Flexible Modeling of Net Survival and Cure by AML Subtype and Age: A French Population-Based Study from FRANCIM
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Statistical Analysis
2.2.1. Statistical Modeling of Excess Mortality Rate and Estimation of Net Survival without Cure Assumption
2.2.2. Statistical Modeling of Cure
3. Results
3.1. Characteristics of the AML Cohort
3.2. Net Survival by AML Subtype at All Ages
3.3. Effect of Age at AML Diagnosis on Net Survival
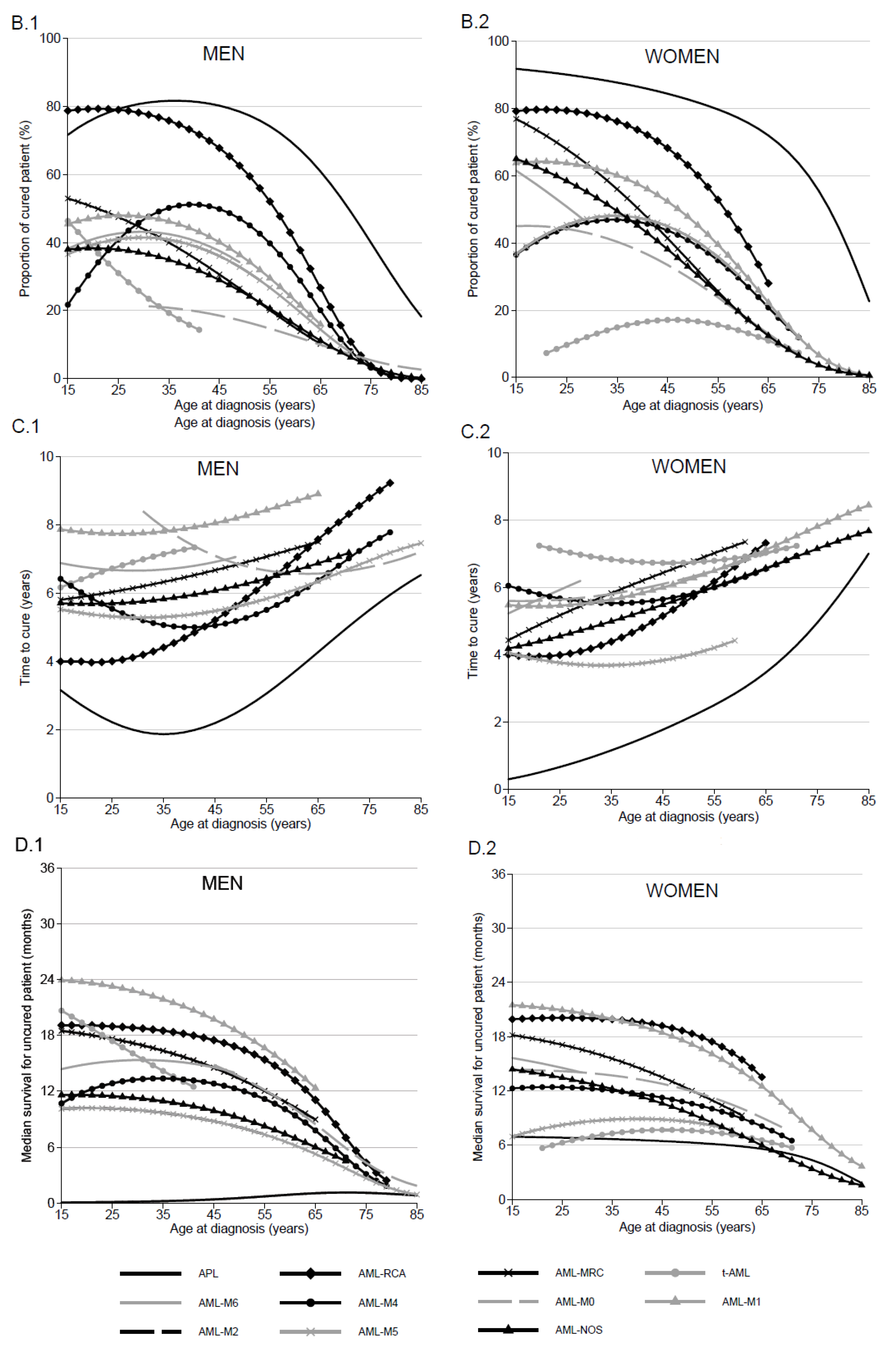
3.4. Proportion of Cured Patients and Time to Cure by AML Subtype, Sex and Age
3.5. Median of Net Survival in “Uncured” Patients by AML Subtype and Age
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 Revision to the World Health Organization Classification of Myeloid Neoplasms and Acute Leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Sant, M.; Allemani, C.; Tereanu, C.; De Angelis, R.; Capocaccia, R.; Visser, O.; Marcos-Gragera, R.; Maynadié, M.; Simonetti, A.; Lutz, J.-M.; et al. Incidence of Hematologic Malignancies in Europe by Morphologic Subtype: Results of the HAEMACARE Project. Blood 2010, 116, 3724–3734. [Google Scholar] [CrossRef]
- Maynadié, M.; De Angelis, R.; Marcos-Gragera, R.; Visser, O.; Allemani, C.; Tereanu, C.; Capocaccia, R.; Giacomin, A.; Lutz, J.-M.; Martos, C.; et al. Survival of European Patients Diagnosed with Myeloid Malignancies: A HAEMACARE Study. Haematologica 2013, 98, 230–238. [Google Scholar] [CrossRef]
- Monnereau, A.; Troussard, X.; Belot, A.; Guizard, A.-V.; Woronoff, A.-S.; Bara, S.; Lapôtre-Ledoux, B.; Iwaz, J.; Tretarre, B.; Maynadié, M.; et al. Unbiased Estimates of Long-Term Net Survival of Hematological Malignancy Patients Detailed by Major Subtypes in France. Int. J. Cancer 2013, 132, 2378–2387. [Google Scholar] [CrossRef]
- Harris, N.L.; Jaffe, E.S.; Diebold, J.; Flandrin, G.; Muller-Hermelink, H.K.; Vardiman, J.; Lister, T.A.; Bloomfield, C.D. The World Health Organization Classification of Neoplastic Diseases of the Hematopoietic and Lymphoid Tissues. Report of the Clinical Advisory Committee Meeting, Airlie House, Virginia, November, 1997. Ann. Oncol. 1999, 10, 1419–1432. [Google Scholar] [CrossRef]
- Vardiman, J.W.; Thiele, J.; Arber, D.A.; Brunning, R.D.; Borowitz, M.J.; Porwit, A.; Harris, N.L.; Le Beau, M.M.; Hellström-Lindberg, E.; Tefferi, A.; et al. The 2008 Revision of the World Health Organization (WHO) Classification of Myeloid Neoplasms and Acute Leukemia: Rationale and Important Changes. Blood 2009, 114, 937–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maynadie, M.; Girodon, F.; Manivet-Janoray, I.; Mounier, M.; Mugneret, F.; Bailly, F.; Favre, B.; Caillot, D.; Petrella, T.; Flesch, M.; et al. Twenty-Five Years of Epidemiological Recording on Myeloid Malignancies: Data from the Specialized Registry of Hematologic Malignancies of Cotee d’Or (Burgundy, France). Haematologica 2011, 96, 55–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhayat, F.; Das-Gupta, E.; Smith, C.; McKeever, T.; Hubbard, R. The Incidence of and Mortality from Leukaemias in the UK: A General Population-Based Study. BMC Cancer 2009, 9, 252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Angelis, R.; Capocaccia, R.; Hakulinen, T.; Soderman, B.; Verdecchia, A. Mixture Models for Cancer Survival Analysis: Application to Population-Based Data with Covariates. Stat. Med. 1999, 18, 441–454. [Google Scholar] [CrossRef]
