Predictors of Single Bronchodilation Treatment Response for COPD: An Observational Study with the Trace Database Cohort
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis and Modeling
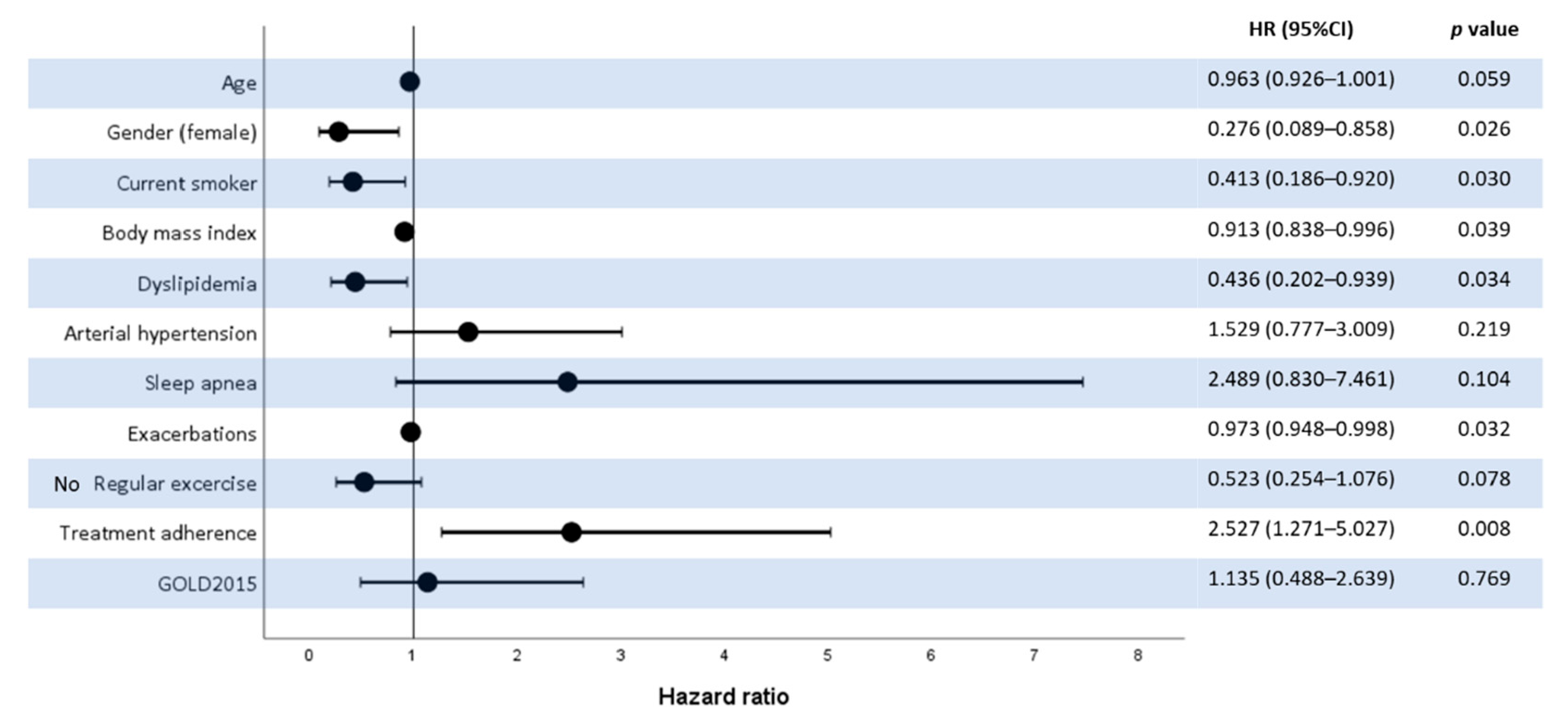
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soler-Cataluna, J.J. Clinical practice guidelines or personalized medicine in chronic obstructive pulmonary disease? Arch. Bronconeumol. 2018, 54, 247–248. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Agusti, A.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Criner, G.J.; Frith, P.; Halpin, D.M.G.; Han, M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: The gold science committee report 2019. Eur. Respir. J. Off. J. Eur. Soc. Clin. Respir. Physiol. 2019, 53, 1900164. [Google Scholar] [CrossRef] [PubMed]
- Donohue, J.F.; Singh, D.; Munzu, C.; Kilbride, S.; Church, A. Magnitude of umeclidinium/vilanterol lung function effect depends on monotherapy responses: Results from two randomised controlled trials. Respir. Med. 2016, 112, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Feldman, G.J.; Sousa, A.R.; Lipson, D.A.; Tombs, L.; Barnes, N.; Riley, J.H.; Patel, S.; Naya, I.; Compton, C.; Alcazar Navarrete, B. Comparative efficacy of once-daily umeclidinium/vilanterol and tiotropium/olodaterol therapy in symptomatic chronic obstructive pulmonary disease: A randomized study. Adv. Ther. 2017, 34, 2518–2533. [Google Scholar] [CrossRef]
- Lopez-Campos, J.L.; Calero-Acuna, C.; Marquez-Martin, E.; Quintana Gallego, E.; Carrasco-Hernandez, L.; Abad Arranz, M.; Ortega Ruiz, F. Double bronchodilation in chronic obstructive pulmonary disease: A crude analysis from a systematic review. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 1867–1876. [Google Scholar] [CrossRef][Green Version]
- Soler-Cataluna, J.J.; Alcazar, B.; Marzo, M.; Perez, J.; Miravitlles, M. Evaluation of changes in control status in copd: An opportunity for early intervention. Chest 2020, 157, 1138–1146. [Google Scholar] [CrossRef]
- Carrasco Hernández, L.; Caballero Eraso, C.; Abad Arranz, M.; Márquez Martín, E.; Calero Acuña, C.; Lopez-Campos, J.L. Time-based register and analysis of copd endpoints (trace) project: Methodology and workflow. Arch. Bronconeumol. 2020. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Schilling, R.S.; Hughes, J.P.; Dingwall-Fordyce, I. Disagreement between observers in an epidemiological study of respiratory disease. Br. Med. J. 1955, 1, 65–68. [Google Scholar] [CrossRef]
- Pocock, S.J.; McMurray, J.J.V.; Collier, T.J. Statistical controversies in reporting of clinical trials: Part 2 of a 4-part series on statistics for clinical trials. J. Am. Coll Cardiol. 2015, 66, 2648–2662. [Google Scholar] [CrossRef]
- Sedgwick, P. Understanding the ecological fallacy. BMJ 2015, 351, h4773. [Google Scholar] [CrossRef]
- Wang, X.; Kattan, M.W. Cohort studies: Design, analysis, and reporting. Chest 2020, 158, S72–S78. [Google Scholar] [CrossRef]
