Effect of Goal-Directed Crystalloid versus Colloid Administration on Perioperative Hemostasis in Partial Hepatectomy: A Randomized, Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
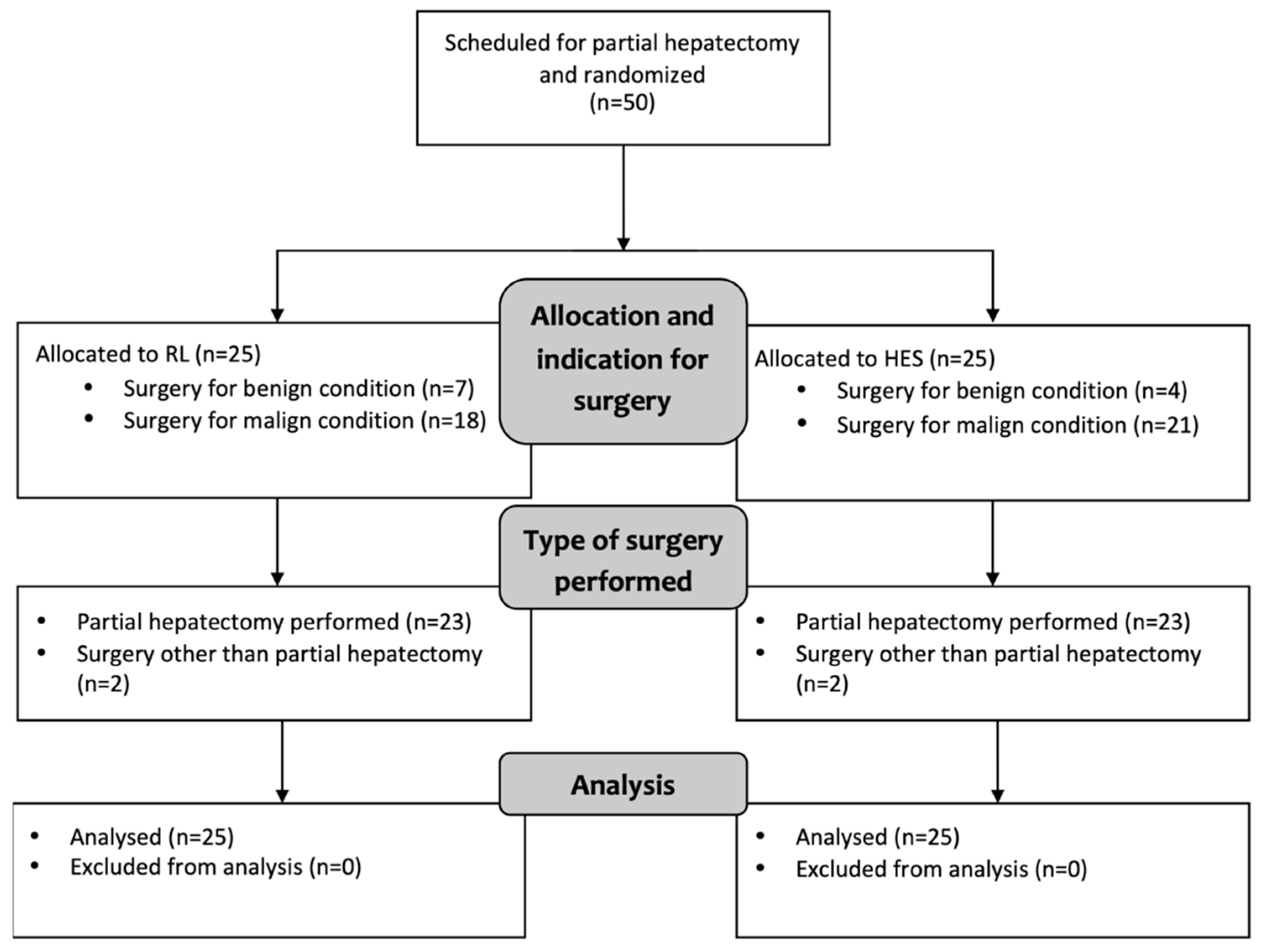
2.2. Study Participants
- 25 patients in the RL group received repeated fluid boluses of 250 mL of lactated Ringer’s solution (Ringer-Lactat; Fresenius Kabi, Germany) when hypovolemia was detected.
