Forensic Analysis of Umbilical and Newborn Blood Gas Values for Infants at Risk of Cerebral Palsy
Abstract
1. Introduction
2. Methods
- Base deficit changes in labor: Previous reports have described the normal BD values prior to labor, changes with stages of labor and fetal heart rate decelerations, as well as normal blood gas values following a vaginal delivery [15,16]. Briefly, the normal fetal arterial BD averages 2 mmol/L prior to labor. The latent phase of labor has minimal change in fetal umbilical artery BD under normal conditions, though BD increases ~1 mmol/L every three-hours of the active phase and ~1 mmol/L per hour of active pushing in the second stage. Early decelerations do not increase fetal umbilical artery BD, though variable decelerations (dependent upon degree and duration) and late decelerations increase BD in a predictive manner [15,16,17,18,19,20,21] The duration of fetal bradycardia (50–70 bpm) corresponds to an increased BD by 1 mmol/L for every 2 minutes. The normal umbilical BDblood values following vaginal delivery approximate 5–6 mmol/L for umbilical artery and 4–5 mmol/L for umbilical vein [22,23].
- Clearance of acid in utero: Fetal acid clearance in utero is primarily across the placenta [24,25], as fetal renal blood flow is markedly reduced compared to postnatal values, and fetal renal acid clearance is immature [26]. As measured in animal models, placental clearance of metabolic acidosis occurs at a rate of ~0.1 mmol/L per minute during periods of normalized fetal blood flow and oxygenation [17,20,21]. Placental acid clearance may be limited under conditions of reduced placental function [19] or pathologies (i.e., placental abruption).
- BD changes during the neonatal period: Under conditions of normal newborn transition or rapid resuscitation, newborns do not increase the level of acidosis from delivery values once spontaneous heart rate exceeds 100 bpm. If born with significant metabolic acidosis, newborns minimally clear acid during the first one to two hours of life due to the early life impairment of hepatic and renal metabolism and clearance mechanisms as well as inhibition by acidosis [27,28]. Ultimately, the ‘’clearance’’ of systemic lactic acidosis depends on the initial peak level and the return and maintenance of spontaneous circulation and appropriate oxygenation. As neonatal cardiac compression produces less than 50% of normal cardiac output [29], newborns experience increasing acidosis during cardiac resuscitation until spontaneous heart rate exceeds 100 bpm. Similarly, severe newborn anemia, hypotension or septic shock will increase neonatal BD.
- Use of BD extracellular fluid (ECF): Blood gas analyzers measure pH, pCO2 and pO2, though bicarbonate values and BD are calculated. Most commercial blood gas analyzers report values as BD, though the calculation is performed for BDblood. However, BDblood values are significantly increased by elevated levels of pCO2 that commonly occur in fetal umbilical artery samples and may be dramatically elevated in cases of end-labor bradycardia. Thus, whereas BDblood values may be appropriate for most children and adults, recent reports have emphasized that BDECF [13,30] should be used rather than BDblood, as BDECF corrects for the altered pCO2 values. With the increasing recognition of BDECF, many of the commercial blood gas analyzers now include this measurement. Alternatively, formulas for the calculation of BDECF from measured pH and pCO2 are readily available. In this manuscript, the radiometer (Radiometer Medical, Brønshøj, Denmark) formula was utilized for all BDECF calculations [31,32].
- Comparison of umbilical artery and vein BD values: Under normal conditions, the (commonly reported) umbilical arterial BDblood is ~1 mmol/L greater than umbilical vein values [23,33], primarily due to the higher umbilical vein pCO2. BDECF values in the artery and vein are commonly similar as metabolic lactic acid is cleared slowly across the placenta. Under conditions of complete umbilical cord occlusion or placental separation from the uterine wall (complete placental abruption), there is no significant flow from the umbilical artery through the placenta to the umbilical vein. Consequently, umbilical venous values at birth represent fetal values at the time of the occurrence of complete cord occlusion or placental separation, while umbilical artery values represent the newborn at the time of birth [15], though the arterial level of acidosis may be impacted by prior occurrences of fetal ischemia or hypoxia. Fetal arterial BD increases by ~0.5 mmHg per minute of complete cord occlusion [20]. Thus, in conditions of complete cord occlusion umbilical venous blood can be completely normal, representing the state of the placenta prior to the sentinel event, though the fetus suffers from severe hypoxia-ischemia [34]. The difference between artery and vein values may be utilized to time the occurrence of cord occlusion or placental separation. Similarly, under conditions of complete umbilical cord occlusion, fetal umbilical artery pCO2 initially increases by ~7 mmHg per minute due to absent placental CO2 clearance [35].
