standards practice task force of the american society of colon and rectal surgeons. Practice parameters for the management of hemorrhoids (revised 2010). Dis. Colon Rectum 2011, 54, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Madoff, R.D.; Fleshman, J.W. Clinical Practice Committee, American Gastroenterological Association American Gastroenterological Association technical review on the diagnosis and treatment of hemorrhoids. Gastroenterology 2004, 126, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, P.; Ellis, C.N.; Gregorcyk, S.; Hyman, N.; Buie, W.D.; Church, J.; Cohen, J.; Fleshner, P.; Kilkenny, J., 3rd; Ko, C.; et al. Practice parameters for the management of hemorrhoids (Revised). Dis. Colon Rectum 2005, 48, 189–194. [Google Scholar] [CrossRef]
- Martinez, M.J.; Bonfill, X.; Moreno, R.M.; Vargas, E.; Capellà, D. Phlebotonics for venous insufficiency. Cochrane Database Syst. Rev. 2005, 3, 003229. [Google Scholar]
- Meyer, O.C. Safety and security of Daflon 500 mg in venous insufficiency and in hemorrhoidal disease. Angiology 1994, 45, 579–584. [Google Scholar] [CrossRef]
- Labrid, C. Pharmacologic properties of Daflon 500 mg. Angiology 1994, 45, 524–530. [Google Scholar]
- Labrid, C. A lymphatic function of Daflon 500 mg. Int. Angiol. 1995, 14, 36–38. [Google Scholar] [PubMed]
- Struckmann, J.R.; Nicolaides, A.N. Flavonoids. A review of the pharmacology and therapeutic efficacy of Daflon 500 mg in patients with chronic venous insufficiency and related disorders. Angiology 1994, 45, 419–428. [Google Scholar] [CrossRef]
- Alonso-Coello, P.; Zhou, Q.; Martinez-Zapata, M.J.; Mills, E.; Heels-Ansdell, D.; Johanson, J.F.; Guyatt, G. Meta-analysis of flavonoids for the treatment of haemorrhoids. Br. J. Surg. 2006, 93, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Khubchandani, I.T. Randomized clinical trial of micronized flavonoids in the early control of bleeding for acute internal haemorrhoids. Tech. Coloproctol. 2001, 5, 57–58. [Google Scholar] [PubMed]
- Misra, M.C.; Parshad, R. Randomized clinical trial of micronized flavonoids in the early control of bleeding from acute internal haemorrhoids. Br. J. Surg. 2000, 87, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.H.; Foo, C.L.; Seow-Choen, F.; Goh, H.S. Prospective randomized controlled trial of a micronized flavonoid fraction to reduce bleeding after haemorrhoidectomy. Br. J. Surg. 1995, 82, 1034–1035. [Google Scholar]
- Babaikere, M.M.; Huang, H.G.; Re, W.N.; Fan, K.; Chu, H.; Ai, E.H.; Li-Mu, M.M.; Wang, Y.R.; Wen, H. How we can improve patients’ comfort after Milligan-Morgan open haemorrhoidectomy. World J. Gastroenterol. 2011, 17, 1448–1456. [Google Scholar]
- Goligher, J.C.; Duthie, H.L.; Nixon, H.H. Surgery of the Anus, Rectum and Colon, 5th ed.; Baillière Tindall: London, UK, 1984; pp. 98–149. [Google Scholar]
- Alonso, P.; Marzo, M.; Mascort, J.J.; Hervas, A.; Vinas, L.; Ferrus, J. Clinical practice guidelines for the management of patients with rectal bleeding. Gastroenterol. Hepatol. 2002, 25, 605–632. [Google Scholar] [CrossRef]
- Corsale, I.; Carrieri, P.; Martellucci, J.; Piccolomini, A.; Verre, L.; Rigutini, M.; Panicucci, S. Flavonoid mixture (diosmin, troxerutin, rutin, hesperidin, quercetin) in the treatment of I–III degree hemorroidal disease: A double-blind multicenter prospective comparative study. Int. J. Colorectal. Dis. 2018, 33, 1595–1600. [Google Scholar] [CrossRef]
- Giannini, I.; Amato, A.; Basso, L.; Tricomi, N.; Marranci, M.; Pecorella, G.; Tafuri, S.; Pennisi, D.; Altomare, D.F. Flavonoids mixture (diosmin, troxerutin, hesperidin) in the treatment of acute hemorrhoidal disease: A prospective, randomized, triple-blind, controlled trial. Tech. Coloproctol. 2015, 19, 339–345. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.M.; Cao, J.D. The impact of micronized purified flavonoid fraction on the treatment of acute haemorrhoidal episodes. Curr. Med. Res. Opin. 2006, 22, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Zagriadskiĭ, E.A.; Bogomazov, A.M.; Golovko, E.B. Conservative Treatment of Hemorrhoids: Results of an Observational Multicenter Study. Adv. Ther. 2018, 35, 1979–1992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).