Reassessment of Poststroke Dysphagia in Rehabilitation Facility Results in Reduction in Diet Restrictions
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
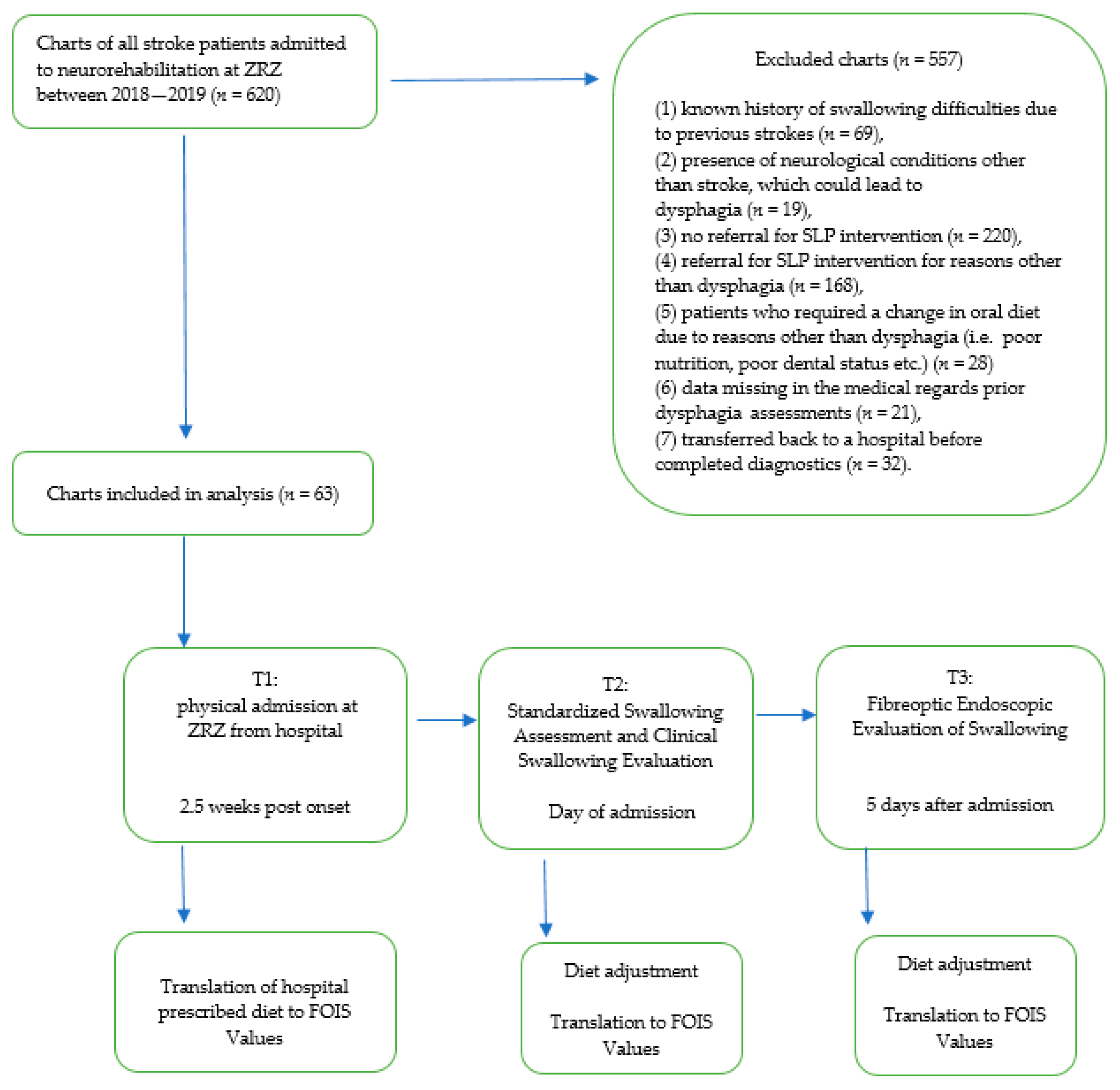
- (1)
- the first diagnosis of stroke and
- (2)
- stable medical condition.