- Umbilical artery and vein blood gas values: On occasion, samples of umbilical artery and vein blood are obtained from the same vessel, or samples are mislabeled. Normal fetal umbilical arterial and venous O2 and CO2 values [34], in conjunction with pH and BD values, can be used to assess if the values are consistent with the identified vessel. Similarly, if blood samples are exposed to an air bubble, umbilical pO2 values will increase and pCO2 values decrease due to atmospheric concentrations.
- Additional criteria for timing hypoxic ischemic injury: In addition to umbilical cord and newborn BD and blood gas values, Apgar scores, nucleated red blood cell count (nRBC), newborn platelet count and evidence of cerebral injury and edema may provide insight into timing. Briefly, as detailed in Neonatal Encephalopathy and Neurologic Outcome, Second Edition, a 5-min Apgar score of 7–10 is classified as reassuring, a score of 4–6 as moderately abnormal, and a score of 0–3 as low in the term infant and late-preterm infant [36]. Thus, 5-min Apgar scores of 7 or more are generally inconsistent with a sentinel hypoxic-ischemic event during labor. Whereas the normal nRBC count in a term infant is 0–4 nRBC/100 white blood cells [37], pre-existing hypoxic injury may produce nRBC counts of ≥26/100 white blood cells [38]. Acute hypoxic-ischemic injury typically results in an increase in nRBC counts, though to values below this level. Erythropoietin mediated stimulation requires ~ 24 h to increase newborn nRBC [39]. Thus, the relatively acute increase in nRBC following a sentinel obstetric event (e.g., uterine rupture) likely reflects release from fetal/newborn hematopoietic stores [40]. Nonspecific increases in nRBC counts may occur as a result of maternal smoking; anemia; hemolysis; and maternal diabetes [41]. Coagulopathy may be a consequence of asphyxia-induced hepatic dysfunction, as the international normalized ratio (INR) of asphyxiated infants may increase significantly on day 1 and 2 of life [42]. Newborn thrombocytopenia (<100,000) generally requires 24 h from a hypoxic injury to be manifest, while cerebral edema, sometimes evident by slit ventricles, generally does not present on an ultrasound or MRI (magnetic resonance imaging) until 18 to 24 h following an insult and generally abates by 5–6 days. Whereas deep gray matter (thalami, basal ganglia) injury is associated with an acute, profound hypoxic injury, cortical watershed regions have been associated with partial, prolonged hypoxic injury in term fetuses [43]. In addition to sites of injury, specific MRI sequences (T1, T2, diffusion weighted images, Flair and spectroscopy) [44] can differentiate myelination, ischemia, cytotoxic edema and other patterns of injury and can aid in timing. Early brain MRI showing ex vacuo brain changes suggests a more chronic timing in injury. Thus, an early brain MRI within the first one to two days of life may be valuable in the timing of an injury if it demonstrates a pattern of edema which would precede labor and delivery.