- Lambert, P.C.; Thompson, J.R.; Weston, C.L.; Dickman, P.W. Estimating and Modeling the Cure Fraction in Population-Based Cancer Survival Analysis. Biostatistics 2007, 8, 576–594. [Google Scholar] [CrossRef] [Green Version]
- Verdecchia, A.; De Angelis, R.; Capocaccia, R.; Sant, M.; Micheli, A.; Gatta, G.; Berrino, F. The Cure for Colon Cancer: Results from the EUROCARE Study. Int. J. Cancer 1998, 77, 322–329. [Google Scholar] [CrossRef]
- Fritz, A.G. International Classification of Diseases for Oncology: ICD-O; World Health Organization: Geneva, Switzerland, 2000; ISBN 978-92-4-154534-1. [Google Scholar]
- Nelson, C.P.; Lambert, P.C.; Squire, I.B.; Jones, D.R. Flexible Parametric Models for Relative Survival, with Application in Coronary Heart Disease. Stat. Med. 2007, 26, 5486–5498. [Google Scholar] [CrossRef] [Green Version]
- Andersson, T.M.L.; Dickman, P.W.; Eloranta, S.; Lambert, P.C. Estimating and Modelling Cure in Population-Based Cancer Studies within the Framework of Flexible Parametric Survival Models. BMC Med. Res. Methodol. 2011, 11, 96. [Google Scholar] [CrossRef] [Green Version]
- Boussari, O.; Romain, G.; Remontet, L.; Bossard, N.; Mounier, M.; Bouvier, A.-M.; Binquet, C.; Colonna, M.; Jooste, V. A New Approach to Estimate Time-to-Cure from Cancer Registries Data. Cancer Epidemiol. 2018, 53, 72–80. [Google Scholar] [CrossRef]
- Sposto, R. Cure Model Analysis in Cancer: An Application to Data from the Children’s Cancer Group. Stat. Med. 2002, 21, 293–312. [Google Scholar] [CrossRef] [PubMed]
- Perme, M.P.; Stare, J.; Estève, J. On Estimation in Relative Survival. Biometrics 2012, 68, 113–120. [Google Scholar] [CrossRef]
- Pigneux, A.; Labopin, M.; Maertens, J.; Cordonnier, C.; Volin, L.; Socié, G.; Blaise, D.; Craddock, C.; Milpied, N.; Bacher, U.; et al. Outcome of Allogeneic Hematopoietic Stem-Cell Transplantation for Adult Patients with AML and 11q23/MLL Rearrangement (MLL-r AML). Leukemia 2015, 29, 2375–2381. [Google Scholar] [CrossRef] [PubMed]
- Schlenk, R.F.; Benner, A.; Krauter, J.; Büchner, T.; Sauerland, C.; Ehninger, G.; Schaich, M.; Mohr, B.; Niederwieser, D.; Krahl, R.; et al. Individual Patient Data-Based Meta-Analysis of Patients Aged 16 to 60 Years with Core Binding Factor Acute Myeloid Leukemia: A Survey of the German Acute Myeloid Leukemia Intergroup. J. Clin. Oncol. J. Am. Soc. Clin. Oncol. 2004, 22, 3741–3750. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Peng, Y.; Wang, X.; Chen, Y.; Jin, L.; Yang, T.; Qian, M.; Ni, W.; Tong, X.; Lan, J. Incidence, Survival, and Risk Factors for Adults with Acute Myeloid Leukemia Not Otherwise Specified and Acute Myeloid Leukemia with Recurrent Genetic Abnormalities: Analysis of the Surveillance, Epidemiology, and End Results (SEER) Database, 2001–2013. Acta Haematol. 2018, 139, 115–127. [Google Scholar] [CrossRef]
- Seymour, J.F.; Döhner, H.; Butrym, A.; Wierzbowska, A.; Selleslag, D.; Jang, J.H.; Kumar, R.; Cavenagh, J.; Schuh, A.C.; Candoni, A.; et al. Azacitidine Improves Clinical Outcomes in Older Patients with Acute Myeloid Leukaemia with Myelodysplasia-Related Changes Compared with Conventional Care Regimens. BMC Cancer 2017, 17, 852. [Google Scholar] [CrossRef]
- Rizzieri, D.A.; Schiller, G.J.; Solomon, S.R.; Newell, L.F.; Erba, H.P.; Ryan, R.J.; Faderl, S.; Cortes, J.E.; Lancet, J.E. Outcomes in Patients with Therapy-Related Acute Myeloid Leukemia (t-AML) Who Achieved Remission with CPX-351 Versus 7+3: Phase 3 Exploratory Analysis. Biol. Blood Marrow Transplant. 2020, 26, S105–S106. [Google Scholar] [CrossRef]