- (1)
- known history of swallowing difficulties due to previous strokes (n = 69),
- (2)
- presence of neurological conditions other than stroke, which could lead to dysphagia (n = 19),
- (3)
- no referral for SLP intervention (n = 220),
- (4)
- referral for SLP intervention for reasons other than dysphagia (n = 168),
- (5)
- patients who required a change in oral diet due to reasons other than dysphagia (i.e., poor nutrition, poor dental status) (n = 28)
- (6)
- data missing in the medical records’ prior dysphagia assessments (n = 21),
- (7)
- transferred back to a hospital before completed diagnostics (n = 32).
2.2. Study Design
2.3. Procedures
2.3.1. Screening Procedures for Presence of Dysphagia and Referral to SLP
- (1)
- listed as a medical diagnosis,
- (2)
- confirmed by previous diagnostic procedures documented in the patient’s records,
- (3)
- diet status prescribed in acute care,
- (4)
- stated by staff in the admission-coordination-report.
2.3.2. Clinical Dysphagia Assessment
2.3.3. Instrumental Assessment
2.3.4. Oral Intake Status
2.3.5. Functional Independence Measurement (FIM)
2.4. Statistical Analysis
3. Results

4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Burgos, R.; Bretón, I.; Cereda, E.; Desport, J.C.; Dziewas, R.; Genton, L.; Gomes, F.; Jésus, P.; Leischker, A.; Muscaritoli, M.; et al. ESPEN guideline clinical nutrition in neurology. Clin. Nutr. 2018, 37, 354–396. [Google Scholar] [CrossRef] [PubMed]
- Martino, R.; Foley, N.; Bhogal, S.; Diamant, N.; Speechley, M.; Teasell, R. Dysphagia after stroke: Incidence, diagnosis, and pulmonary complications. Stroke 2005, 36, 2756–2763. [Google Scholar] [CrossRef]
- Hinchey, J.A.; Shephard, T.; Furie, K.; Smith, D.; Wang, D.; Tonn, S.; Stroke Practice Improvement Network Investigators. Formal dysphagia screening protocols prevent pneumonia. Stroke 2005, 36, 1972–1976. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.; Emery, P.W.; Weekes, C.E. Risk of Malnutrition Is an Independent Predictor of Mortality, Length of Hospital Stay, and Hospitalization Costs in Stroke Patients. J. Stroke Cerebrovasc. Dis. 2016, 25, 25799–25806. [Google Scholar] [CrossRef]
- Muehlemann, N.; Jouaneton, B.; de Léotoing, L.; Chalé, J.J.; Fernandes, J.; Kägi, G.; Sarikaya, H.; Arnold, M. Hospital costs impact of post ischemic stroke dysphagia: Database analyses of hospital discharges in France and Switzerland. PLoS ONE 2019, 14, e0210313. [Google Scholar] [CrossRef]
- Sørensen, R.T.; Rasmussen, R.S.; Overgaard, K.; Lerche, A.; Johansen, A.M.; Lindhardt, T. Dysphagia screening and intensified oral hygiene reduce pneumonia after stroke. J. Neurosci. Nurs. 2013, 45, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Eltringham, S.A.; Kilner, K.; Gee, M.; Sage, K.; Bray, B.D.; Pownall, S.; Smith, C.J. Impact of Dysphagia Assessment and Management on Risk of Stroke-Associated Pneumonia: A Systematic Review. Cerebrovasc. Dis. 2018, 46, 99–107. [Google Scholar] [CrossRef]
- Deutsche Gesellschaft für Neurologie. Available online: www.dgn.org/leitlinien (accessed on 24 January 2021).