- 25 patients in the HES group received repeated fluid boluses of 250 mL of 6% hydroxyethyl starch 130/0.4 (Voluven; Fresenius Kabi, Germany) in case of hypovolemia.
2.3. Anesthetic Procedures
2.4. Measurements and Outcome Parameters
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. ROTEM Measurements and Plasma Fibrinogen Levels
3.3. Conventional Coagulation Tests
3.4. Estimated Blood Loss, Cell Counts, and Procoagulant Medication
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, T.E.; Myles, P.S. Perioperative Fluid Therapy for Major Surgery. Anesthesiology 2019, 130, 825–832. [Google Scholar] [CrossRef]
- Feldheiser, A.; Pavlova, V.; Bonomo, T.; Jones, A.; Fotopoulou, C.; Sehouli, J.; Wernecke, K.D.; Spies, C. Balanced crystalloid compared with balanced colloid solution using a goal-directed haemodynamic algorithm. Br. J. Anaesth. 2013, 110, 231–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, A.E.; Jia, Y.; Soltesz, E.; Leung, S.; Yilmaz, H.O.; Mao, G.; Timur, A.A.; Kottke-Marchant, K.; Rogers, H.J.; Ma, C.; et al. Effect of 6% hydroxyethyl starch 130/0.4 on kidney and haemostatic function in cardiac surgical patients: A randomised controlled trial. Anaesthesia 2020, 75, 1180–1190. [Google Scholar] [CrossRef] [Green Version]
- Zampieri, F.G.; Cavalcanti, A.B. Hydroxyethyl Starch for Fluid Replacement Therapy in High-Risk Surgical Patients. JAMA 2020, 323, 217. [Google Scholar] [CrossRef]
- Zarychanski, R.; Turgeon, A.F. Re-framing the question: Should hydroxyethyl starch be used in clinical practice? Can. J. Anesth. Can. d’anesthésie 2019, 66, 21–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raiman, M.; Mitchell, C.G.; Biccard, B.M.; Rodseth, R.N. Comparison of hydroxyethyl starch colloids with crystalloids for surgical patients. Eur. J. Anaesthesiol. 2016, 33, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, M.C.; Buggy, D.J.I. Pendulum swings again: Crystalloid or colloid fluid therapy? Br. J. Anaesth. 2014, 113, 335–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myburgh, J.A.; Finfer, S.; Bellomo, R.; Billot, L.; Cass, A.; Gattas, D.; Glass, P.; Lipman, J.; Liu, B.; McArthur, C.; et al. Hydroxyethyl Starch or Saline for Fluid Resuscitation in Intensive Care. N. Engl. J. Med. 2012, 367, 1901–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perner, A.; Haase, N.; Guttormsen, A.B.; Tenhunen, J.; Klemenzson, G.; Åneman, A.; Madsen, K.R.; Møller, M.H.; Elkjær, J.M.; Poulsen, L.M.; et al. Hydroxyethyl Starch 130/0.42 versus Ringer’s Acetate in Severe Sepsis. N. Engl. J. Med. 2012, 367, 124–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The European Medicines Agency. Hydroxyethyl-Starch Solutions (HES) No Longer to Be Used in Patients with Sepsis or Burn Injuries or in Critically Ill Patients. 2013. Available online: www.ema.europa.eu/en/documents/referral/hydroxyethyl-starch-solutions-hes-no-longer-be-used-patients-sepsis-burn-injuries-critically-ill_en (accessed on 31 January 2021).