- Finn, L.; Dalovisio, A.; Foran, J. Older Patients with Acute Myeloid Leukemia: Treatment Challenges and Future Directions. Ochsner J. 2017, 17, 398–404. [Google Scholar] [PubMed]
- Juliusson, G.; Antunovic, P.; Derolf, A.; Lehmann, S.; Möllgård, L.; Stockelberg, D.; Tidefelt, U.; Wahlin, A.; Höglund, M. Age and Acute Myeloid Leukemia: Real World Data on Decision to Treat and Outcomes from the Swedish Acute Leukemia Registry. Blood 2009, 113, 4179–4187. [Google Scholar] [CrossRef] [Green Version]
- Masetti, R.; Rondelli, R.; Fagioli, F.; Mastronuzzi, A.; Pierani, P.; Togni, M.; Menna, G.; Pigazzi, M.; Putti, M.C.; Basso, G.; et al. Infants with Acute Myeloid Leukemia Treated According to the Associazione Italiana Di Ematologia e Oncologia Pediatrica 2002/01 Protocol Have an Outcome Comparable to That of Older Children. Haematologica 2014, 99, e127–e129. [Google Scholar] [CrossRef]
- Hossain, M.J.; Xie, L.; Caywood, E.H. Prognostic Factors of Childhood and Adolescent Acute Myeloid Leukemia (AML) Survival: Evidence from Four Decades of US Population Data. Cancer Epidemiol. 2015, 39, 720–726. [Google Scholar] [CrossRef]
- Hossain, M.J.; Xie, L. Sex Disparity in Childhood and Young Adult Acute Myeloid Leukemia (AML) Survival: Evidence from US Population Data. Cancer Epidemiol. 2015, 39, 892–900. [Google Scholar] [CrossRef] [Green Version]
- Roman, E.; Smith, A.; Appleton, S.; Crouch, S.; Kelly, R.; Kinsey, S.; Cargo, C.; Patmore, R. Myeloid Malignancies in the Real-World: Occurrence, Progression and Survival in the UK’s Population-Based Haematological Malignancy Research Network 2004–2015. Cancer Epidemiol. 2016, 42, 186–198. [Google Scholar] [CrossRef] [Green Version]
- Utuama, O.; Mukhtar, F.; Pham, Y.T.-H.; Dabo, B.; Manani, P.; Moser, J.; Michael-Asalu, A.; Tran, C.T.; Le, L.C.; Le, T.V.; et al. Racial/Ethnic, Age and Sex Disparities in Leukemia Survival among Adults in the United States during 1973–2014 Period. PLoS ONE 2019, 14, e0220864. [Google Scholar] [CrossRef]
- Brown, C.A.; Youlden, D.R.; Aitken, J.F.; Moore, A.S. Therapy-Related Acute Myeloid Leukemia Following Treatment for Cancer in Childhood: A Population-Based Registry Study. Pediatric Blood Cancer 2018, 65, e27410. [Google Scholar] [CrossRef]
- Yu, X.Q.; De Angelis, R.; Andersson, T.M.L.; Lambert, P.C.; O’Connell, D.L.; Dickman, P.W. Estimating the Proportion Cured of Cancer: Some Practical Advice for Users. Cancer Epidemiol. 2013. [Google Scholar] [CrossRef]
- Lacombe, F.; Arnoulet, C.; Maynadié, M.; Lippert, E.; Luquet, I.; Pigneux, A.; Vey, N.; Casasnovas, O.; Witz, F.; Béné, M.C. Early Clearance of Peripheral Blasts Measured by Flow Cytometry during the First Week of AML Induction Therapy as a New Independent Prognostic Factor: A GOELAMS Study. Leukemia 2009, 23, 350–357. [Google Scholar] [CrossRef]
- Guénot, C.; Lacombe, F.; Allou, K.; Dumezy, F.; Feuillard, J.; Geneviève, F.; Guérin, E.; Guy, J.; Maynadié, M.; Ballon, O.W.; et al. Peripheral Blood Minimal/Measurable Residual Disease Assessed in Flow Cytometry in Acute Myeloblastic Leukemia. Leukemia 2019. [Google Scholar] [CrossRef]