- Ford, I.; Norrie, J. Pragmatic trials. N. Engl. J. Med. 2016, 375, 454–463. [Google Scholar] [CrossRef]
- Abad-Arranz, M.; Moran-Rodriguez, A.; Mascaros Balaguer, E.; Quintana Velasco, C.; Abad Polo, L.; Nunez Palomo, S.; Gonzalvez Rey, J.; Fernandez Vargas, A.M.; Hidalgo Requena, A.; Helguera Quevedo, J.M.; et al. Quantification of inaccurate diagnosis of copd in primary care medicine: An analysis of the coach clinical audit. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 1187–1194. [Google Scholar] [CrossRef]
- Abad-Arranz, M.; Moran-Rodriguez, A.; Mascaros Balaguer, E.; Quintana Velasco, C.; Abad Polo, L.; Nunez Palomo, S.; Gonzalvez Rey, J.; Fernandez Vargas, A.M.; Hidalgo Requena, A.; Helguera Quevedo, J.M.; et al. Community assessment of copd health care (coach) study: A clinical audit on primary care performance variability in copd care. BMC Med. Res. Methodol. 2018, 18, 68. [Google Scholar] [CrossRef]
- Calle Rubio, M.; Rodriguez Hermosa, J.L.; Soler-Cataluna, J.J.; Lopez-Campos, J.L.; Alcazar Navarrete, B.; Soriano, J.B.; Rodriguez Gonzalez-Moro, J.M.; Fuentes Ferrer, M.E.; Miravitlles, M.; Grupo EPOCONSUL. Medical care according to risk level and adaptation to spanish copd guidelines (gesepoc): The epoconsul study. Arch. Bronconeumol. 2018, 54, 270–279. [Google Scholar] [CrossRef]
- Lopez-Campos, J.L.; Navarrete, B.A.; Soriano, J.B.; Soler-Cataluna, J.J.; Gonzalez-Moro, J.M.R.; Ferrer, M.E.F.; Rubio, M.C. Determinants of medical prescriptions for copd care: An analysis of the epoconsul clinical audit. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 2279–2288. [Google Scholar] [CrossRef]
- Perez, T.A.; Castillo, E.G.; Ancochea, J.; Pastor Sanz, M.T.; Almagro, P.; Martinez-Camblor, P.; Miravitlles, M.; Rodriguez-Carballeira, M.; Navarro, A.; Lamprecht, B.; et al. Sex differences between women and men with copd: A new analysis of the 3cia study. Respir. Med. 2020, 171, 106105. [Google Scholar] [CrossRef]
- Zysman, M.; Burgel, P.R.; Court-Fortune, I.; Brinchault-Rabin, G.; Nesme-Meyer, P.; Surpas, P.; Deslee, G.; Perez, T.; Le Rouzic, O.; Jebrak, G.; et al. Relationship between gender and survival in a real-life cohort of patients with copd. Respir. Res. 2019, 20, 191. [Google Scholar] [CrossRef]
- Cote, C.G.; Chapman, K.R. Diagnosis and treatment considerations for women with copd. Int. J. Clin. Pract. 2009, 63, 486–493. [Google Scholar] [CrossRef]
- Soriano, J.B.; Alfageme, I.; Miravitlles, M.; de Lucas, P.; Soler-Cataluna, J.J.; Garcia-Rio, F.; Casanova, C.; Rodriguez Gonzalez-Moro, J.M.; Cosio, B.G.; Sanchez, G.; et al. Prevalence and determinants of copd in spain: Episcan II. Arch. Bronconeumol. 2020, 57, 61–69. [Google Scholar] [CrossRef]
- Whittaker, H.R.; Pimenta, J.M.; Jarvis, D.; Kiddle, S.J.; Quint, J.K. Characteristics associated with accelerated lung function decline in a primary care population with chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 3079–3091. [Google Scholar] [CrossRef]