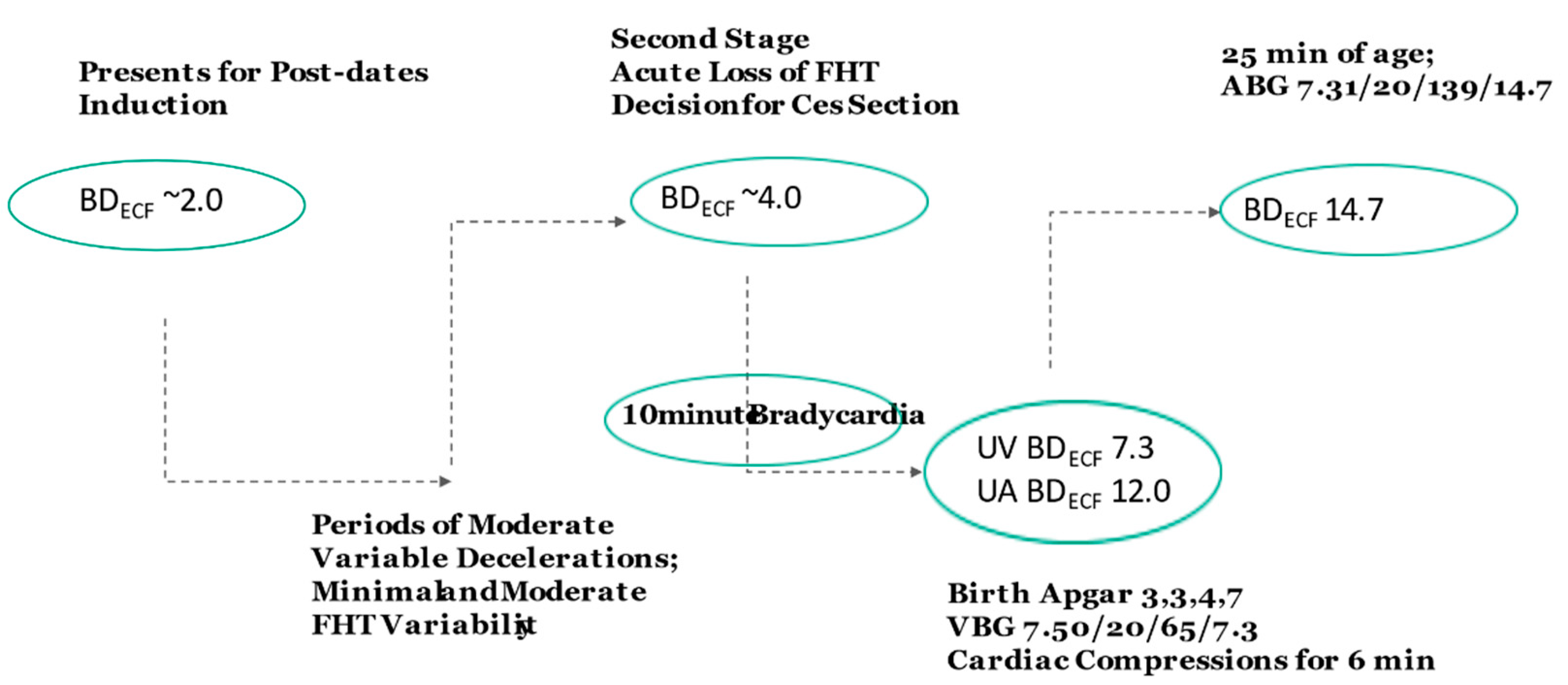
3. Case Studies
4. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Himmelmann, K.; Uvebrant, P. The panorama of cerebral palsy in Sweden part XII shows that patterns changed in the birth years 2007–2010. Acta Paediatr. 2018, 107, 462–468. [Google Scholar] [CrossRef]
- Blair, E.; Langdon, K.; McIntyre, S.; Lawrence, D.; Watson, L. Survival and mortality in cerebral palsy: Observations to the sixth decade from a data linkage study of a total population register and National Death Index. BMC Neurol. 2019, 19, 111. [Google Scholar] [CrossRef] [PubMed]
- Whitney, D.G.; Kamdar, N.S.; Ng, S.; Hurvitz, E.A.; Peterson, M.D. Prevalence of high-burden medical conditions and health care resource utilization and costs among adults with cerebral palsy. Clin Epidemiol. 2019, 11, 469–481. [Google Scholar] [CrossRef]
- Whitney, D.; Kamdar, N.; Hirth, R.A.; Hurvitz, E.A.; Peterson, M.D. Economic burden of paediatric-onset disabilities among young and middle-aged adults in the USA: A cohort study of privately insured beneficiaries. BMJ Open 2019, 9, e030490. [Google Scholar] [CrossRef]
- Pulgar, S.; Bains, S.; Gooch, J.; Chambers, H.; Noritz, G.H.; Wright, E.; Sawhney, T.G.; Pyenson, B.; Ferro, C. Prevalence, Patterns, and Cost of Care for Children with Cerebral Palsy Enrolled in Medicaid Managed Care. J. Manag. Care Spec. Pharm. 2019, 25, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Kancherla, V.; Amendah, D.D.; Grosse, S.D.; Yeargin-Allsopp, M.; Van Naarden Braun, K. Medical expenditures attributable to cerebral palsy and intellectual disability among Medicaid-enrolled children. Res. Dev. Disabil. 2012, 33, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Badawi, N.; Keogh, J.M. Causal pathways in cerebral palsy. J. Paediatr. Child Health 2013, 49, 5–8. [Google Scholar] [CrossRef]
- Sartwelle, T.P. Electronic Fetal Monitoring: A Defense Lawyer’s View. Rev. Obstet. Gynecol. 2012, 5, e121–e125. [Google Scholar] [PubMed]
- Sartwelle, T.P.; Johnston, J.C.; Arda, B. A half century of electronic fetal monitoring and bioethics: Silence speaks louder than words. Matern. Health Neonatol. Perinatol. 2017, 3, 21. [Google Scholar] [CrossRef]