- Ganzel, C.; Sun, Z.; Cripe, L.D.; Fernandez, H.F.; Douer, D.; Rowe, J.M.; Paietta, E.M.; Ketterling, R.; O’Connell, M.J.; Wiernik, P.H.; et al. Very Poor Long-Term Survival in Past and More Recent Studies for Relapsed AML Patients: The ECOG-ACRIN Experience. Am. J. Hematol. 2018, 93, 1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jastaniah, W.; Bayoumy, M.; Alsultan, A.; Al Daama, S.; Ballourah, W.; Al-Anzi, F.; Al Shareef, O.; Al Sudairy, R.; Abrar, M.B.; Al Ghemlas, I. Identifying Prognostic Factors That Influence Outcome of Childhood Acute Myeloid Leukemia in First Relapse in Saudi Arabia: Results of the Multicenter SAPHOS Study. Clin. Lymphoma Myeloma Leuk. 2018, 18, 773–780. [Google Scholar] [CrossRef]
- Andersson, T.M.-L.; Lambert, P.C.; Derolf, A.R.; Kristinsson, S.Y.; Eloranta, S.; Landgren, O.; Björkholm, M.; Dickman, P.W. Temporal Trends in the Proportion Cured among Adults Diagnosed with Acute Myeloid Leukaemia in Sweden 1973-2001, a Population-Based Study. Br. J. Haematol. 2010, 148, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Andersson, T.M.-L.; Rachet, B.; Björkholm, M.; Lambert, P.C. Survival and Cure of Acute Myeloid Leukaemia in England, 1971-2006: A Population-Based Study. Br. J. Haematol. 2013, 162, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Schnegg-Kaufmann, A.; Feller, A.; Baldomero, H.; Rovo, A.; Manz, M.G.; Gregor, M.; Efthymiou, A.; Bargetzi, M.; Hess, U.; Spertini, O.; et al. Improvement of Relative Survival in Elderly Patients with Acute Myeloid Leukaemia Emerging from Population-Based Cancer Registries in Switzerland between 2001 and 2013. Cancer Epidemiol. 2018, 52, 55–62. [Google Scholar] [CrossRef]
- Thein, M.S.; Ershler, W.B.; Jemal, A.; Yates, J.W.; Baer, M.R. Outcome of Older Patients with Acute Myeloid Leukemia. Cancer 2013, 119, 2720–2727. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 Revision of the World Health Organization Classification of Lymphoid Neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [Green Version]
- Voso, M.T.; Ottone, T.; Lavorgna, S.; Venditti, A.; Maurillo, L.; Lo-Coco, F.; Buccisano, F. MRD in AML: The Role of New Techniques. Front. Oncol. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Specific Myeloid Morphologies | ICD-O-3 Morphology Codes | All | Men | Women |
|---|---|---|---|---|
| N | N (%) | N (%) | ||
| All AML | - | 9453 | 5001 (53) | 4452 (47) |
| Acute promyelocytic leukemia with t(15;17) (q22;q12) (APL) | 9866/3 | 575 | 310 (54) | 265 (46) |
| AML with recurrent cytogenetic abnormalities (AML-RCA) | - | 406 | 214 (53) | 192 (47) |
| Acute myeloid leukemia with t(6;9)(p23;q34.1); DEK-NUP214 | 9865/3 | 6 | 3 (50) | 3 (50) |
| Acute myeloid leukemia with inv(3)(q21.3q26.2) or t(3;3)(q21.3;q26.2); GATA2, MECOM | 9869/3 | 6 | 3 (50) | 3 (50) |
| Acute myeloid leukemia with inv(16)(p13.1q22) or t(16;16)(p13,1;q22); CBFB-MYH11 | 9871/3 | 173 | 86 (50) | 87 (50) |
| Acute myeloid leukemia with t(8;21)(q22;q22); RUNX1-RUNX1T1 | 9896/3 | 135 | 82 (61) | 53 (39) |
| Acute myeloid leukemia with t(9,11)(p22;q23); MLLT3-MLL | 9897/3 | 84 | 39 (46) | 45 (54) |
| Myeloid leukemia associated with Down syndrome | 9898/3 | 2 | 1 (50) | 1 (50) |
| AML with myelodysplasia-related changes (AML-MRC) | - | 969 | 521 (54) | 448 (46) |
| Acute Myeloid Leukemia with multilineage dysplasia | 9895/3 | 903 | 483 (53) | 420 (47) |
| Refractory anaemia with excess blasts in transformation | 9984/3 | 66 | 38 (58) | 28 (42) |
| Therapy-related AML; NOS (t-AML) | 9920/3 | 613 | 300 (49) | 313 (51) |
| Acute panmyelosis with myelofibrosis (APMF) | 9931/3 | 85 | 63 (74) | 22 (26) |
| Acute biphenotypic leukemia | - | 109 | 66 (61) | 43 (39) |
| Acute biphenotypic leukemia | 9805/3 | 82 | 48 (59) | 34 (41) |
