Sepsis Induces Physical and Mental Impairments in a Mouse Model of Post-Intensive Care Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Cecal Slurry
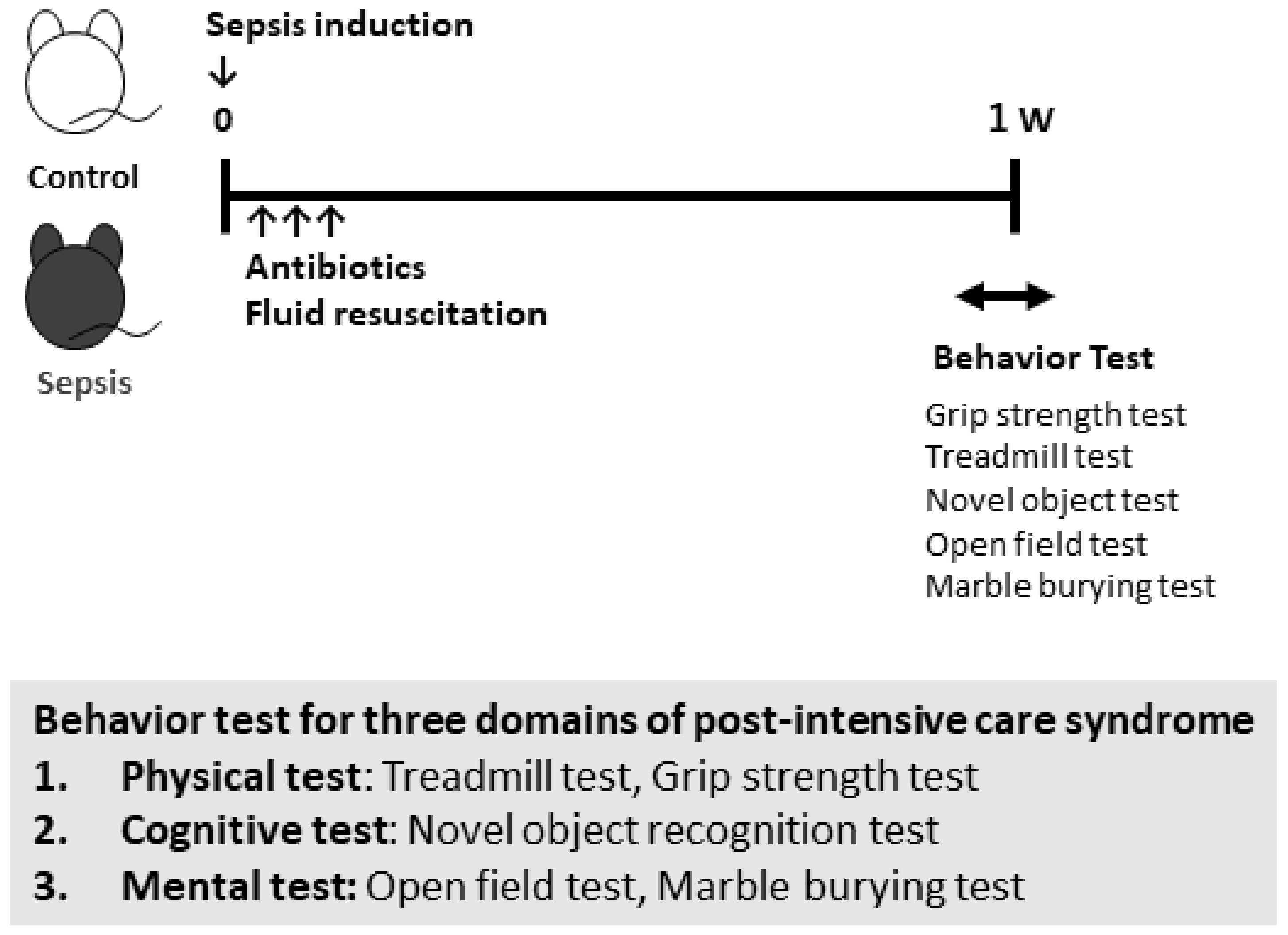
2.2. Animal Housing and Study Design
2.3. Behavioral Procedures
2.4. Physical Examination
2.4.1. Grip Strength Test (GST)
2.4.2. Treadmill Test
2.5. Cognitive Examination
Novel Object Recognition Test (NORT)
2.6. Mental Examination
2.6.1. Open Field Test (OFT)
2.6.2. Marble Burying Test (MBT)
2.7. Statistical Analyses
3. Results
3.1. Cecal Slurry Injection Induces Sublethal Outcomes in Mice
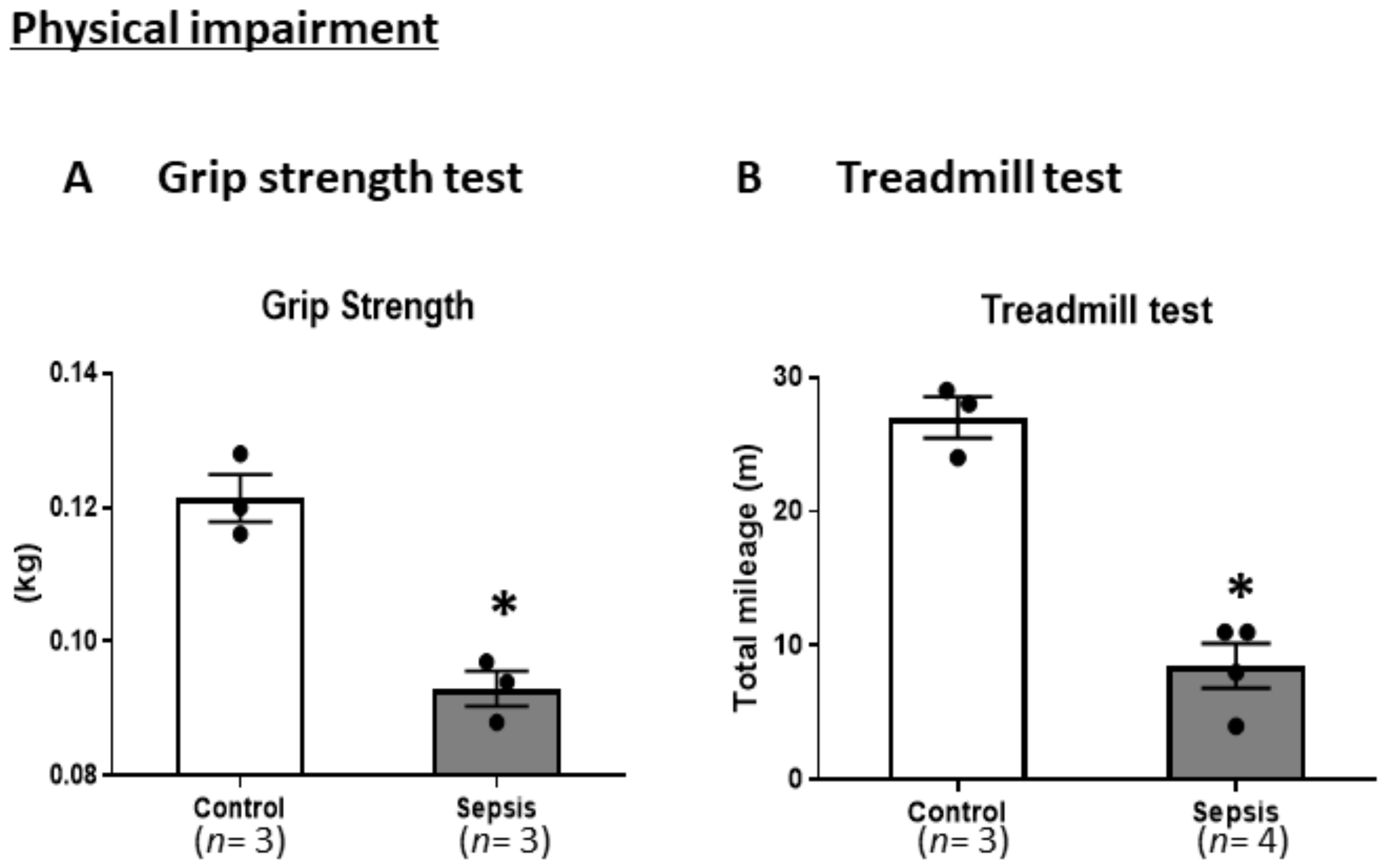
3.2. Sepsis Induces Physical Impairments
3.3. Sepsis Does Not Induce Cognitive Impairments
3.4. Sepsis Induces Mental Impairments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carson, S.S.; Cox, C.E.; Holmes, G.M.; Howard, A.; Carey, T.S. The changing epidemiology of mechanical ventilation: A population-based study. J. Intensive Care Med. 2006, 21, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Needham, D.M.; Bronskill, S.E.; Calinawan, J.R.; Sibbald, W.J.; Pronovost, P.J.; Laupacis, A. Projected incidence of mechanical ventilation in Ontario to 2026: Preparing for the aging baby boomers. Crit. Care Med. 2005, 33, 574–579. [Google Scholar] [CrossRef]
- Spragg, R.G.; Bernard, G.R.; Checkley, W.; Curtis, J.R.; Gajic, O.; Guyatt, G.; Hall, J.; Israel, E.; Jain, M.; Needham, D.M.; et al. Beyond mortality: Future clinical research in acute lung injury. Am. J. Respir. Crit. Care Med. 2010, 181, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Needham, D.M.; Davidson, J.; Cohen, H.; Hopkins, R.O.; Weinert, C.; Wunsch, H.; Zawistowski, C.; Bemis-Dougherty, A.; Berney, S.C.; Bienvenu, O.J.; et al. Improving long-term outcomes after discharge from intensive care unit: Report from a stakeholders' conference. Crit. Care Med. 2012, 40, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Prescott, H.C.; Angus, D.C. Enhancing recovery from sepsis: A review. JAMA 2018, 319, 62–75. [Google Scholar] [CrossRef]