- Sartwelle, T.P.; Johnston, J.C.; Arda, B. Perpetuating Myths, Fables, and Fairy Tales: A Half Century of Electronic Fetal Monitoring. Surg. J. N. Y. 2015, 1, e28–e34. [Google Scholar] [CrossRef]
- Clark, S.L.; Nageotte, M.P.; Garite, T.J.; Freeman, R.K.; Miller, D.A.; Simpson, K.R.; Belfort, M.A.; Dildy, G.A.; Parer, J.T.; Berkowitz, R.L.; et al. Intrapartum management of category II fetal heart rate tracings: Towards standardization of care. Am. J. Obstet. Gynecol. 2013, 209, 89–97. [Google Scholar] [CrossRef]
- Clark, S.L.; Hamilton, E.F.; Garite, T.J.; Timmins, A.; Warrick, P.A.; Smith, S. The limits of electronic fetal heart rate monitoring in the prevention of neonatal metabolic acidemia. Am. J. Obstet. Gynecol. 2017, 216, 163 e161–163 e166. [Google Scholar] [CrossRef]
- Ross, M.G. Threshold of metabolic acidosis associated with newborn cerebral palsy: Medical legal implications. Am. J. Obstet. Gynecol. 2019, 220, 348–353. [Google Scholar] [CrossRef]
- Kelly, R.; Ramaiah, S.M.; Sheridan, H.; Cruickshank, H.; Rudnicka, M.; Kissack, C.; Becher, J.C.; Stenson, B.J. Dose-dependent relationship between acidosis at birth and likelihood of death or cerebral palsy. Arch Dis. Child. Fetal Neonatal Ed. 2017, 103, F567–F572. [Google Scholar] [CrossRef] [PubMed]
- Hagelin, A.; Leyon, J. The effect of labor on the acid-base status of the newborn. Acta Obstet. Gynecol. Scand. 1998, 77, 841–844. [Google Scholar] [PubMed]
- Lazarević, B.; Ljubić, A.; Stević, R.; Sulović, V.; Rosić, B.; Radunović, N.; Ilić, S. Respiratory gases and acid base parameter of the fetus during the second and third trimester. Clin. Exp. Obstet. Gynecol. 1991, 18, 81–84. [Google Scholar]
- Ross, M.G.; Gala, R. Use of umbilical artery base excess: Algorithm for the timing of hypoxic injury. Am. J. Obstet. Gynecol. 2002, 187, 1–9. [Google Scholar] [CrossRef]
- Ross, M.G. Base excess during cord occlusion. Am. J. Obstet. Gynecol. 2003, 189, 1811–1812. [Google Scholar] [CrossRef]
- Amaya, K.E.; Matushewski, B.; Durosier, L.D.; Frasch, M.G.; Richardson, B.S.; Ross, M.G. Accelerated acidosis in response to variable fetal heart rate decelerations in chronically hypoxic ovine fetuses. Am. J. Obstet. Gynecol. 2016, 214, e270–e271. [Google Scholar] [CrossRef]