- McCurtin, A.; Boland, P.; Kavanagh, M.; Lisiecka, D.; Roche, C.; Galvin, R. Do stroke clinical practice guideline recommendations for the intervention of thickened liquids for aspiration support evidence based decision making? A systematic review and narrative synthesis. J. Eval. Clin. Pract. 2020, 26, 1744–1760. [Google Scholar] [CrossRef] [PubMed]
- Smithard, D.G.; Fairfield, C.; Roffe, C.; Enderby, P. European Society for Swallowing Disorders Position Statements and Meeting Abstracts 2012: Dysphagia management in the acute phase of stroke. Dysphagia 2007, 28, 307. [Google Scholar]
- Braun, T.; Juenemann, M.; Viard, M.; Meyer, M.; Fuest, S.; Reuter, I.; Kaps, M.; Prosiegel, M.; Tanislav, C. What is the value of fibre-endoscopic evaluation of swallowing (FEES) in neurological patients? A cross-sectional hospital-based registry study. BMJ Open 2018, 8, e019016. [Google Scholar] [CrossRef]
- Lippert, W.C.; Chadha, R.; Sweigart, J.R. Things We Do for No Reason: The Use of Thickened Liquids in Treating Hospitalized Adult Patients with Dysphagia. J. Hosp. Med. 2019, 14, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.G.; Yoo, D.H. A comparison of the swallowing function and quality of life by oral intake level in stroke patients with dysphagia. J. Phys. Ther. Sci. 2017, 29, 1552–1554. [Google Scholar] [CrossRef]
- Crary, M.A.; Mann, G.D.; Groher, M.E. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch. Phys. Med. Rehabil. 2005, 86, 1516–1520. [Google Scholar] [CrossRef]
- Perry, L.; Love, C.P. Screening for dysphagia and aspiration in acute stroke: A systematic review. Dysphagia 2001, 16, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Daniels, S.K.; Brailey, K.; Priestly, D.H.; Herrington, L.R.; Weisberg, L.A.; Foundas, A.L. Aspiration in patients with acute stroke. Arch. Phys. Med. Rehabil. 1998, 79, 14–19. [Google Scholar] [CrossRef]
- Logemann, J.A.; Veis, S.; Colangelo, L. A screening procedure for oropharyngeal dysphagia. Dysphagia 1999, 14, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Langmore, S.E. Endoscopic Evaluation and Treatment of Swallowing Disorders, 2nd ed.; Thieme Medical Publishers: New York, NY, USA, 2001. [Google Scholar]
- Rosenbek, J.C.; Robbins, J.A.; Roecker, E.B.; Coyle, J.L.; Wood, J.L. A penetration-aspiration scale. Dysphagia 1996, 11, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Linacre, J.M.; Heinemann, A.W.; Wright, B.D.; Granger, C.V.; Hamilton, B.B. The structure and stability of the Functional Independence Measure. Arch. Phys. Med. Rehabil. 1994, 75, 127–132. [Google Scholar] [CrossRef]
- Heckert, K.D.; Komaroff, E.; Adler, U.; Barrett, A.M. Postacute reevaluation may prevent Dysphagia-associated morbidity. Stroke 2009, 40, 1381–1385. [Google Scholar] [CrossRef] [PubMed]
- Nip, W.F.; Perry, L.; McLaren, S.; Mackenzie, A. Dietary intake, nutritional status and rehabilitation outcomes of stroke patients in hospital. J. Hum. Nutr. Diet. 2011, 24, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Carnaby-Mann, G.; Lenius, K. The bedside examination in dysphagia. Phys. Med. Rehabil. Clin. N. Am. 2008, 19, 747–768. [Google Scholar] [CrossRef] [PubMed]