- Prescott, H.C.; Angus, D.C. Postsepsis Morbidity. JAMA 2018, 319, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwashyna, T.J.; Ely, E.W.; Smith, D.M.; Langa, K.M. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 2010, 304, 1787–1794. [Google Scholar] [CrossRef] [Green Version]
- Osuchowski, M.F.; Ayala, A.; Bahrami, S.; Bauer, M.; Boros, M.; Cavaillon, J.M.; Chaudry, I.H.; Coopersmith, C.M.; Deutschman, C.S.; Drechsler, S.; et al. Minimum Quality Threshold in Pre-Clinical Sepsis Studies (MQTiPSS): An international expert consensus initiative for improvement of animal modeling in sepsis. Shock 2018, 50, 377–380. [Google Scholar] [CrossRef] [Green Version]
- Saito, M.; Inoue, S.; Yamashita, K.; Kakeji, Y.; Fukumoto, T.; Kotani, J. IL-15 improves aging-induced persistent t cell exhaustion in mouse models of repeated sepsis. Shock 2020, 53, 228–235. [Google Scholar] [CrossRef]
- Starr, M.E.; Steele, A.M.; Saito, M.; Hacker, B.J.; Evers, B.M.; Saito, H. A new cecal slurry preparation protocol with improved long-term reproducibility for animal models of sepsis. PLoS ONE 2014, 9, e115705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, A.M.; Patel, S.P.; Smith, J.D.; Balasuriya, B.K.; Mori, S.F.; Hawk, G.S.; Stromberg, A.J.; Kuriyama, N.; Kaneki, M.; Rabchevsky, A.G.; et al. Chronic muscle weakness and mitochondrial dysfunction in the absence of sustained atrophy in a preclinical sepsis model. eLife 2019, 8. [Google Scholar] [CrossRef]
- Ono, Y.; Maejima, Y.; Saito, M.; Sakamoto, K.; Horita, S.; Shimomura, K.; Inoue, S.; Kotani, J. TAK-242, a specific inhibitor of Toll-like receptor 4 signalling, prevents endotoxemia-induced skeletal muscle wasting in mice. Sci. Rep. 2020, 10, 694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satow, A.; Maehara, S.; Ise, S.; Hikichi, H.; Fukushima, M.; Suzuki, G.; Kimura, T.; Tanak, T.; Ito, S.; Kawamoto, H.; et