- Ross, M.G.; Jessie, M.; Amaya, K.; Matushewski, B.; Durosier, L.D.; Frasch, M.G.; Richardson, B.S. Correlation of arterial fetal base deficit and lactate changes with severity of variable heart rate decelerations in the near-term ovine fetus. Am. J. Obstet. Gynecol. 2013, 208, e281–e286. [Google Scholar] [CrossRef]
- Frasch, M.G.; Mansano, R.Z.; McPhaul, L.; Gagnon, R.; Richardson, B.S.; Ross, M.G. Measures of acidosis with repetitive umbilical cord occlusions leading to fetal asphyxia in the near-term ovine fetus. Am. J. Obstet Gynecol. 2009, 200, e201–e207. [Google Scholar] [CrossRef] [PubMed]
- Victory, R.; Penava, D.; Da Silva, O.; Natale, R.; Richardson, B. Umbilical cord pH and base excess values in relation to adverse outcome events for infants delivering at term. Am. J. Obstet. Gynecol. 2004, 191, 2021–2028. [Google Scholar] [CrossRef] [PubMed]
- Arikan, G.M.; Scholz, H.S.; Haeusler, M.C.; Giuliani, A.; Haas, J.; Weiss, P.A. Low fetal oxygen saturation at birth and acidosis. Obstet. Gynecol. 2000, 95, 565–571. [Google Scholar]
- Hooper, S.B. Fetal metabolic responses to hypoxia. Reprod Fertil Dev. 1995, 7, 527–538. [Google Scholar] [CrossRef]
- Hooper, S.B.; Walker, D.W.; Harding, R. Oxygen, glucose, and lactate uptake by fetus and placenta during prolonged hypoxemia. Am. J. Physiol. 1995, 268 Pt 2, R303–R309. [Google Scholar] [CrossRef]
- Elbourne, I.; Lumbers, E.R.; Hill, K.J. The secretion of organic acids and bases by the ovine fetal kidney. Exp. Physiol. 1990, 75, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Beath, S.V. Hepatic function and physiology in the newborn. Semin Neonatol. 2003, 8, 337–346. [Google Scholar] [CrossRef]
- Bellomo, R. Bench-to-bedside review: Lactate and the kidney. Crit. Care. 2002, 6, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, J.S. Mechanical cardiopulmonary resuscitation for patients with cardiac arrest. World J. Emerg. Med. 2011, 2, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Berend, K. Diagnostic Use of Base Excess in Acid-Base Disorders. N. Engl. J. Med. 2018, 378, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Reference Manual for ABLTM 700 Series; Radiometer Medical: Brønshøj, Denmark, 2003.