| Mixed-phenotype acute leukemia with t(9;22)(q34.1;q11.2) | 9806/3 | 5 | 2 (40) | 3 (60) |
| Mixed-phenotype acute leukemia B/myeloid, NOS | 9808/3 | 11 | 8 (73) | 3 (27) |
| Mixed-phenotype acute leukemia T/myeloid, NOS | 9809/3 | 11 | 8 (73) | 3 (27) |
| Pure erythroid leukemia (AML-M6) | 9840/3 | 279 | 180 (65) | 99 (35) |
| Acute Myelomonocytic leukemia (AML-M4) | 9867/3 | 844 | 439 (52) | 405 (48) |
| Acute basophilic leukemia- | 9870/3 | 3 | 2 (67) | 1 (33) |
| Acute myeloid leukemia with minimal differentiation (AML-M0) | 9872/3 | 372 | 221 (59) | 151 (41) |
| Acute Myeloid leukemia without maturation (AML-M1) | 9873/3 | 888 | 418 (47) | 470 (53) |
| Acute Myeloid leukemia with maturation (AML-M2) | 9874/3 | 1171 | 634 (54) | 537 (46) |
| Acute monoblastic and monocytic leukemia (AML-M5) | 9891/3 | 825 | 451 (55) | 374 (45) |
| Acute megakarioblastic leukemia- | 9910/3 | 63 | 39 (62) | 24 (38) |
| Myeloid sarcoma- | 9930/3 | 50 | 26 (52) | 24 (48) |
| Acute Myeloid leukemia, not otherwise specified (AML-NOS) | 9861/3 | 2201 | 1117 (53) | 1084 (47) |
| Specific AML Morphologies | Number at Diagnosis | Age at Diagnosis, Median (Min–Max) | Number at 5 Year | Number at 10 Year | Number Death at 10 Year | Percentage of Loss to Follow-up (%) |
|---|---|---|---|---|---|---|
| MEN | ||||||
| Acute promyelocytic leukemia with t(15;17) (q22;q12) (APL) | 310 | 57 (15–92) | 146 | 66 | 123 | 2.2 |
| AML with recurrent cytogenetic abnormalities (AML-RCA) | 214 | 53 (15–91) | 85 | 32 | 107 | 0 |
| AML with myelodysplasia-related changes (AML-MRC) | 521 | 74 (15–97) | 39 | 13 | 482 | <1 |
| Therapy-related AML; NOS (t-AML) | 300 | 72 (16–93) | 15 | 3 | 274 | <1 |
| Acute panmyelosis with myelofibrosis (APMF) | 63 | 72 (36–88) | 6 | 2 | 58 | 1.6 |
| Acute biphenotypic leukemia- | 66 | 58 (15–85) | 17 | 8 | 43 | 3 |
| Pure erythroid leukemia (AML-M6) | 180 | 68 (23–98) | 21 | 10 | 151 | 1.1 |
| Acute Myelomonocytic leukemia (AML-M4) | 439 | 69 (15–93) | 73 | 35 | 358 | <1 |
| Acute basophilic leukemia- | 2 | 75 (70–80) | 0 | 0 | 2 | 0 |
| Acute myeloid leukemia with minimal differentiation (AML-M0) | 221 | 72 (15–94) | 16 | 6 | 201 | <1 |
| Acute Myeloid leukemia without maturation (AML-M1) | 418 | 67 (18–93) | 71 | 28 | 322 | <1 |
| Acute Myeloid leukemia with maturation (AML-M2) | 634 | 70 (15–97) | 98 | 39 | 526 | <1 |
| Acute monoblastic and monocytic leukemia (AML-M5) | 451 | 67 (15–101) | 54 | 21 | 379 | 0 |
| Acute megakarioblastic leukemia - | 39 | 64 (24–90) | 2 | 1 | 36 | 2.6 |
| Myeloid sarcoma - | 26 | 58 (18–87) | 6 | 2 | 20 | 0 |
| Acute Myeloid leukemia, not otherwise specified (AML-NOS) | 1117 | 73 (15–98) | 103 | 64 | 1018 | <1 |
| WOMEN | ||||||
| Acute promyelocytic leukemia with t(15;17) (q22;q12) (APL) | 265 | 52 (15–96) | 154 | 85 | 74 | 1.5 |
| AML with recurrent cytogenetic abnormalities (AML-RCA) | 192 | 53 (16–90) | 81 | 35 | 99 | 2 |
| AML with myelodysplasia-related changes (AML-MRC) | 448 | 76 (15–98) | 42 | 20 | 407 | <1 |
| Therapy-related AML; NOS (t-AML) | 313 | 70 (15–93) | 20 | 6 | 282 | <1 |
| Acute panmyelosis with myelofibrosis (APMF) | 22 | 68 (39–88) | 11 | 1 | 14 | 0 |
| Acute biphenotypic leukemia- | 43 | 60 (20–93) | 7 | 0 | 32 | 0 |
| Pure erythroid leukemia (AML-M6) | 99 | 70 (19–98) | 10 | 3 | 90 | 0 |
| Acute Myelomonocytic leukemia (AML-M4) | 405 | 68 (15–97) | 68 | 32 | 318 | 1.8 |
| Acute basophilic leukemia - | 1 | 75 (75–75) | 0 | 0 | 1 | 0 |