al. Pharmacological effects of the metabotropic glutamate receptor 1 antagonist compared with those of the metabotropic glutamate receptor 5 antagonist and metabotropic glutamate receptor 2/3 agonist in rodents: Detailed investigations with a selective allosteric metabotropic glutamate receptor 1 antagonist, FTIDC [4-[1-(2-fluoropyridine-3-yl)-5-methyl-1H-1,2,3-triazol-4-yl]-N-isopropyl-N-methy l-3,6-dihydropyridine-1(2H)-carboxamide]. J. Pharmacol. Exp. Ther. 2008, 326, 577–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seldeen, K.L.; Berman, R.N.; Pang, M.; Lasky, G.; Weiss, C.; MacDonald, B.A.; Thiyagarajan, R.; Redae, Y.; Troen, B.R. Vitamin D insufficiency reduces grip strength, grip endurance and increases frailty in aged C57Bl/6J mice. Nutrients 2020, 12, 3005. [Google Scholar] [CrossRef]
- Pan, S.; Wu, Y.; Pei, L.; Li, S.; Song, L.; Xia, H.; Wang, Y.; Yu, Y.; Yang, X.; Shu, H.; et al. BML-111 reduces neuroinflammation and cognitive impairment in mice with sepsis via the SIRT1/NF-kappaB signaling pathway. Front. Cell. Neurosci. 2018, 12, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, M.; Fujinami, Y.; Ono, Y.; Ohyama, S.; Fujioka, K.; Yamashita, K.; Inoue, S.; Kotani, J. Infiltrated regulatory T cells and Th2 cells in the brain contribute to attenuation of sepsis-associated encephalopathy and alleviation of mental impairments in mice with polymicrobial sepsis. Brain Behav. Immun. 2020. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Han, W.; Wang, C.; Sui, D.; Bian, J.; Bo, L.; Deng, X. Upregulation of heme oxygenase-1 by hemin alleviates sepsis-induced muscle wasting in mice. Oxid. Med. Cell. Longev. 2018, 2018, 8927104. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Li, T.M.; Liu, X.R.; Bai, Y.P.; Li, J.; Tang, N.; Wang, X.B. MicroRNA-140 inhibits skeletal muscle glycolysis and atrophy in endotoxin-induced sepsis in mice via the WNT signaling pathway. Am. J. Physiol. Cell Physiol. 2019, 317, C189–C199. [Google Scholar] [CrossRef]