- Mokarami, P.; Wiberg, N.; Olofsson, P. An overlooked aspect on metabolic acidosis at birth: Blood gas analyzers calculate base deficit differently. Acta Obstet. Gynecol. Scand. 2012, 91, 574–579. [Google Scholar] [CrossRef]
- Helwig, J.T.; Parer, J.T.; Kilpatrick, S.J.; Laros, R.K., Jr. Umbilical cord blood acid-base state: What is normal? Am. J. Obstet. Gynecol. 1996, 174, 1807–1812. [Google Scholar] [CrossRef]
- Pomerance, J. Umbilical cord blood gas casebook. J. Perinatol. 2002, 22, 504–505. [Google Scholar] [CrossRef] [PubMed]
- Fujii, E.Y.; Takahashi, N.; Kodama, Y.; Roman, C.; Ferriero, D.M.; Parer, J.T. Hemodynamic changes during complete umbilical cord occlusion in fetal sheep related to hippocampal neuronal damage. Am. J. Obstet. Gynecol. 2003, 188, 413–418. [Google Scholar] [CrossRef]
- ACoOa, American College of Obstetrics and Gynecology. Task Force on Neonatal Encephalopathy; American Academy of Pediatrics. Neonatal Encephalopathy and Neurologic Outcome, 2nd ed.; Americn College of Obstetricians and Gynecologists: Washington, DC, USA, 2014. [Google Scholar]
- Nicolaides, K.H.; Thilaganathan, B.; Mibashan, R.S. Cordocentesis in the investigation of fetal erythropoiesis. Am. J. Obstet. Gynecol. 1989, 161, 1197–1200. [Google Scholar] [CrossRef]
- Hankins, G.D.; Koen, S.; Gei, A.F.; Lopez, S.M.; Van Hook, J.W.; Anderson, G.D. Neonatal organ system injury in acute birth asphyxia sufficient to result in neonatal encephalopathy. Obstet. Gynecol. 2002, 99 Pt 1, 688–691. [Google Scholar]
- Christensen, R.D.; Lambert, D.K.; Richards, D.S. Estimating the nucleated red blood cell ‘emergence time’ in neonates. J. Perinatol. 2014, 34, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Bedrick, A.D. Nucleated red blood cells and fetal hypoxia: A biologic marker whose ‘timing’ has come? J. Perinatol. 2014, 34, 85–86. [Google Scholar] [CrossRef]
- Hermansen, M.C. Nucleated red blood cells in the fetus and newborn. Arch Dis. Child. Fetal Neonatal Ed. 2001, 84, F211–F215. [Google Scholar] [CrossRef]
- Sweetman, D.; Kelly, L.A.; Zareen, Z.; Nolan, B.; Murphy, J.; Boylan, G.; Donoghue, V.; Molloy, E.J. Coagulation Profiles Are Associated with Early Clinical Outcomes in Neonatal Encephalopathy. Front. Pediatr. 2019, 7, 399. [Google Scholar] [CrossRef]
- Triulzi, F.; Parazzini, C.; Righini, A. Patterns of damage in the mature neonatal brain. Pediatr. Radiol. 2006, 36, 608–620. [Google Scholar] [CrossRef]
- Varghese, B.; Xavier, R.; Manoj, V.C.; Aneesh, M.K.; Priya, P.S.; Kumar, A.; Sreenivasan, V.K. Magnetic resonance imaging spectrum of perinatal hypoxic-ischemic brain injury. Indian J. Radiol Imaging 2016, 26, 316–327. [Google Scholar] [CrossRef]
- Wyckoff, M.H.; Aziz, K.; Escobedo, M.B.; Kapadia, V.S.; Kattwinkel, J.; Perlman, J.M.; Simon, W.M.; Weiner, G.M.; Zaichkin, J.G. Part 13: Neonatal Resuscitation: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015, 132 (Suppl. 2), S543–S560. [Google Scholar] [CrossRef]
- Redline, R.W. Cerebral palsy in term infants: A clinicopathologic analysis of 158 medicolegal case reviews. Pediatr. Dev. Pathol. 2008, 11, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Ross, M. How to differentiate maternal from fetal heart rate patterns on electronic fetal monitoring. OBG Manag. 2018, 30, 40–46. [Google Scholar]
- Stoll, T. The importance of guidelines for court-appointed experts in medical malpractice litigation. Anaesthesist 2019, 68, 633–636. [Google Scholar] [CrossRef] [PubMed]