- Wiedermann, C.J.; Eisendle, K. Comparison of hydroxyethyl starch regulatory summaries from the Food and Drug Administration and the European Medicines Agency. J. Pharm. Policy Pract. 2017, 10, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Futier, E.; Garot, M.; Godet, T.; Biais, M.; Verzilli, D.; Ouattara, A.; Huet, O.; Lescot, T.; Lebuffe, G.; Dewitte, A.; et al. Effect of Hydroxyethyl Starch vs Saline for Volume Replacement Therapy on Death or Postoperative Complications Among High-Risk Patients Undergoing Major Abdominal Surgery. JAMA 2020, 323, 225. [Google Scholar] [CrossRef]
- Kabon, B.; Sessler, D.I.; Kurz, A.; Maheshwari, K.; Babazade, R.; Fiffick, A.; Gazmuri, I.; Ghobrial, M.; Honar, H.; Kot, M.; et al. Effect of Intraoperative Goal-directed Balanced Crystalloid versus Colloid Administration on Major Postoperative Morbidity. Anesthesiology 2019, 130, 728–744. [Google Scholar] [CrossRef] [Green Version]
- Joosten, A.; Delaporte, A.; Mortier, J.; Ickx, B.; Van Obbergh, L.; Vincent, J.-L.; Cannesson, M.; Rinehart, J.; Van der Linden, P. Long-term Impact of Crystalloid versus Colloid Solutions on Renal Function and Disability-free Survival after Major Abdominal Surgery. Anesthesiology 2019, 130, 227–236. [Google Scholar] [CrossRef]
- Rasmussen, K.C.; Secher, N.H.; Pedersen, T. Effect of perioperative crystalloid or colloid fluid therapy on hemorrhage, coagulation competence, and outcome. Medicine 2016, 95, e4498. [Google Scholar] [CrossRef] [PubMed]
- Kozek-Langenecker, S.A. Fluids and coagulation. Curr. Opin. Crit. Care 2015, 21, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Gratz, J.; Oberladstätter, D.; Schöchl, H. Trauma-Induced Coagulopathy and Massive Bleeding: Current Hemostatic Concepts and Treatment Strategies. Hamostaseologie 2020. [Google Scholar] [CrossRef]
- Schramko, A.; Suojaranta-Ylinen, R.; Niemi, T.; Pesonen, E.; Kuitunen, A.; Raivio, P.; Salmenpera, M. The use of balanced HES 130/0.42 during complex cardiac surgery; effect on blood coagulation and fluid balance: A randomized controlled trial. Perfusion 2015, 30, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Skhirtladze, K.; Base, E.M.; Lassnigg, A.; Kaider, A.; Linke, S.; Dworschak, M.; Hiesmayr, M.J. Comparison of the effects of albumin 5%, hydroxyethyl starch 130/0.4 6%, and Ringer’s lactate on blood loss and coagulation after cardiac surgery. Br. J. Anaesth. 2014, 112, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Schramko, A.; Suojaranta-Ylinen, R.; Kuitunen, A.; Raivio, P.; Kukkonen, S.; Niemi, T. Hydroxyethylstarch and gelatin solutions impair blood coagulation after cardiac surgery: A prospective randomized trial. Br. J. Anaesth. 2010, 104, 691–697. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gotzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 Explanation and Elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c869. [Google Scholar] [CrossRef] [Green Version]
- Gan, T.J.; Soppitt, A.; Maroof, M.; El-Moalem, H.; Robertson, K.M.; Moretti, E.; Dwane, P.; Glass, P.S.A. Goal-directed Intraoperative Fluid Administration Reduces Length of Hospital Stay after Major Surgery. Anesthesiology 2002, 97, 820–826. [Google Scholar] [CrossRef]