- Williams, A.; Wang, J.J.; Wang, L.; Sun, X.; Fischer, J.E.; Hasselgren, P.O. Sepsis in mice stimulates muscle proteolysis in the absence of IL-6. Am. J. Physiol. 1998, 275, R1983–R1991. [Google Scholar] [CrossRef]
- Steiner, J.L.; Pruznak, A.M.; Deiter, G.; Navaratnarajah, M.; Kutzler, L.; Kimball, S.R.; Lang, C.H. Disruption of genes encoding eIF4E binding proteins-1 and -2 does not alter basal or sepsis-induced changes in skeletal muscle protein synthesis in male or female mice. PLoS ONE 2014, 9, e99582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, C.; Yao, R.Q.; Zhang, H.; Feng, Y.W.; Yao, Y.M. Sepsis-associated encephalopathy: A vicious cycle of immunosuppression. J. Neuroinflamm. 2020, 17, 14. [Google Scholar] [CrossRef] [PubMed]
- Kealy, J.; Murray, C.; Griffin, E.W.; Lopez-Rodriguez, A.B.; Healy, D.; Tortorelli, L.S.; Lowry, J.P.; Watne, L.O.; Cunningham, C. Acute inflammation alters brain energy metabolism in mice and humans: Role in suppressed spontaneous activity, impaired cognition, and delirium. J. Neurosci. 2020, 40, 5681–5696. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.H.; Qiu, L.L.; Tang, H.; Ju, L.S.; Sun, X.R.; Zhang, H.; Jia, M.; Zuo, Z.Y.; Shen, J.C.; Yang, J.J. Sepsis-induced selective parvalbumin interneuron phenotype loss and cognitive impairments may be mediated by NADPH oxidase 2 activation in mice. J. Neuroinflamm. 2015, 12, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esquerdo, K.F.; Sharma, N.K.; Brunialti, M.K.C.; Baggio-Zappia, G.L.; Assunção, M.; Azevedo, L.C.P.; Bafi, A.T.; Salomao, R. Inflammasome gene profile is modulated in septic patients, with a greater magnitude in non-survivors. Clin. Exp. Immunol. 