- Lange, P.; Celli, B.; Agusti, A.; Boje Jensen, G.; Divo, M.; Faner, R.; Guerra, S.; Marott, J.L.; Martinez, F.D.; Martinez-Camblor, P.; et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N. Engl. J. Med. 2015, 373, 111–122. [Google Scholar] [CrossRef]
- Lopez-Campos, J.L.; Quintana Gallego, E.; Carrasco Hernandez, L. Status of and strategies for improving adherence to copd treatment. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 1503–1515. [Google Scholar] [CrossRef]
- Sonnex, K.; Alleemudder, H.; Knaggs, R. Impact of smoking status on the efficacy of inhaled corticosteroids in chronic obstructive pulmonary disease: A systematic review. BMJ Open 2020, 10, e037509. [Google Scholar] [CrossRef]
- Josephs, L.; Culliford, D.; Johnson, M.; Thomas, M. Improved outcomes in ex-smokers with copd: A uk primary care observational cohort study. Eur. Respir. J. Off. J. Eur. Soc. Clin. Respir. Physiol. 2017, 49, 1602114. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Campos, J.L.; Ruiz-Duque, B.; Carrasco-Hernandez, L.; Caballero-Eraso, C. Integrating comorbidities and phenotype-based medicine in patient-centered medicine in copd. J. Clin. Med. 2020, 9, 2745. [Google Scholar] [CrossRef] [PubMed]
- Yohannes, A.M.; Dryden, S.; Casaburi, R.; Hanania, N.A. Long-term benefits of pulmonary rehabilitation in copd patients: A 2-year follow-up study. Chest 2020, 159, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Bourbeau, J.; Gagnon, S.; Ross, B. Pulmonary rehabilitation. Clin. Chest Med. 2020, 41, 513–528. [Google Scholar] [CrossRef]
- Walsh, A.; Perrem, L.; Khashan, A.S.; Henry, M.T.; Ni Chroinin, M. Statins versus placebo for people with chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2019, 7, CD011959. [Google Scholar] [CrossRef]
- Lin, C.M.; Yang, T.M.; Yang, Y.H.; Tsai, Y.H.; Lee, C.P.; Chen, P.C.; Chen, W.C.; Hsieh, M.J. Statin use and the risk of subsequent hospitalized exacerbations in copd patients with frequent exacerbations. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 289–299. [Google Scholar] [CrossRef]
- Rea, F.; Calusi, G.; Franchi, M.; Vetrano, D.L.; Roberto, G.; Bonassi, S.; Kirchmayer, U.; Chinellato, A.; Bettiol, A.; Sultana, J.; et al. Adherence of elderly patients with cardiovascular disease to statins and the risk of exacerbation of chronic obstructive pulmonary disease: Evidence from an italian real-world investigation. Drugs Aging 2018, 35, 1099–1108. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, T.; Wang, Z.; Yu, F.; Xu, Q.; Guo, W.; Wu, C.; He, J. Body mass index and mortality in chronic obstructive pulmonary disease: A dose-response meta-analysis. Medicine 2016, 95, e4225. [Google Scholar] [CrossRef]
- Franssen, F.M.; O’Donnell, D.E.; Goossens, G.H.; Blaak, E.E.; Schols, A.M. Obesity and the lung: 5. Obesity and copd. Thorax 2008, 63, 1110–1117. [Google Scholar] [CrossRef]