- Reddy, S.K.; Barbas, A.S.; Turley, R.S.; Steel, J.L.; Tsung, A.; Marsh, J.W.; Geller, D.A.; Clary, B.M. A standard definition of major hepatectomy: Resection of four or more liver segments. HPB 2011, 13, 494–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, J.D.; Lupkiewicz, S.M.; Palenik, L.; Lopez, L.M.; Ariet, M. Determination of ideal body weight for drug dosage calculations. Am. J. Hosp. Pharm. 1983, 40, 1016–1019. [Google Scholar] [CrossRef]
- Whiting, D.; DiNardo, J.A. TEG and ROTEM: Technology and clinical applications. Am. J. Hematol. 2014, 89, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Innerhofer, P.; Fries, D.; Margreiter, J.; Klingler, A.; Kühbacher, G.; Wachter, B.; Oswald, E.; Salner, E.; Frischhut, B.; Schobersberger, W. The Effects of Perioperatively Administered Colloids and Crystalloids on Primary Platelet-Mediated Hemostasis and Clot Formation. Anesth. Analg. 2002, 95, 858–865. [Google Scholar] [CrossRef]
- Kozek-Langenecker, S.A. Effects of Hydroxyethyl Starch Solutions on Hemostasis. Anesthesiology 2005, 103, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Schaden, E.; Wetzel, L.; Kozek-Langenecker, S.; Thaler, U.; Scharbert, G. Effect of the carrier solution for hydroxyethyl starch on platelet aggregation and clot formation. Br. J. Anaesth. 2012, 109, 572–577. [Google Scholar] [CrossRef] [Green Version]
- Solomon, C.; Schöchl, H.; Ranucci, M.; Schött, U.; Schlimp, C.J. Comparison of fibrin-based clot elasticity parameters measured by free oscillation rheometry (ReoRox ®) versus thromboelastometry (ROTEM ®). Scand. J. Clin. Lab. Invest. 2015, 75, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Gratz, J.; Ponschab, M.; Iapichino, G.E.; Schlimp, C.J.; Cadamuro, J.; Grottke, O.; Zipperle, J.; Oberladstätter, D.; Gabriel, C.; Ziegler, B.; et al. Comparison of fresh frozen plasma vs. coagulation factor concentrates for reconstitution of blood. Eur. J. Anaesthesiol. 2020, 37, 879–888. [Google Scholar] [CrossRef]
- Gratz, J.; Schlimp, C.J.; Honickel, M.; Hochhausen, N.; Schöchl, H.; Grottke, O. Sufficient Thrombin Generation Despite 95% Hemodilution: An In Vitro Experimental Study. J. Clin. Med. 2020, 9, 3805. [Google Scholar] [CrossRef]
- Spahn, D.R.; Bouillon, B.; Cerny, V.; Duranteau, J.; Filipescu, D.; Hunt, B.J.; Komadina, R.; Maegele, M.; Nardi, G.; Riddez, L.; et al. The European guideline on management of major bleeding and coagulopathy following trauma: Fifth edition. Crit. Care 2019, 23, 98. [Google Scholar] [CrossRef] [Green Version]
- Yates, D.R.A.; Davies, S.J.; Milner, H.E.; Wilson, R.J.T. Crystalloid or colloid for goal-directed fluid therapy in colorectal surgery. Br. J. Anaesth. 2014, 112, 281–289. [Google Scholar] [CrossRef] [Green Version]
- Hartog, C.S.; Reuter, D.; Loesche, W.; Hofmann, M.; Reinhart, K. Influence of hydroxyethyl starch (HES) 130/0.4 on hemostasis as measured by viscoelastic device analysis: A systematic review. Intensive Care Med. 2011, 37, 1725–1737. [Google Scholar] [CrossRef]