2017, 189, 232–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witteveen, E.; Hoogland, I.C.; Wieske, L.; Weber, N.C.; Verhamme, C.; Schultz, M.J.; van Schaik, I.N.; Horn, J. Assessment of intensive care unit-acquired weakness in young and old mice: An E. coli septic peritonitis model. Muscle Nerve 2016, 53, 127–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Illendula, M.; Osuru, H.P.; Ferrarese, B.; Atluri, N.; Dulko, E.; Zuo, Z.; Lunardi, N. Surgery, anesthesia and intensive care environment induce delirium-like behaviors and impairment of synaptic function-related gene expression in aged mice. Front. Aging Neurosci. 2020, 12, 542421. [Google Scholar] [CrossRef]
- Goto, S. The Biological Mechanisms of Aging: A Historical and Critical Overview. In Aging Mechanisms: Longevity, Metabolism, and Brain Aging; Mori, N., Mook-Jung, I., Eds.; Springer: Tokyo, Japan, 2015; pp. 3–27. [Google Scholar]
- Kawakami, D.; Fujitani, S.; Morimoto, T.; Dote, H.; Takita, M.; Takaba, A.; Hino, M.; Nakamura, M.; Irie, H.; Adachi, T.; et al. Prevalence of post-intensive care syndrome among Japanese intensive care unit patients: A prospective, multicenter, observational J-PICS study. Crit. Care 2021, 25, 69. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujinami, Y.; Inoue, S.; Ono, Y.; Miyazaki, Y.; Fujioka, K.; Yamashita, K.; Kotani, J. Sepsis Induces Physical and Mental Impairments in a Mouse Model of Post-Intensive Care Syndrome. J. Clin. Med. 2021, 10, 1593. https://doi.org/10.3390/jcm10081593
Fujinami Y, Inoue S, Ono Y, Miyazaki Y, Fujioka K, Yamashita K, Kotani J. Sepsis Induces Physical and Mental Impairments in a Mouse Model of Post-Intensive Care Syndrome. Journal of Clinical Medicine. 2021; 10(8):1593. https://doi.org/10.3390/jcm10081593
Chicago/Turabian StyleFujinami, Yoshihisa, Shigeaki Inoue, Yuko Ono, Yusuke Miyazaki, Kazumichi Fujioka, Kimihiro Yamashita, and Joji Kotani. 2021. "Sepsis Induces Physical and Mental Impairments in a Mouse Model of Post-Intensive Care Syndrome" Journal of Clinical Medicine 10, no. 8: 1593. https://doi.org/10.3390/jcm10081593