- de Reuver, P.R.; Dijkgraaf, M.G.; Gevers, S.K.; Gouma, D.J.; Group, B.S. Poor agreement among expert witnesses in bile duct injury malpractice litigation: An expert panel survey. Ann. Surg. 2008, 248, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, S.; Backe, B.; Lydersen, S.; Ovrebo, K.; Oian, P. The consistency of experts’ evaluation of obstetric claims for compensation. BJOG 2015, 122, 948–953. [Google Scholar] [CrossRef][Green Version]
- Petty, T.; Stephenson, L.; Campbell, P.; Stephenson, T. Outcome Bias in Clinical Negligence Medico-legal Cases. J. Law Med. 2019, 26, 825–830. [Google Scholar] [PubMed]
- Hankins, G.D.; MacLennan, A.H.; Speer, M.E.; Strunk, A.; Nelson, K. Obstetric litigation is asphyxiating our maternity services. Obstet. Gynecol. 2006, 107, 1382–1385. [Google Scholar] [CrossRef]
- Weber, C.E.; Talbot, L.J.; Geller, J.M.; Kuo, M.C.; Wai, P.Y.; Kuo, P.C. Comparing 20 years of national general surgery malpractice claims data: Obesity versus morbid obesity. Am. J. Surg. 2013, 205, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Depp, R. Perinatal asphyxia: Assessing its causal role and timing. Semin. Pediatr. Neurol. 1995, 2, 3–36. [Google Scholar] [CrossRef]
- Shields, J.R.; Schifrin, B.S. Perinatal antecedents of cerebral palsy. Obstet. Gynecol. 1988, 71 Pt 1, 899–905. [Google Scholar] [CrossRef]
- Schifrin, B.S.; Hamilton-Rubinstein, T.; Shields, J.R. Fetal heart rate patterns and the timing of fetal injury. J. Perinatol. 1994, 14, 174–181. [Google Scholar] [PubMed]
- Towell, M.E. The rationale for biochemical moni-toring of the fetus. J. Perinat Med. 1988, 16 (Suppl. 1), 55–70. [Google Scholar] [CrossRef]
- Neacsu, A.; Herghelegiu, C.G.; Voinea, S.; Dimitriu, M.C.T.; Ples, L.; Bohiltea, R.E.; Braila, A.D.; Nastase, L.; Bacalbasa, N.; Chivu, L.I.; et al. Umbilical cord lactate compared with pH as predictors of intrapartum asphyxia. Exp. Ther. Med. 2021, 21, 80. [Google Scholar] [CrossRef] [PubMed]
- Pierre, F. Medical-legal aspects: The obstetrician as a defendant or as an expert. J. Gynecol. Obstet. Biol. Reprod. Paris 2003, 32 (Suppl. 1), S114–S118. [Google Scholar]
- Sandler, P.; Goldstein, L.N. The effect of different forms of heparin on point-of-care blood gas analysis. S. Afr. Med. J. 2018, 108, 224–229. [Google Scholar] [CrossRef]
- Shipley, L.; Gale, C.; Sharkey, D. Trends in the incidence and management of hypoxic-ischaemic encephalopathy in the therapeutic hypothermia era: A national population study. Arch Dis. Child. Fetal Neonatal. Ed. 2021. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ushiro, S.; Suzuki, H.; Ueda, S. Japan Obstetric Compensation System for Cerebral Palsy: Strategic system of data aggregation, investigation, amelioration and no-fault compensation. J. Obstet. Gynaecol. Res. 2019, 45, 493–513. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ross, M.G. Forensic Analysis of Umbilical and Newborn Blood Gas Values for Infants at Risk of Cerebral Palsy. J. Clin. Med. 2021, 10, 1676. https://doi.org/10.3390/jcm10081676
Ross MG. Forensic Analysis of Umbilical and Newborn Blood Gas Values for Infants at Risk of Cerebral Palsy. Journal of Clinical Medicine. 2021; 10(8):1676. https://doi.org/10.3390/jcm10081676
Chicago/Turabian StyleRoss, Michael G. 2021. "Forensic Analysis of Umbilical and Newborn Blood Gas Values for Infants at Risk of Cerebral Palsy" Journal of Clinical Medicine 10, no. 8: 1676. https://doi.org/10.3390/jcm10081676
APA StyleRoss, M. G. (2021). Forensic Analysis of Umbilical and Newborn Blood Gas Values for Infants at Risk of Cerebral Palsy. Journal of Clinical Medicine, 10(8), 1676. https://doi.org/10.3390/jcm10081676