- Tynngård, N.; Berlin, G.; Samuelsson, A.; Berg, S. Low dose of hydroxyethyl starch impairs clot formation as assessed by viscoelastic devices. Scand. J. Clin. Lab. Invest. 2014, 74, 344–350. [Google Scholar] [CrossRef]
- Rasmussen, K.C.; Johansson, P.I.; Højskov, M.; Kridina, I.; Kistorp, T.; Thind, P.; Nielsen, H.B.; Ruhnau, B.; Pedersen, T.; Secher, N.H. Hydroxyethyl Starch Reduces Coagulation Competence and Increases Blood Loss During Major Surgery. Ann. Surg. 2014, 259, 249–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittermayr, M.; Streif, W.; Haas, T.; Fries, D.; Velik-Salchner, C.; Klingler, A.; Oswald, E.; Bach, C.; Schnapka-Koepf, M.; Innerhofer, P. Hemostatic Changes After Crystalloid or Colloid Fluid Administration During Major Orthopedic Surgery: The Role of Fibrinogen Administration. Anesth. Analg. 2007, 105, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Ahn, S.W.; Song, J.W.; Shim, J.K.; Yoo, K.-J.; Kwak, Y.L. Effect of Hydroxyethyl Starch 130/0.4 on Blood Loss and Coagulation in Patients With Recent Exposure to Dual Antiplatelet Therapy Undergoing Off-Pump Coronary Artery Bypass Graft Surgery. Circ. J. 2011, 75, 2397–2402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ejaz, A.; Spolverato, G.; Kim, Y.; Margonis, G.A.; Gupta, R.; Amini, N.; Frank, S.M.; Pawlik, T.M. Impact of Blood Transfusions and Transfusion Practices on Long-Term Outcome Following Hepatopancreaticobiliary Surgery. J. Gastrointest. Surg. 2015, 19, 887–896. [Google Scholar] [CrossRef]
- Zdolsek, H.J.; Vegfors, M.; Lindahl, T.L.; Törnquist, T.; Bortnik, P.; Hahn, R.G. Hydroxyethyl starches and dextran during hip replacement surgery: Effects on blood volume and coagulation. Acta Anaesthesiol. Scand. 2011, 55, 677–685. [Google Scholar] [CrossRef]
- Hung, M.-H.; Zou, C.; Lin, F.-S.; Lin, C.-J.; Chan, K.-C.; Chen, Y. New 6% hydroxyethyl starch 130/0.4 does not increase blood loss during major abdominal surgery—A randomized, controlled trial. J. Formos. Med. Assoc. 2014, 113, 429–435. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.-L.; Yu, B.-W. Effects of Acute Hypervolemic Fluid Infusion of Hydroxyethyl Starch and Gelatin on Hemostasis and Possible Mechanisms. Clin. Appl. Thromb. 2010, 16, 91–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozek-Langenecker, S.A.; Ahmed, A.B.; Afshari, A.; Albaladejo, P.; Aldecoa, C.; Barauskas, G.; De Robertis, E.; Faraoni, D.; Filipescu, D.C.; Fries, D.; et al. Management of severe perioperative bleeding. Eur. J. Anaesthesiol. 2017, 34, 332–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| RL (n = 25) | HES (n = 25) | |
|---|---|---|
| Gender (male/female) | 13/12 | 15/10 |
| Age (years) | 56 ± 16 | 58 ± 12 |
| BMI (kg m−2) | 25 ± 4 | 26 ± 6 |
| ASA status (I/II/III) | 5/14/6 | 6/8/11 |
| Liver resection (minor/major) | 14/9 | 19/4 |
| Duration of surgery (min) | 212 ± 79 | 228 ± 86 |
| Preoperative chemotherapy | 10 | 12 |
| FIBTEM MCF (mm) | 23 ± 6 | 21 ± 7 |
| EXTEM CT (s) | 57 ± 11 | 60 ± 10 |
| EXTEM MCF (mm) | 66 ± 7 | 66 ± 6 |