- Daniels, S.K.; Ballo, L.A.; Mahoney, M.C.; Foundas, A.L. Clinical predictors of dysphagia and aspiration risk: Outcome measures in acute stroke patients. Arch. Phys. Med. Rehabil. 2000, 81, 1030–1033. [Google Scholar] [CrossRef] [PubMed]
- McCullough, G.H.; Rosenbek, J.C.; Wertz, R.T.; McCoy, S.; Mann, G.; McCullough, K. Utility of clinical swallowing examination measures for detecting aspiration post-stroke. J. Speech. Lang. Hear. Res. 2005, 48, 1280–1293. [Google Scholar] [CrossRef]
- Warnecke, T.; Teismann, I.; Oelenberg, S.; Hamacher, C.; Ringelstein, E.B.; Schäbitz, W.R.; Dziewas, R. The safety of fiberoptic endoscopic evaluation of swallowing in acute stroke patients. Stroke 2009, 40, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Juenemann, M.; Viard, M.; Meyer, M.; Reuter, I.; Prosiegel, M.; Kaps, M.; Tanislav, C. Adjustment of oral diet based on flexible endoscopic evaluation of swallowing (FEES) in acute stroke patients: A cross-sectional hospital-based registry study. BMC Neurol. 2019, 19, 282. [Google Scholar] [CrossRef]
- Bax, L.; McFarlane, M.; Green, E.; Miles, A. Speech-language pathologist-led fiberoptic endoscopic evaluation of swallowing: Functional outcomes for patients after stroke. J. Stroke Cerebrovasc. Dis. 2014, 23, e195–e200. [Google Scholar] [CrossRef]
- Cohen, D.L.; Roffe, C.; Beavan, J.; Blackett, B.; Fairfield, C.A.; Hamdy, S.; Havard, D.; McFarlane, M.; McLauglin, C.; Randall, M.; et al. Post-stroke dysphagia: A review and design considerations for future trials. Int. J. Stroke 2016, 11, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Teuschl, Y.; Trapl, M.; Ratajczak, P.; Matz, K.; Dachenhausen, A.; Brainin, M. Systematic dysphagia screening and dietary modifications to reduce stroke-associated pneumonia rates in a stroke-unit. PLoS ONE 2018, 13, e0192142. [Google Scholar] [CrossRef] [PubMed]
- Brogan, E.; Langdon, C.; Brookes, K.; Budgeon, C.; Blacker, D. Respiratory infections in acute stroke: Nasogastric tubes and immobility are stronger predictors than dysphagia. Dysphagia 2014, 29, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Brady, S.L.; Rao, N.; Gibbons, P.J.; Williams, L.; Hakel, M.; Pape, T. Face-to-face versus online training for the interpretation of findings in the fiberoptic endoscopic exam of the swallow procedure. Adv. Med. Educ. Pract. 2018, 9, 433–441. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total Cohort (n = 63) | NCOD (n = 22) | COD (n = 41) | p (NCOD vs. COD) |
|---|---|---|---|---|
| Age | 75.25 (10.08) | 73.73 (11.49) | 76.27 (8.32) | p > 0.05 |
| Male | 44 | 12 | 32 | p < 0.05 |
| Female | 19 | 10 | 9 | p > 0.05 |
| Stroke type | ||||
| p < 0.05 | ||||
| Ischemic | 53 | 18 | 35 | p > 0.05 |
| Hemorrhage | 10 | 4 | 6 | |
| FIM points at admission | 49.32 (21.91) | 42.72 (18.91) | 49.37 (22.08) | p > 0.05 |
| FIM points at discharge | 71.44 (31.10) | 68.50 (13.15) | 74.50 (17.40) | p < 0.05 |
| Dysphagia assessment prior to NR | ||||
| Cinical diagnostic only | 8 | 3 | 5 | p > 0.05 |
| Instrumental diagnostic only | 0 | 0 | 0 | p > 0.05 |
| Clinical and instrumental diagnostic | 11 | 5 | 6 | p > 0.05 |
| Assessment not specified. | ||||