| Acute myeloid leukemia with minimal differentiation (AML-M0) | 151 | 72 (15–97) | 20 | 5 | 128 | 0 |
| Acute Myeloid leukemia without maturation (AML-M1) | 470 | 68 (17–94) | 107 | 43 | 343 | <1 |
| Acute Myeloid leukemia with maturation (AML-M2) | 537 | 72 (15–103) | 91 | 37 | 424 | <1 |
| Acute monoblastic and monocytic leukemia (AML-M5) | 374 | 70 (15–96) | 61 | 27 | 301 | 2.1 |
| Acute megakarioblastic leukemia- | 24 | 75 (25–85) | 2 | 1 | 22 | 0 |
| Myeloid sarcoma- | 24 | 67 (19–92) | 3 | 2 | 21 | 0 |
| Acute Myeloid leukemia, not otherwise specified (AML-NOS) | 1084 | 76 (15–102) | 114 | 88 | 961 | <1 |
| % (95% CI) | 1-Year Net Survival | 5-Year Net Survival | 10-Year Net Survival | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Age | Age = 25 | Age = 50 | Age = 75 | All Age | Age = 25 | Age = 50 | Age = 75 | All Age | Age = 25 | Age = 50 | Age = 75 | |
| MEN | ||||||||||||
| Acute promyelocytic leukemia with t(15;17) (q22;q12) (APL) | 74 (69–79) | 85 (77–94) | 85 (79–91) | 55 (45–67) | 68 (63–74) | 82 (74–91) | 81 (74–88) | 46 (35–59) | 64 (58–71) | 79 (70–89) | 78 (70–86) | 40 (28–56) |
| AML with recurrent cytogenetic abnormalities (AML-RCA) | 72 (68–76) | 96 (92–99) | 84 (78–90) | 25 (16–41) | 56 (51–62) | 86 (78–94) | 66 (57–76) | 10 (4–26) | 48 (42–56) | 75 (63–89) | 56 (45–69) | 9 (3–30) |
| AML with myelodysplasia-related changes (AML-MRC) | 36 (33–40) | 79 (67–94) | 65 (58–72) | 34 (30–39) | 10 (8–13) | 54 (34–85) | 32 (24–41) | 6 (4–9) | 7 (5–10) | 47 (28–82) | 25 (18–35) | 3 (2–6) |
| Therapy-related AML; NOS (t-AML) | 40 (35–45) | 67 (48–93) | 49 (40–59) | 39 (33–46) | 9 (6–13) | 40 (19–82) | 16 (10–26) | 7 (4–13) | 4 (2–7) | 36 (16–82) | 9 (4–19) | 2 (1–5) |
| Pure erythroid leukemia (AML-M6) | 44 (38–51) | 68 (43–100) | 67 (57–77) | 34 (26–44) | 19 (14–25) | 52 (24–100) | 42 (32–56) | 5 (2–13) | 12 (7–18) | 49 (22–100) | 31 (20–47) | 1 (0–9) |
| Acute Myelomonocytic leukemia (AML-M4) | 41 (38–45) | 72 (60–85) | 73 (68–79) | 22 (17–29) | 21 (18–25) | 44 (30–64) | 49 (42–58) | 4 (2–8) | 19 (15–23) | 40 (26–62) | 45 (38–55) | 3 (1–8) |
| Acute myeloid leukemia with minimal differentiation (AML-M0) | 39 (34–46) | 78 (62–97) | 63 (54–74) | 32 (25–41) | 11 (7–16) | 25 (9–69) | 20 (12–32) | 8 (4–14) | 9 (5–16) | 16 (3–74) | 15 (7–29) | 7 (3–17) |
| Acute Myeloid leukemia without maturation (AML-M1) | 47 (43–51) | 71 (58–85) | 75 (69–81) | 30 (25–38) | 26 (22–29) | 51 (37–70) | 53 (46–62) | 5 (3–10) | 21 (17–26) | 47 (33–68) | 47 (39–57) | 2 (1–7) |
| Acute Myeloid leukemia with maturation (AML-M2) | 50 (47–54) | 84 (77–93) | 76 (72–81) | 39 (34–44) | 21 (18–24) | 56 (44–73) | 45 (39–53) | 9 (6–13) | 14 (12–18) | 52 (38–70) | 36 (29–45) | 3 (2–7) |
| Acute monoblastic and monocytic leukemia (AML-M5) | 36 (32–40) | 72 (63–83) | 59 (53–67) | 20 (15–26) | 18 (15–22) | 44 (33–58) | 34 (28–43) | 7 (4–12) | 16 (13–21) | 36 (25–52) | 30 (23–40) | 7 (4–13) |
| Acute Myeloid leukemia, not otherwise specified (AML-NOS) | 31 (29–34) | 67 (58–77) | 57 (52–62) | 26 (23–30) | 11 (9–13) | 42 (32–55) | 29 (24–34) | 5 (3–7) | 9 (7–11) | 39 (29–53) | 25 (20–30) | 3 (2–5) |
| WOMEN | ||||||||||||
| Acute promyelocytic leukemia with t(15;17) (q22;q12) (APL) | 84 (80–88) | 95 (90–99) | 89 (85–94) | 70 (60–81) | 77 (72–82) | 92 (86–98) | 84 (78–91) | 58 (46–72) | 74 (68–79) | 90 (83–98) | 81 (74–89) | 52 (39–69) |