| INTEM CT (s) | 156 ± 53 | 152 ± 35 |
| INTEM MCF (mm) | 67 ± 6 | 68 ± 4 |
| PT (%) | 103 ± 27 | 104 ± 21 |
| aPTT (s) | 34.5 ± 4.9 | 36.4 ± 4.3 |
| Plasma fibrinogen (g L−1) | 4.04 ± 1.32 | 4.01 ± 1 |
| Hemoglobin (g L−1) | 127 ± 13 | 128 ± 20 |
| Platelet count (G L−1) | 247 ± 91 | 231 ± 98 |
| RL (n = 25) | HES (n = 25) | p | |
|---|---|---|---|
| Total fluid volume (L) | 2.95 (1.55) | 2.9 (1.57) | 0.47 |
| Crystalloid volume (L) | 2.95 (1.55) | 1.66 (0.87) | <0.001 |
| Colloid volume (L) | - | 1.12 ± 0.49 | - |
| Lowest temperature (°C) | 35.9 ± 0.5 | 35.9 ± 0.5 | 0.82 |
| Lowest pH | 7.31 ± 0.03 | 7.32 ± 0.04 | 0.42 |
| Lowest Ca2+ (mmol L−1) | 1.16 ± 0.05 | 1.16 ± 0.05 | 0.53 |
| Estimated blood loss (mL) | 470 ± 299 | 604 ± 351 | 0.16 |
| Summary Measures | 24 h Postoperative | |||||
|---|---|---|---|---|---|---|
| RL (n = 25) | HES (n = 25) | p | RL (n = 25) | HES (n = 25) | p | |
| FIBTEM MCF (mm) | 20 ± 2 | 14 ± 6 | 0.03 | 23 ± 7 | 23 ± 8 | 0.76 |
| EXTEM CT (s) | 57 ± 4 | 70 ± 7 | 0.003 | 60 ± 12 | 63 ± 7 | 0.15 |
| EXTEM MCF (mm) | 64 ± 1 | 60 ± 5 | 0.10 | 66 ± 6 | 64 ± 6 | 0.26 |
| INTEM CT (s) | 154 ± 10 | 155 ± 9 | 0.92 | 164 ± 48 | 152 ± 32 | 0.33 |
| INTEM MCF (mm) | 64 ± 2 | 62 ± 3 | 0.19 | 66 ± 5 | 67 ± 4 | 0.81 |
| Plasma fibrinogen (g L−1) | 3.08 ± 0.37 | 2.65 ± 0.64 | 0.18 | 3.59 ± 0.95 | 3.69 ± 0.82 | 0.95 |
| PT (%) | 78 ± 7 | 68 ± 13 | 0.09 | 63 ± 19 | 67 ± 17 | 0.35 |
| aPTT (s) | 34.8 ± 1.4 | 38.0 ± 2.9 | 0.04 | 37.2 ± 3.6 | 36.3 ± 3.8 | 0.43 |
| Hemoglobin (g L−1) | 111 ± 3 | 106 ± 12 | 0.34 | 107 ± 15 | 109 ± 14 | 0.53 |
| Platelet count (G L−1) | 202 ± 18 | 155 ± 28 | 0.006 | 201 ± 68 | 185 ± 53 | 0.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gratz, J.; Zotti, O.; Pausch, A.; Wiegele, M.; Fleischmann, E.; Gruenberger, T.; Krenn, C.G.; Kabon, B. Effect of Goal-Directed Crystalloid versus Colloid Administration on Perioperative Hemostasis in Partial Hepatectomy: A Randomized, Controlled Trial. J. Clin. Med. 2021, 10, 1651. https://doi.org/10.3390/jcm10081651
Gratz J, Zotti O, Pausch A, Wiegele M, Fleischmann E, Gruenberger T, Krenn CG, Kabon B. Effect of Goal-Directed Crystalloid versus Colloid Administration on Perioperative Hemostasis in Partial Hepatectomy: A Randomized, Controlled Trial. Journal of Clinical Medicine. 2021; 10(8):1651. https://doi.org/10.3390/jcm10081651
Chicago/Turabian StyleGratz, Johannes, Oliver Zotti, André Pausch, Marion Wiegele, Edith Fleischmann, Thomas Gruenberger, Claus G. Krenn, and Barbara Kabon. 2021. "Effect of Goal-Directed Crystalloid versus Colloid Administration on Perioperative Hemostasis in Partial Hepatectomy: A Randomized, Controlled Trial" Journal of Clinical Medicine 10, no. 8: 1651. https://doi.org/10.3390/jcm10081651
APA StyleGratz, J., Zotti, O., Pausch, A., Wiegele, M., Fleischmann, E., Gruenberger, T., Krenn, C. G., & Kabon, B. (2021). Effect of Goal-Directed Crystalloid versus Colloid Administration on Perioperative Hemostasis in Partial Hepatectomy: A Randomized, Controlled Trial. Journal of Clinical Medicine, 10(8), 1651. https://doi.org/10.3390/jcm10081651