| No documentation of | 6 | 2 | 4 | p > 0.05 |
| dysphagia assessment | 38 | 12 | 26 | p < 0.05 |
| Feeding tube dependency at admission to NR | ||||
| PEG-Tube | 27 | 10 | 17 | p > 0.05 |
| NG-Tube | 14 | 8 | 6 | p > 0.05 |
| Time from admission to clinical assessment in days | 0 (0.82) | 0 (0.815) | 0 (0.825) | p > 0.05 |
| Time from admission to first FEES in days | 5 (3.95) | 4 (1.98) | 5 (2.01) | p > 0.05 |
| Length of stay in rehabilitation in days | 38 (20) | 39 (20.5) | 38 (19.5) | p > 0.05 |
| Pneumonia | 24 | 8 | 16 | p < 0.05 |
| Functional Oral Intake Scale | |||
|---|---|---|---|
| T1 | T2 | T3 | |
| Differential values 2.59 | Differential values 2.79 | Differential values 3.67 | |
| T1 | 0.210 | 0.001 | |
| T2 | 0.210 | 0.001 | |
| T3 | 0.001 | 0.001 | |
| Functional Oral Intake Scale at T1 | ||||
|---|---|---|---|---|
| Groups | PAS I–II | PAS III–V | PAS VI–VIII | PAS VIII |
| Differential values 4.000 | Differential values 3.875 | Differential values 1.902 | Differential values 1.815 | |
| PAS I–II | 0.999 | 0.103 | 0.094 | |
| PAS III–V | 0.999 | 0.001 | 0.001 | |
| PAS VI–VIII | 0.103 | 0.001 | 0.997 | |
| PAS VIII | 0.094 | 0.001 | 0.997 | |
| Functional Oral Intake Scale at T2 | ||||
| Groups | Differential values 5.500 | Differential values 4.083 | Differential values 2.195 | Differential values 2.000 |
| PAS I–II | 0.391 | 0.001 | 0.001 | |
| PAS III–V | 0.391 | 0.001 | 0.001 | |
| PAS VI–VIII | 0.001 | 0.001 | 0.964 | |
| PAS VIII | 0.001 | 0.001 | 0.964 | |
| Functional Oral Intake Scale at T3 | ||||
| Groups | Differential values 7.000 | Differential values 5.250 | Differential values 2.902 | Differential values 2.482 |
| PAS I–II | 0.243 | 0.001 | 0.001 | |
| PAS III–V | 0.243 | 0.001 | 0.001 | |
| PAS VI–VIII | 0.001 | 0.001 | 0.758 | |
| PAS VIII | 0.001 | 0.001 | 0.758 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pekacka-Egli, A.M.; Kazmierski, R.; Lutz, D.; Pekacka-Falkowska, K.; Maszczyk, A.; Windisch, W.; Spielmanns, M. Reassessment of Poststroke Dysphagia in Rehabilitation Facility Results in Reduction in Diet Restrictions. J. Clin. Med. 2021, 10, 1714. https://doi.org/10.3390/jcm10081714
Pekacka-Egli AM, Kazmierski R, Lutz D, Pekacka-Falkowska K, Maszczyk A, Windisch W, Spielmanns M. Reassessment of Poststroke Dysphagia in Rehabilitation Facility Results in Reduction in Diet Restrictions. Journal of Clinical Medicine. 2021; 10(8):1714. https://doi.org/10.3390/jcm10081714
Chicago/Turabian StylePekacka-Egli, Anna Maria, Radoslaw Kazmierski, Dietmar Lutz, Katarzyna Pekacka-Falkowska, Adam Maszczyk, Wolfram Windisch, and Marc Spielmanns. 2021. "Reassessment of Poststroke Dysphagia in Rehabilitation Facility Results in Reduction in Diet Restrictions" Journal of Clinical Medicine 10, no. 8: 1714. https://doi.org/10.3390/jcm10081714
APA StylePekacka-Egli, A. M., Kazmierski, R., Lutz, D., Pekacka-Falkowska, K., Maszczyk, A., Windisch, W., & Spielmanns, M. (2021). Reassessment of Poststroke Dysphagia in Rehabilitation Facility Results in Reduction in Diet Restrictions. Journal of Clinical Medicine, 10(8), 1714. https://doi.org/10.3390/jcm10081714