| AML with recurrent cytogenetic abnormalities (AML-RCA) | 68 (64–73) | 92 (86–99) | 84 (78–91) | 34 (24–49) | 52 (47–58) | 84 (74–94) | 67 (58–78) | 9 (4–20) | 46 (40–53) | 79 (68–92) | 60 (49–73) | 4 (1–18) |
| AML with myelodysplasia-related changes (AML-MRC) | 32 (28–36) | 87 (77–99) | 67 (61–75) | 29 (25–35) | 12 (10–15) | 73 (55–97) | 41 (33–51) | 6 (4–9) | 9 (7–12) | 68 (48–96) | 33 (25–44) | 3 (2–6) |
| Therapy-related AML; NOS (t-AML) | 34 (29–39) | 37 (19–74) | 47 (39–57) | 28 (23–36) | 11 (8–15) | 12 (3–53) | 20 (14–30) | 7 (4–12) | 8 (5–13) | 9 (2–49) | 16 (10–26) | 5 (2–10) |
| Pure erythroid leukemia (AML-M6) | 33 (26–42) | 84 (66–100) | 58 (45–76) | 24 (15–37) | 12 (7–19) | 57 (29–100) | 25 (14–46) | 6 (2–17) | 8 (4–17) | 43 (15–100) | 16 (6–42) | 3 (1–20) |
| Acute Myelomonocytic leukemia (AML-M4) | 42 (39–47) | 72 (61–85) | 67 (61–74) | 28 (22–35) | 23 (19–27) | 48 (35–67) | 44 (37–53) | 9 (6–14) | 20 (16–24) | 45 (31–65) | 40 (32–49) | 6 (3–12) |
| Acute myeloid leukemia with minimal differentiation (AML-M0) | 43 (37–51) | 76 (64–90) | 65 (54–78) | 33 (25–44) | 17 (12–23) | 48 (32–73) | 33 (21–50) | 6 (3–13) | 13 (9–20) | 42 (26–70) | 27 (16–45) | 3 (1–11) |
| Acute Myeloid leukemia without maturation (AML-M1) | 51 (48–55) | 85 (76–94) | 81 (77–86) | 36 (30–42) | 29 (26–33) | 56 (42–76) | 58 (51–65) | 12 (8–18) | 26 (22–31) | 49 (33–71) | 53 (45–62) | 11 (6–18) |
| Acute Myeloid leukemia with maturation (AML-M2) | 48 (45–52) | 84 (77–93) | 78 (73–83) | 41 (37–47) | 23 (21–26) | 68 (57–82) | 54 (48–62) | 9 (7–13) | 18 (15–21) | 66 (53–81) | 46 (39–54) | 3 (1–6) |
| Acute monoblastic and monocytic leukemia (AML-M5) | 39 (35–43) | 67 (54–82) | 65 (58–73) | 28 (22–35) | 22 (19–26) | 49 (36–67) | 46 (38–55) | 9 (6–14) | 17 (14–21) | 42 (27–63) | 37 (29–48) | 4 (2–9) |
| Acute Myeloid leukemia, not otherwise specified (AML-NOS) | 29 (27–31) | 81 (74–88) | 62 (57–66) | 23 (20–26) | 13 (11–14) | 62 (52–73) | 35 (30–41) | 5 (3–7) | 11 (10–13) | 59 (49–70) | 32 (27–38) | 4 (2–6) |
| Age Class with Cure Assumption Accepted | Proportion of Cured Patient (%) | Time to Cure (Years) | Median Survival for Uncured Patient (Months) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age = 25 | Age = 50 | Age = 75 | Age = 25 | Age = 50 | Age = 75 | Age = 25 | Age = 50 | Age = 75 | ||
| MEN | ||||||||||
| Acute promyelocytic leukemia with t(15;17) (q22;q12) (APL) | 15–85 | 79 (68–87) | 78 (69–85) | 40 (28–52) | 2 (0–8) | 3 (0–7) | 6 (0–15) | 0 (0–0) | 1 (0–1) | 1 (0–2) |
| AML with recurrent cytogenetic abnormalities (AML-RCA) | 15–85 | 79 (66–88) | 61 (49–71) | 4 (1–12) | 4 (0–9) | 6 (0–12) | 9 (0–39) | 19 (11–27) | 17 (10–23) | 4 (2–7) |
| AML with myelodysplasia-related changes (AML-MRC) | 15–66 | 47 (22–70) | 25 (18–34) | no cure | 6 (0–20) | 7 (0–15) | no cure | 18 (12–24) | 13 (10–17) | no cure |
| Therapy-related AML; NOS (t-AML) | 15–42 | 31 (8–58) | no cure | no cure | 7 (0–29) | no cure | no cure | 17 (10–25) | no cure | no cure |
| Pure erythroid leukemia (AML-M6) | 15–50 | 43 (8–75) | 33 (21–46) | no cure | 7 (0–35) | 7 (0–18) | no cure | 15 (5–25) | 14 (8–20) | no cure |
| Acute Myelomonocytic leukemia (AML-M4) | 15–80 | 41 (24–57) | 46 (38–54) | 3 (1–6) | 6 (0–18) | 5 (0–11) | 7 (0–25) | 13 (9–17) | 12 (9–15) | 3 (2–4) |
| Acute myeloid leukemia with minimal differentiation (AML-M0) | 30–85 | no cure | 17 (9–27) | 5 (2–11) | no cure | 6 (0–20) | 7 (0–26) | no cure | 14 (10-17) | 4 (3–6) |