- Parameswaran, K.; Todd, D.C.; Soth, M. Altered respiratory physiology in obesity. Can. Respir. J. 2006, 13, 203–210. [Google Scholar] [CrossRef]
| Factors | Total (n = 764) | LABD Monotherapy (n = 128) | Other Treatments (n = 636) | p-Value * |
|---|---|---|---|---|
| Age (years) | 69 (62.76) | 66 (62.73) | 70 (61.77) | 0.017 |
| Gender (male) | 6,590,020 (86.3) | 108 (84.4%) | 551 (86.6%) | 0.498 |
| Comorbidities (Charlson) | 2.26 (1.6) | 2.13 (1.4) | 2.28 (1.6) | 0.309 |
| Hypertension | 389 (51.0%) | 53 (41.7%) | 336 (52.9%) | 0.028 |
| FEV1 (mL) | 1320 (720) | 1700 (757.5) | 1260 (617.5) | <0.001 |
| GOLD 1 | 71 (9.3%) | 25 (19.7%) | 46 (7.2%) | <0.001 |
| GOLD 2 | 411 (53.8%) | 89 (69.5%) | 322 (50.6%) | <0.001 |
| GOLD 3 | 227 (29.7%) | 13 (10.2%) | 214 (33.6%) | <0.001 |
| GOLD 4 | 55 (7.2%) | 1 (0.8%) | 54 (8.5%) | 0.002 |
| MRC 0 | 144 (18.9%) | 49 (38.6%) | 95 (15.0%) | <0.001 |
| MRC 1 | 393 (51.4%) | 61 (47.2%) | 332 (52.2%) | 0.384 |
| MRC 2 | 150 (19.7%) | 17 (13.4%) | 133 (20.9%) | 0.047 |
| MRC 3 | 55 (7.2%) | 1 (0.8%) | 54 (8.5%) | 0.002 |
| MRC 4 | 18 (2.4%) | 0 (0.0%) | 18 (2.8%) | 0.054 |
| Cough | 451 (59.2%) | 63 (49.6%) | 388 (61.1%) | 0.021 |
| Expectoration | 407 (53.4%) | 57 (44.9%) | 350 (55.1%) | 0.044 |
| Exacerbations during the previous 12 months | 0.9 (1.1) | 0.6 (0.9) | 0.9 (1.1) | <0.001 |
| Hospital admission during the previous 12 months | 0.1 (0.4) | 0.08 (0.2) | 0.1 (0.4) | 0.124 |
| Perform daily exercise | 212 (27.8%) | 46 (36.2%) | 166 (26.1%) | 0.027 |
| Received flu vaccine | 526 (69.0%) | 75 (59.1%) | 451 (71.0%) | 0.011 |
| On oxygen therapy | 83 (10.9%) | 2 (1.6%) | 81 (12.8%) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, L.C.; Eraso, C.C.; Ruiz-Duque, B.; Arranz, M.A.; Martín, E.M.; Calero Acuña, C.; Lopez-Campos, J.L. Predictors of Single Bronchodilation Treatment Response for COPD: An Observational Study with the Trace Database Cohort. J. Clin. Med. 2021, 10, 1708. https://doi.org/10.3390/jcm10081708
Hernández LC, Eraso CC, Ruiz-Duque B, Arranz MA, Martín EM, Calero Acuña C, Lopez-Campos JL. Predictors of Single Bronchodilation Treatment Response for COPD: An Observational Study with the Trace Database Cohort. Journal of Clinical Medicine. 2021; 10(8):1708. https://doi.org/10.3390/jcm10081708
Chicago/Turabian StyleHernández, Laura Carrasco, Candela Caballero Eraso, Borja Ruiz-Duque, María Abad Arranz, Eduardo Márquez Martín, Carmen Calero Acuña, and Jose Luis Lopez-Campos. 2021. "Predictors of Single Bronchodilation Treatment Response for COPD: An Observational Study with the Trace Database Cohort" Journal of Clinical Medicine 10, no. 8: 1708. https://doi.org/10.3390/jcm10081708
APA StyleHernández, L. C., Eraso, C. C., Ruiz-Duque, B., Arranz, M. A., Martín, E. M., Calero Acuña, C., & Lopez-Campos, J. L. (2021). Predictors of Single Bronchodilation Treatment Response for COPD: An Observational Study with the Trace Database Cohort. Journal of Clinical Medicine, 10(8), 1708. https://doi.org/10.3390/jcm10081708