| Acute Myeloid leukemia without maturation (AML-M1) | 15–66 | 45 (28–61) | 47 (38–56) | no cure | 6 (0–18) | 6 (0–12) | no cure | 13 (8–18) | 13 (10–17) | no cure |
| Acute Myeloid leukemia with maturation (AML-M2) | 15–56 | 48 (32–62) | 35 (28–43) | no cure | 8 (0–17) | 8 (3–13) | no cure | 23 (18–28) | 18 (15–21) | no cure |
| Acute monoblastic and monocytic leukemia (AML-M5) | 15–85 | 41 (28–53) | 32 (24–40) | 4 (2–8) | 5 (0–14) | 6 (0–12) | 7 (0–21) | 10 (8–13) | 8 (6–10) | 3 (2–4) |
| Acute Myeloid leukemia, not otherwise specified (AML-NOS) | 15–72 | 38 (27–49) | 25 (20–30) | no cure | 6 (0–15) | 6 (1–12) | no cure | 11 (9–14) | 9 (7–11) | no cure |
| WOMEN | ||||||||||
| Acute promyelocytic leukemia with t(15;17)(q22;q12) (APL) | 15–85 | 90 (80–95) | 82 (73–88) | 55 (41–68) | 1 (0–2) | 2 (0–5 | 5 (0–16) | 7 (0–14) | 6 (0–13) | 4 (0–9) |
| AML with recurrent cytogenetic abnormalities (AML-RCA) | 15–65 | 79 (65–88) | 62 (50–72) | no cure | 4 (0–9) | 6 (0–12) | no cure | 20 (12–28) | 19 (12–25) | no cure |
| AML with myelodysplasia-related changes (AML-MRC) | 15–62 | 68 (39–85) | 33 (24–43) | no cure | 5 (0–15) | 7 (0–14) | no cure | 17 (11–23) | 12 (9–16) | no cure |
| Therapy-related AML; NOS (t-AML) | 20–72 | 10 (1–31) | 17 (10–25) | no cure | 7 (0–46) | 7 (0–18) | no cure | 6 (3–10) | 8 (6–10) | no cure |
| Pure erythroid leukemia (AML-M6) | 15–30 | 51 (10–82) | no cure | no cure | 6 (0–36) | no cure | no cure | 15 (7–22) | no cure | no cure |
| Acute Myelomonocytic leukemia (AML-M4) | 15–72 | 45 (28–60) | 40 (32–48) | no cure | 6 (0–17) | 6 (0–12) | no cure | 12 (10–15) | 11 (9–12) | no cure |
| Acute myeloid leukemia with minimal differentiation (AML-M0) | 15–70 | 44 (23–63) | 29 (16–44) | no cure | 6 (0–20) | 6 (0–20) | no cure | 14 (8–20) | 12 (8–17) | no cure |
| Acute Myeloid leukemia without maturation (AML-M1) | 15–85 | 51 (33–67) | 55 (46–62) | 9 (6–14) | 6 (0–17) | 5 (0–10) | 8 (0–19) | 17 (13–21) | 15 (12–18) | 5 (4–7) |
| Acute Myeloid leukaemia with maturation (AML-M2) | 15–58 | 64 (48–76) | 47 (39–54) | no cure | 5 (0–12) | 6 (2–10) | no cure | 21 (16–26) | 17 (14–21) | no cure |
| Acute monoblastic and monocytic leukemia (AML-M5) | 15–60 | 45 (29–60) | 41 (32–50) | no cure | 4 (0–13) | 4 (0–9) | no cure | 8 (5–12) | 9 (6–11) | no cure |
| Acute Myeloid leukemia, not otherwise specified (AML-NOS) | 15–85 | 58 (47–68) | 32 (26–37) | 4 (2–5) | 5 (0–10) | 6 (1–10) | 7 (0–16) | 13 (11–16) | 10 (8–11) | 3 (3–4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mounier, M.; Romain, G.; Callanan, M.; Alla, A.D.; Boussari, O.; Maynadié, M.; Colonna, M.; Jooste, V., for FRANCIM Network. Flexible Modeling of Net Survival and Cure by AML Subtype and Age: A French Population-Based Study from FRANCIM. J. Clin. Med. 2021, 10, 1657. https://doi.org/10.3390/jcm10081657
Mounier M, Romain G, Callanan M, Alla AD, Boussari O, Maynadié M, Colonna M, Jooste V for FRANCIM Network. Flexible Modeling of Net Survival and Cure by AML Subtype and Age: A French Population-Based Study from FRANCIM. Journal of Clinical Medicine. 2021; 10(8):1657. https://doi.org/10.3390/jcm10081657
Chicago/Turabian StyleMounier, Morgane, Gaëlle Romain, Mary Callanan, Akoua Denise Alla, Olayidé Boussari, Marc Maynadié, Marc Colonna, and Valérie Jooste for FRANCIM Network. 2021. "Flexible Modeling of Net Survival and Cure by AML Subtype and Age: A French Population-Based Study from FRANCIM" Journal of Clinical Medicine 10, no. 8: 1657. https://doi.org/10.3390/jcm10081657






